Abstract
Ampelopsis grossedentata (AG) is mainly distributed in Chinese provinces and areas south of the Yangtze River Basin. It is mostly concentrated or scattered in mountainous bushes or woods with high humidity. Approximately 57 chemical components of AG have been identified, including flavonoids, phenols, steroids and terpenoids, volatile components, and other chemical components. In vitro studies have shown that the flavone of AG has therapeutic properties such as anti-bacteria, anti-inflammation, anti-oxidation, enhancing immunity, regulating glucose and lipid metabolism, being hepatoprotective, and being anti-tumor with no toxicity. Through searching and combing the related literature, this paper comprehensively and systematically summarizes the research progress of AG, including morphology, traditional and modern uses, chemical composition and structure, and pharmacological and toxicological effects, with a view to providing references for AG-related research.
Keywords: Ampelopsis grossedentata, China medicinal plants, phytochemistry
1. Botanical Description
There are approximately 30 species of vitaceae and ampelopsis plants in the world, and approximately 17 species and mutations are distributed in China, most of which are endemic to China and mainly distributed in hillside shrubs and forests in the southwest, south, and northeast of China [1]. Ampelopsis grossedentata (AG) is a genus of ampelopsis in the family Rutaceae of the angiosperm family Magnoliaceae [2]. The roots of the whole plant are slender, fibrous, and partially curved. They have climbing, branched stems with longitudinal ribs on the surface. Moreover, they are glabrous, and the nodes are dilated. The tendrils are bifurcated and spaced two internodes apart on opposite sides of the leaves [3]. The leaves are bipinnate, petioles are 1.5–3.0 cm long, stipules are caducous, the upper leaves of the branches are almost sessile, apical leaflets have petioles, lateral leaflets are sessile, and both sides of the leaves are glabrous. The cymes arise from leaf axils or branch apices opposite the leaves. The calyx is discoid and 2.2 mm in diameter. They have five oblong-shaped petals and five stamens, and the flower disc is shallowly cup-shaped. The fruit is a berry, which is nearly spherical and purple-black when mature, with a diameter of 3–6 mm. Their flowering period is from June to September, and the fruiting period is from July to November [4].
2. Use
2.1. Traditional Uses
AG has a long history of use in China as an ancient medicinal and food homologous plant. The Chinese folk drink AG dates back 600 years to the period of “Divine Farmer“ testing a hundred varieties of herbs.” People of the Zhuang and Yao nationalities were the first to utilize it [5]. Afterward, it was extensively used in the Tujia, Lahu, Dong, Jino, and Hakka nationalities. AG was first recorded in the Classic of Tea [6], which has the functions of clearing heat and detoxifying, relieving cough and phlegm, promoting blood circulation and dredging collaterals, protecting the liver, and dispelling rheumatism [7]. Since then, ancient texts such as “Ying Shan Zheng Yao” [8] and “Cao Mu Bian Fang” [9] have used this name to record AG and its effects. The usage of AG was recorded in the “Chinese Materia Medica,” mostly for internal use, using 15–30 g of AG in decoction or tea making. The nickname and traditional use of rattan tea vary in each region, and the specific content is presented in Table 1.
Table 1.
Effects of rattan tea in different regions.
| Nationality/Region | Nickname | Site of Use | Role of Tradition |
|---|---|---|---|
| Fujian Hakka | Ampelopsis grossedentata | Stem and leaf | Clearing heat and moisturizing the lung, anti-inflammation and detoxification, reducing blood pressure and fat, and eliminating fatigue [10]. |
| SHE-Minority in Sanming, Fujian Province | AG | Young stems and leaves | Heat stroke, mouth sores, aphonia, toothache, equine dental sores, and foot eczema [11]. |
| Guangxi (Zhuang nationality) | Sweet tea | Leaves and shoots | Good medicine for clearing heat and moisturizing the lung, eliminating phlegm and cough, and stopping bleeding and swelling [12]. |
| Hubei (Tujia Family) | Musty Tea | Leaves and shoots | Drinking tea can prevent and treat hypertension, and external application of fresh plants can treat Carbuncle swelling [13]. |
| Xiangxi (Hmong) | AG | Young leaf | Cool and quench thirst, as one of the teas for “oil tea” [14]. |
| Yao nationality | Tian Po tea, AG | The whole plant was used as medicine | Treatment of throat swelling and pain, cold and fever, icteric hepatitis, sore boils, anti-inflammatory, antibacterial, reducing three high, liver protection, liver protection, antioxidant, anti-tumor [15,16]. |
| Fanjing Mountain area, Guizhou province | AG, Sweet tea, White tea, Bang Bang tea |
Tender stem and leaves | Prevention and treatment of hypertension, treatdamp-heat dysentery, pruritus of the skin, and ulcer or ulcer. It has the functions of nourishing the liver and kidney, moistening the lungs, relieving coughs, relieving drowsiness, and promoting sobriety [6]. |
| JiNuo nationality | AG | ★ | Chewing and swallowing to treat toothache [17]. |
| Dong nationality | AG | The whole plant was used as medicine | Beverage tea and treat skin and external diseases [18]. |
| LaHu nationality | AG | ★ | Daily consumption of tea [18]. |
| Hengdong County, Hunan Province | AG | ★ | People used to treat cuts, falls, swollen gums, oral ulcers, gastric ulcers, influenza, pneumonia, hypertension, diabetes, osteoporosis, hemorrhoids, constipation, anti-drinking poisoning, and cardiovascular diseases [19]. |
| Yingde city, Lianzhou city, Guangdong province | Wild AG, AG, White tea, Lai Li tea, Nectar tea | ★ | Treat colds and fevers, sore throats, icteric hepatitis, sore boils, hypertension, hyperlipidemia, etc. [20]. |
Note: ★ It shows that there is no detailed record of the use site of AG in this area.
2.2. Modern Uses
Common products on the market include various AG drinks and “Hua An Baimaohou” boxed products refined from the tender stems and leaves of AG [21]. Luo Anling [22] combined AG, Lobed Kudzuvine root, and corn oligopeptide into a compound formula and conducted an intervention test on the drunk mouse model. The results showed that the compound formula down-regulated the expression levels of NF-κB and TNF-α, thereby improving liver tissue damage and reducing the extent of liver cell damage caused by alcohol. Zhang et al. [23] formulated a creamy yellow soymilk-AG compound drink composite drink with a 5:1 ratio of soymilk and AG juice volume with 0.03% sucralose and 0.2% carboxymethyl cellulose as a stabilizer. The formulation had a bean fragrance, a slight AG fragrance, and high nutritional and therapeutic value. The AG and ginseng capsules prepared from AG, Codonopsis ginseng, Lycium barbarum, and starch have the effects of lowering blood glucose, blood lipids, and blood pressure, enhancing immunity, and delaying aging [24]. The compound tea bag made of AG, Qingqian willow, and mulberry leaves lowers blood glucose, and the compound tea bag is more convenient to brew. The liquid when tea bags are brewed in boiling water is transparent, has the unique aroma of AG, and is rich in total flavonoids and polysaccharides, which have good market prospects [25]. In the study of Zheng et al. [26], AG was extracted with water at 80 °C for 30 min. Sucrose, salt, and citric acid were added at 7.40%, 0.09%, and 0.04%, respectively, and a sucrose-type AG beverage was prepared. The formula can be blended into a xylitol-type AG beverage by adding 9.08%, 0.08%, and 0.05% of xylitol, salt, and citric acid, respectively.
3. Phytochemistry
AG is rich in flavonoids, phenols, steroids, terpenoids, water-soluble polysaccharides, and other volatile components, including dihydromyricetin, myricetin, grossedentatasin, grossedentataside, quercetin, rutinum, quercetin-3-O-β-D-glucoside, gallic acid, gallicin, ethyl gallate, and gallic-β-D-glucoside [27,28,29,30], of which flavonoids are the most abundant compounds (up to 43%) [31], while dihydromyricetin (up to 30%, m/m) is the most abundant among the flavonoids [32,33]. This section summarizes the distribution, location, and molecular and structural formulae of the various compounds in AG by referring to other literature [34] (Table 2 and Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7).
Table 2.
Various chemical compositions 1 in AG.
| Active Component | Molecular Formula | Distribution | References | |
|---|---|---|---|---|
| Flavonoids | ||||
| 1 | Myricetin | C15H10O8 | tender stem and leaf | [35] |
| 2 | Kaempferol | C15H10O6 | stem and leaf | [36] |
| 3 | Quercetin | C15H10O7 | tender stem and leaf | [37] |
| 4 | Myricetin-3′-O-β-D-xylopyranoside | C15H9O7 | leaf | [38] |
| 5 | Dihydrokaempferol | C15H12O6 | leaf | [38] |
| 6 | Dihydroquercetin | C15H12O7 | stem and leaf | [36] |
| 7 | Dihydromyricetin | C15H12O8 | tender stem and leaf | [39] |
| 8 | Taxifolin | C15H12O7 | stem and leaf | [27] |
| 9 | (2R,3S)-5,7,3′,4′,5′-pentahydroxyflavanonol | C15H12O8 | stem and leaf | [40] |
| 10 | 6,7-dihydroxy-3′-methoxy-4′,5′-methylenedioxyisoflavone | C17H12O7 | stem and leaf | [41] |
| 11 | 6,7-dihydroxy-3′-methoxy-4, 5′-methylenedioxyisoflavone 6-O-β-D-glucopyranoside | C17H11O6 | stem and leaf | [41] |
| 12 | 6,7-dihydroxy-3′-methoxy-4′,5′-methylenedioxyisoflavone 6-O-α-L-rhamnopyranoside | C17H11O6 | stem and leaf | [41] |
| 13 | 6,7-dihydroxy-3′-methoxy-4′,5′-methylenedioxyisoflavone 6-O-β-D-xylopyranosyl-(1-6)-β-D-glucopyranoside | C17H11O6 | stem and leaf | [41] |
| 14 | Hesperetin | C15H13O6 | stem and leaf | [36] |
| 15 | 5,7,3′,4′,5′-pentahydroxyflavanone | C15H12O7 | stem and leaf | [40] |
| 16 | Grossedentatasin | C16H15O3 | stem and leaf | [28] |
| 17 | Grossedentataside | C16H15O3 | stem and leaf | [28] |
| 18 | Luteolin | C15H10O6 | stem | [42] |
| 19 | Vitexin | C15H9O5 | stem | [42] |
| 20 | Myricetrin | C15H9O7 | aerial part | [43] |
| 21 | Rutinum | C15H9O6 | tender stem and leaf | [37] |
| 22 | Myricetin-3-O-β-D-galactopyranoside | C15H9O7 | stem and leaf | [42] |
| 23 | Apigenin | C15H10O5 | stem and leaf | [36] |
| 24 | 5,7-dihydroxy-3′4′-dihydroxyflavone-3-O-6″-rhamnose | C15H9O6 | leaf | [44] |
| 25 | 5,7-dihydroxy-3′4′5′-trihydroxyflavone-3-O-6″-rhamnose | C15H9O7 | leaf | [44] |
| 26 | Afzelechin | C15H9O6 | stem and leaf | [27,38] |
| 27 | Astragalin | C15H9O5 | stem and leaf | [27,38] |
| 28 | Quercetin-3-O-α-L-rhamnopyranoside | C15H9O6 | stem and leaf | [27,38] |
| 29 | Quercetin-3-O-β-D-glucoside | C15H9O6 | stem and leaf | [27,38] |
| 30 | Myricetin-3-O-β-D- galactoside | C15H9O7 | stem and leaf | [27,38] |
| 31 | Bellidifolin | C14H10O6 | stem and leaf | [43] |
| Phenols | ||||
| 32 | Gallic acid | C7H6O5 | tender stem and leaf | [45] |
| 33 | Gallicin | C8H8O5 | stem and leaf | [27] |
| 34 | Ethyl gallate | C9H10O5 | tender stem and leaf | [30] |
| 35 | Gallic-β-D-glucose | C7H5O5 | tender stem and leaf | [30] |
| Steroids and terpenoids | ||||
| 36 | Stigmasterol | C29H48O | tender stem and leaf | [45] |
| 37 | β-sitosterol | C29H50O | tender stem and leaf | [45] |
| 38 | Oleanolic acid | C30H48O3 | stem and leaf | [36] |
| 39 | Ambrein | C30H52O | aerial part | [43] |
| Volatile components and other compounds | ||||
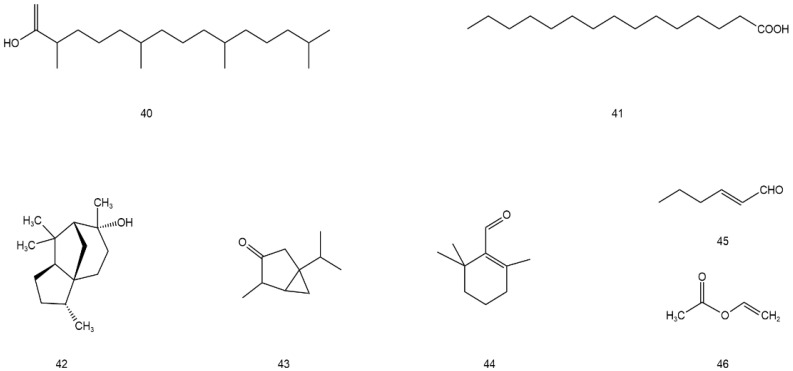
| 40 | Phytol | C20H40O | tender stem and leaf | [46] |
| 41 | n-Hexadecanoic acid | C15H30O2 | tender stem and leaf | [46] |
| 42 | Cedrol | C15H26O | tender stem and leaf | [46] |
| 43 | β-thujone | C10H16O | stem and leaf | [47] |
| 44 | β-cyclocitral | C10H16O | stem and leaf | [48] |
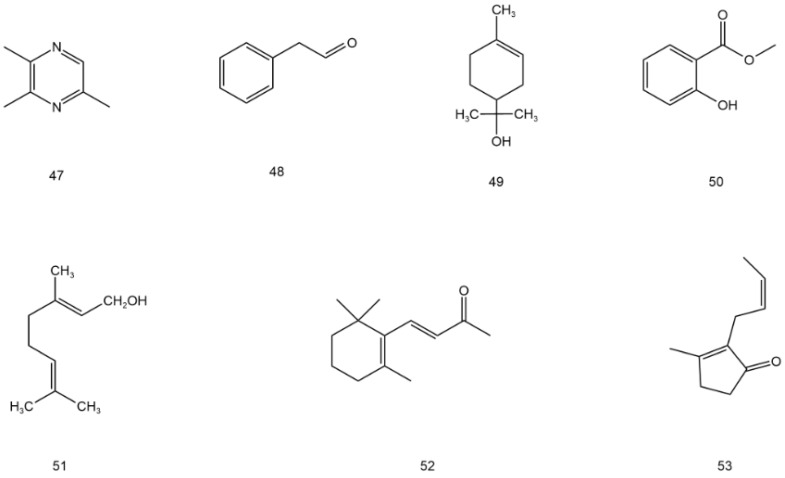
| 45 | (E)-2-hexenal | C6H10O | stem and leaf | [49] |
| 46 | (Z)-3-hexenyl hexanote | C4H6O2 | stem and leaf | [49] |
| 47 | Trimethyl pyrazine | C7H10N2 | stem and leaf | [49] |
| 48 | Phenylacetaldehyde | C8H8O | stem and leaf | [49] |
| 49 | α-terpinol | C10H20O | stem and leaf | [49] |
| 50 | Methyl salicylate | C8H8O3 | stem and leaf | [49] |
| 51 | Geraniol | C10H18O | stem and leaf | [49] |
| 52 | β-ionone | C13H20O | stem and leaf | [49] |
| 53 | (Z)-jasmone | C10H14O | stem and leaf | [49] |
| 54 | 6,10,14-trimethyl-2-pen-tadecanone | C18H36O | stem and leaf | [49] |
| 55 | Nerolidol | C15H26O | stem and leaf | [49] |
| 56 | Palmitic acid | C15H30O2 | stem and leaf | [49] |
| 57 | Emodin | C17H14O3 | stem and leaf | [49] |
1 Two-dimensional structural formula of various components was drawn using Kingdraw software, v5.0.
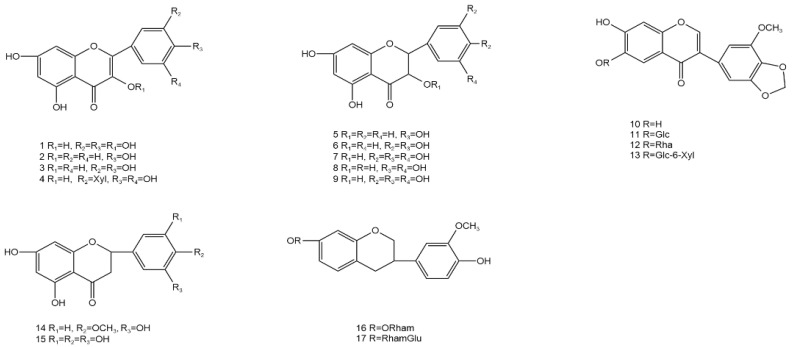
Figure 1.
Structural formula of flavonoids in AG (1–17).
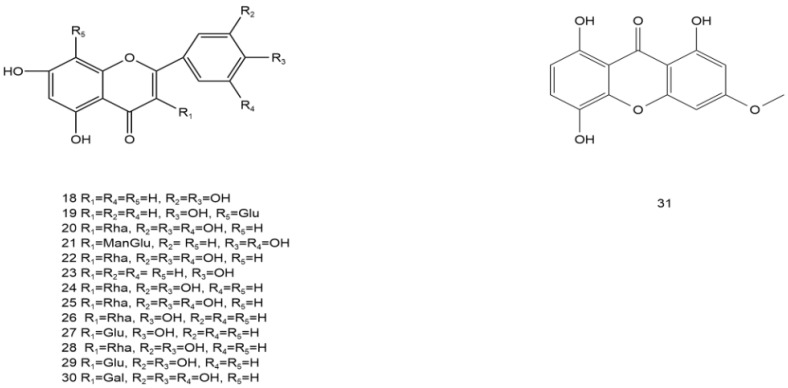
Figure 2.
Structural formula of flavonoids in AG (18–31).
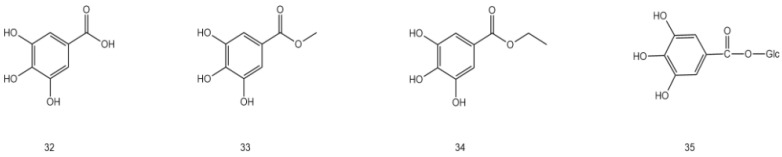
Figure 3.
Structural formula of phenolic compounds in AG.
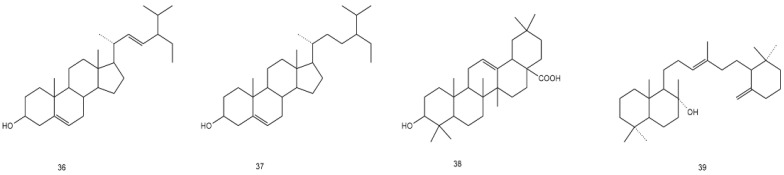
Figure 4.
Structural formula of steroids and terpenoids in AG.
Figure 5.
Structural formula of volatile components and other compounds in AG (40–46).
Figure 6.
Structural formula of volatile components and other compounds in AG (47–53).
Figure 7.
Structural formula of volatile components and other compounds in AG (54–57).
3.1. Flavonoids
Approximately 30 flavonoids have been isolated from AG, including dihydromyricetin, quercetin, myricetin, hesperidin, apigenin, and ampelopsin, among which 3-dihydroxyquercetin and dihydromyricetin are isomers of each other. Among the components, dihydromyricetin has the highest content and is the main active ingredient. The total flavonoid content of different leaf types also varies, with the highest content of 31.66% in medium-sized leaves and as high as 43.4% to 45.52% in tender stems and leaves.
Zhou [39], Zhou [50], and Liu et al. [51] were the first to isolate dihydromyricetin from a mixture of young stems and leaves of AG, which is only found in Ampelopsis of the grape family and is a chemical taxonomic characteristic of this genus [17]. Yuan et al. [43] first isolated two compounds, myricetin and myricetrines, from the aerial part of AG, and the contents of total flavonoids, dihydromyricetin, and myricetin in AG were 43.4–44.0%, 37.4–38.5%, and 1.72–1.78%, respectively. Qin et al. [52] extracted and isolated two flavonoid components, myricetin and dihydromyricetin, from the fine powder of AG of Yao nationality in Guangxi, and myricetin was isolated from this plant for the first time.
3.2. Phenols
Phenols are a pharmacodynamic component of AG. Phenolic compounds have been isolated, including gallic acid, gallicin, ethyl gallate, gallic-β-D-glucose, catechin, epicatechin, and epigallocatechin. Bai et al. [40] used column chromatography to isolate and identify eight phenols in AG samples in Zhangjiajie. These include dihydromyricetin (I), 5,7,3′,4′,5′-pentahydroxydihydroflavone (II), gallic β-D-glucoside (III), gallic acid (IV), ethyl gallate (V), myricetrin (VI), (2R, 3S)-5,7,3′,4′,5′-pentahydroxydihydroflavonol (VII), and myricetin (VIII). Wang et al. [45] first isolated and identified gallic acid using chromatography and spectral analysis. Wang [27] first isolated gallicin from the ethyl acetate extract of AG. Zhang [30] isolated gallic β-D-glucose from the mixture of spring and summer tender stems and leaves of AG.
3.3. Steroids and Terpenoids
Three steroidal compounds and one terpenoid have been isolated from AG, including stigmasterol, β-sitosterol, oleanolic acid, and the terpenoid ambrein [34]. Yuan et al. [43] first isolated ambrein (terpenoids) and β-sitosterol (steroids) from the aboveground part of AG. Wang et al. [45] first isolated stigmasterol using chromatography and spectral analysis. He et al. [36] isolated oleanolic acid using repeated silica gel, polyamide column chromatography, and recrystallization.
3.4. Water-Soluble Polysaccharide
Pan [53] extracted selenium-rich polysaccharide from fermented AG in Zhangjiajie Bachong Village using hot water extraction and ethanol precipitation. Under optimal extraction conditions, the extraction ratio of polysaccharides was 11.26%. Furthermore, the polysaccharide was composed of Man, GlcUA, Glc, Gal, and Xyl, which had the excellent scavenging ability of the 2,2-biphenyl-1-picrylhydrazyl (DPPH) free radical. Four proteins, AGP-3, AGP-4, ALPS, and ASPS, were further isolated from the water-soluble polysaccharides [54,55]. Zou et al. [56] used the hot water extraction method to extract the polysaccharide of AG. The results showed that in August, the polysaccharide content of the stems and leaves of AG in Huaihua City, Hunan Province, was the highest at 1.41% and 2.79%, respectively.
3.5. Volatile Components and Other Compounds
Three major volatile oil components, phytol, n-Hexadecanoic acid, and cedrol, were isolated from AG samples (Provided by the Science and Technology Commission of Yongding District, Zhangjiajie, China) using gas chromatography-mass spectrometry (GC-MS) analysis [46]. Zhang et al. [47] isolated and identified 63 volatile oil components of AG using solid phase microextraction/GC-MS, including ethanol, 1,3-di-tert-butyl benzene, 2-methyl decane, 2,4-dimethyl-1-decene, 2,4-dimethyl-1-heptene, 7-methy-11-undecene, 2,6-dimethyl-nonane, sabinene, α-pinene, ethylformate, tridecane, β-thujone, 1-undecene, 2,3,5,8-tetramethyl-decane, and 4.6-dimethyl-dodecane.
4. Pharmacological Properties
4.1. Anti-Inflammatory and Analgesia
Dihydromyricetin (purified moecule), the main active ingredient in AG, has a good anti-inflammatory and analgesic effect. Intraperitoneal injection of dihydromyricetin (purified moecule) into collagen-induced arthritis (CIA) mice showed a significant reduction in erythema and swelling of the paws after dihydromyricetin treatment, and the results of the pathological analysis of the knee joint and peripheral blood cytokine assay confirmed the anti-arthritic effect of dihydromyricetin [57]. Wu et al. [58] further demonstrated that dihydromyricetin (purified moecule) is protective against CIA in mice by blocking the phosphorylation of I-κB kinase and impeding the activation of nuclear factor-κB (NF-κB) to inhibit the formation of osteoclasts. Jia et al. [59] reported that dihydromyricetin (purified moecule) effectively inhibited rainfarin-induced production and expression of several pro-inflammatory cytokines (IL-1β, TNF-α, and IL-17) in mouse bone marrow-derived macrophages, thereby attenuating the pancreatic and systemic inflammatory responses in mice with acute pancreatitis (AP) induced by rainfarin injection. In a model of foot and plantar swelling and acute inflammation in rats caused by carrageenan gum, dihydromyricetin (purified moecule) significantly reduced carrageenan-induced paw foot swelling in rats, and the effect was similar to the anti-inflammatory effect of the positive control drugs indomethacin, dexamethasone, and methotrexate [60]. Cheng et al. observed that AG (crude plant extract) protects against ulcerative colitis (UC) in mice by inhibiting IRAK1/TAF6/ NF-κB-mediated inflammatory signaling pathways [61].
4.2. Anti-Oxidation
Various anti-oxidation experiments have shown that the antioxidant activity of flavonoid-rich AG extract is similar to that of tert-butylhydroquinone [62] and has high scavenging activity against 1,1-Diphenyl-2-picrylhydrazyl (DPPH) radicals [63]. AG extract and dihydromyricetin (purified moecule) exhibited strong DPPH radical scavenging capacity and high oxygen radical absorbance capacity during in vitro experiments, and this antioxidant activity may act through the activation of the cellular Nrf2/Keap 1 pathway [64]. The antioxidant effect of various AG components differs, among which the strongest scavenging ability for DPPH free radicals was observed in Myricetin (purified moecule), the strongest scavenging ability for O2− was observed in dihydromyricetin, and the scavenging effect for hydroxyl radicals (OH-) was in the order myricetin > dihydromyricetin > total flavonoids of AG [65], and dihydromyricetin also had strong antioxidant activity in soybean oil, cooked beef, and sausage [66,67]. After constructing a hyperlipidemia (HLP) model in rats, AG flavonoid suspension was instilled, and the results showed that AG reduced serum total cholesterol, triglyceride, and low-density lipoprotein levels, increased high-density lipoprotein levels, reduced lipid peroxidation [68], improved the body’s antioxidant capacity, and improved blood lipid levels in HLP rats.
4.3. Reduction of Blood Sugar, Blood Pressure, and Blood Lipid Levels
Some studies have demonstrated the therapeutic efficacy of AG (crude plant extract) in preventing and treating triple highs (blood glucose, blood pressure, and blood lipids) in patients with adult type 2 diabetes mellitus (T2DM) who supplemented their daily diet with AG. The results showed a significant decrease in fasting plasma glucose, glycated albumin, cystatin C, and retinol-binding protein-4 levels in participants supplemented with AG, indicating that AG supplementation improves glycemic and renal function parameters in patients with adult T2DM and has a good hypoglycemic effect [69]. Furthermore, AG (crude plant extract) ameliorates glucose and lipid metabolism disorders in type 2 diabetic rats by inhibiting gluconeogenesis through the Akt/Foxo1/Pck2 signaling pathway and fatty acid synthesis through the SREBP1c/Fasn signaling pathway [70]. A hypertensive rat model was artificially created by surgery, followed by continuous gavage of an aqueous solution of AG (crude plant extract) and feeding for 30 days. Finally, blood pressure and heart rate values were detected, and the results showed that AG effectively lowered blood pressure but did not affect heart rate [71].
4.4. Liver and Kidney Protection
The treatment of hamsters on a high-fat diet with AG (crude plant extract) and dihydropyrimethamine (purified moecule) reduced high-fat-induced weight gain and liver lipid deposition and lowered serum TG and TC levels, indicating that AG and dihydropyrimethamine have a protective effect on hamster liver during a high-fat diet [72]. Three animal models of hyperuricemia induced by yeast, hypoxanthine, and potassium oxyzincate were constructed and treated with AG total flavonoids by gavage after successful modeling. Compared with the control group, the serum uric acid (UA) content, xanthine oxidase (XOD), and adenosine deaminase (ADA) activities of mice in the AG total flavonoids group were significantly reduced. Total flavonoids of AG exerted anti-hyperuricemia effects by reducing serum UA levels, inhibiting XOD and ADA enzyme activities, and improving UA metabolism, with certain effects on improving kidney injury and preserving kidney function [73]. Wu et al. [74] established a mouse model of hyperuricemia by intraperitoneal injection of potassium oxyzincate and gavaged AG extract for 14 days. The serum UA, creatinine, urea nitrogen, aspartate amino transaminase, glutamate amino transaminase, and liver XOD levels of mice in the AG extract group showed a decreasing trend. The pathological histomorphological examination showed that AG significantly improved the liver and kidney tissues of mice with hyperuricemia, indicating that it effectively lowered UA levels and protected the liver and kidney in mice with hyperuricemia. Human experiments have also shown that AG extract reduces postprandial UA levels by inhibiting XOD activity and promoting the excretion of UA [75].
4.5. Tumor Suppression and Anti-Tumor Activity
Studies have demonstrated that dihydromyricetin (purified moecule) has anti-cancer, anti-tumor, and atherosclerotic properties [76]. Xylene was used to induce acute inflammation in the auricles of mice, followed by the administration of different doses of AG total flavonoids. The swelling of the auricles was inhibited in all groups of mice treated with AG total flavonoids, and the higher the concentration of AG total flavonoids, the higher the inhibition rate of swelling in the auricles of mice [77]. Zhang et al. reported that dihydromyricetin (purified moecule) inhibited the migration and invasion of SK-Hep-1 and MHCC97L hepatocellular carcinoma cell lines by downregulating the expression of MMP-9 protein [78]. The compound dihydromyricetin isolated from AG healing tissues exhibited desirable cytotoxic activity against mouse breast cancer (4T1), human lung adenocarcinoma (A549), and human non-small cell lung cancer (NCI-H1975) cell lines [79], providing a new direction for developing anti-cancer drugs. Dihydromyricetin (purified moecule) also inhibits the growth of cholangiocarcinoma cell lines by increasing the expression of miR-455-3p, which ultimately inhibits tumor growth [80].
Dihydromyricetin (purified moecule) inhibits the expression of nasopharyngeal carcinoma cell lines by blocking the Wnt/β-linked protein signaling pathway and regulating the expression of downstream proteins in nasopharyngeal carcinoma [81], thus serving as a novel agent for treating nasopharyngeal carcinoma. In addition, it downregulates the expression of cyclins A1, D1, SMAD3, and SMAD4 to inhibit cell cycle arrest and ultimately inhibit the proliferation of human choriocarcinoma cells [82]. In contrast, the inhibition of ovarian cancer (OC) cells is mainly due to the upregulation of cleaved cystatin-3 and Bax/Bcl-2 in OC cells, which ultimately induces apoptosis [83]. Dihydromyricetin can also be combined with multiple drugs (adriamycin, nedaplatin, oxaliplatin, nedaplatin, erlotinib, and paclitaxel) to increase the sensitivity of cancer cells to drugs, reverse multidrug resistance, and exert synergistic anti-cancer effects [84].
4.6. Antibacterial
AG extract and its active ingredient, dihydromyricetin (purified moecule), showed good antibacterial activity against Staphylococcus aureus [85]. Xiao-Nian Xiao evaluated the antibacterial ability of dihydromyricetin (purified moecule) extracted from AG using the disc diffusion method [86] and showed that dihydromyricetin exhibited desirable antibacterial activity against five food-borne bacteria (Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Salmonella paratyphi, and Pseudomonas aeruginosa), causing lysis of bacterial cell walls, leakage of intracellular components, and inhibition of the bacterial tricarboxylic acid cycle pathway, ultimately leading to bacterial death. In vitro antibacterial tests with AG powder and dihydromyricetin (purified moecule) against Aeromonas vivax and Aeromonas hydrophila showed good antibacterial effects [87]. Muhammad Umair et al. [88] used the agar pore diffusion method to evaluate the antibacterial activity of AG extracts and isolated six compounds from AG extracts. Approximately 5,7,8,3,4-pentahydroxyisoflavone (C15H10O7) exhibited the strongest antibacterial activity against Bacillus cereus (AS11846) and Staphylococcus aureus (CMCCB26003) and can be used as a potent antibacterial agent in the food processing industry.
5. Toxicology
Many trials have verified the safety of the clinical use of AG. Zhong et al. [89] administered the total flavonoids of Guangxi AG to rats at doses of 1.5 g/kg and 0.3 g/kg for 12 weeks. Two weeks after the Guangxi AG was discontinued, the appearance, behavior, body weight, organ coefficient, and blood biochemistry indices of the rats were not significantly different from those of the control group. Furthermore, no obvious lesions related to drug toxicity were observed in the pathological examination, and there was no delayed toxic reaction after discontinuing the drug, indicating that Guangxi AG flavonoid had no significant toxic effect on rats after long-term administration. In another study [90], three gavages (0.2 mL/10 g/bodyweight) were administered three hours apart to mice that were observed for two weeks. Three mutagenicity tests were negative. The mice had good growth and development, with no adverse effects on body weight or food utilization. Furthermore, the routine blood and white blood cell levels of the dose group were not significantly different from those of the control group. Furthermore, no substantial pathological changes were observed in the organs, indicating that the Enshi selenium-rich AG used in the test was safe and non-toxic.
6. Conclusions
This review summarizes the various uses of AG and its morphology, phytochemistry, pharmacology, and toxicology. Furthermore, we summarized the use of AG in different regions and the molecular and structural formulas of various components based on previous studies. AG is mainly used to treat jaundice-type hepatitis, cold, and fever and lower blood lipid and sugar levels. Moreover, single compounds of AG, such as flavonoids and dihydromyricetin, have various biological activities, including anti-inflammatory, analgesic, anti-tumor, and antibacterial properties.
Dihydromyricetin significantly inhibits certain cancer cells, suggesting that dihydromyricetin, a component of AG, may serve as a novel anti-cancer drug or as a synergistic drug with existing anti-cancer drugs, providing a new direction for anti-cancer and anti-tumor therapy.
Acknowledgments
All the authors thank Yan Liu for her assistance in revising the grammar of the manuscript. We thank all the members for their generous participation.
Author Contributions
R.-R.W.: conceptualization; formal analysis; investigation; methodology; writing—original draft. X.L.: conceptualization; funding acquisition; supervision; validation; writing—review and editing. Y.-H.C. and X.P.: conceptualization; investigation; supervision; writing—review and editing. G.-F.L.: conceptualization; funding acquisition; supervision; validation. Z.Y. and Z.-K.L.: resources; supervision; validation; writing—review and editing. Y.W. and Z.-Y.L.: conceptualization; resources; supervision; validation; writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Source databases for these publications include the Science Citation Index (SCI), SCI Expanded, and the Chinese Science Citation Database.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was financially supported by the Hunan Provincial Graduate Innovation Project (Grant No. QL20230182).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Zheng X.J., Zhang W.T., Xie L.Y. Advances in the pharmacological effects of Ampelopsis. Fujian J. Med. 2013;35:152–154. [Google Scholar]
- 2.Li Y.J., Zhong Z.X. An introduction to the research and application of Ampelopsis grossedentata. Chin. Ethn. Folk. Med. 2013;22:26–27. [Google Scholar]
- 3.Liu J.X., Zhou T.D. Biopharmacological study of Ampelopsis grossedentata. Chin. Herb. Med. 1999;6:459–463. [Google Scholar]
- 4.Chen Z.Z. Wild natural tea-like plant substitute tea-wild Ampelopsis grossedentata. Guangxi Trop. Agric. 2003;3:27–28. [Google Scholar]
- 5.Food and Drug Administration of Guangxi Zhuang Autonomous Region . Quality Standard of Zhuang Medicine in Guangxi Zhuang Autonomous Region. Volume 1 Guangxi Science and Technology Press; Nanning, China: 2008. [Google Scholar]
- 6.Lai F. The current situation of research and development of sweet tea resources in China and the direction of development in the new century. Tea Newsl. 2003;3:25–28. [Google Scholar]
- 7.Yang Z.G. Calling tea is not “tea” of Ampelopsis grossedentata. Life World. 2019;8:74–83. doi: 10.1016/j.lfs.2019.04.036. [DOI] [Google Scholar]
- 8.Hu S.H. Ying Shan Zheng Yao. China Chinese Medicine Publishing House; Beijing, China: 2009. [Google Scholar]
- 9.Liu S.S. Cao Mu Bian Fang. Chongqing Publishing House; Chongqing, China: 2009. [Google Scholar]
- 10.Xiong L.Z., Lin L.P., Xu W., Huang Z.H. The literature examination and microscopic identification of Hakka Ampelopsis grossedentata. Fujian Tradit. Chin. Med. 2022;53:26–29. [Google Scholar]
- 11.Song W.W., Dai Q.L., Huang M.E., Lei F.X. A preliminary investigation of the folk herb “Ampelopsis grossedentata”. Hunan J. Tradit. Chin. Med. 1996;5:41. [Google Scholar]
- 12.Wei J.F., Huang X.C. Identification of the original plant of Guangxi sweet tea. Chin. Med. Mater. 1996;1:16–17. [Google Scholar]
- 13.Song B.Z., Wan D.R. Survey of common botanicals (Vitaceae) of the Tujia family in Hubei Province. Chin. J. Ethn. Folk. Med. 2003;2:117–118. [Google Scholar]
- 14.Cao T.R. Daily beverage plants of the Hmong compatriots. J. Plants. 1994;2:12–13. [Google Scholar]
- 15.Jiang C.W. Research progress on the efficacy of Yao medicine Ampelopsis grossedentata. Zhuang Yao Med. Natly. Res. 2021;1:52–55+183. [Google Scholar]
- 16.Yang H. Research on the chemical composition and pharmacology of Yao nationality Ampelopsis grossedentata. Fujian Tea. 2020;42:9–10. [Google Scholar]
- 17.Yang S.L., Guo S.R., Zheng P.C. JiNuo Nationality Medicine. Yunnan Science and Technology Press; Kunming, China: 2001. p. 300. [Google Scholar]
- 18.Xu L.J., Ma P., Xiao W., Peng Y., He C.N., Xiao P.G. Preliminary investigation on the ancient and modern application history of different tea-Ampelopsis grossedentata. China Mod. Tradit. Chin. Med. 2012;14:62–66. [Google Scholar]
- 19.Chen X.W., Dong B.P., Chen J.M., Xiao C.F., Li B.S., Luo D.S. Research on the medicinal value of Ampelopsis grossedentata for drinking. Hunan Agric. Sci. 2007;6:180–181. [Google Scholar]
- 20.Li J.C., Li S.Y., Wang Y., Wang W.N. Chemical composition, pharmacological effects and quality marker (Q-marker) prediction analysis of Ampelopsis grossedentata. J. Southwest Univ. Natl. (Nat. Sci. Ed.) 2021;47:254–266. [Google Scholar]
- 21.Pan L.H., Luo S.Z. Research on the application of Ampelopsis grossedentata in soft drinks. Beverag. Ind. 2005;1:37–38. [Google Scholar]
- 22.Luo A.L. Master’s Thesis. Shanghai Jiaotong University; Shanghai, China: 2019. Study on the Hepatoprotective Effect of a Compound Formula of Pueraria Lobata, Ampelopsis grossedentata and Corn Oligopeptide on Alcohol in Mice. [Google Scholar]
- 23.Zhang S.X., Gao M., Liang L.L., Cheng Y., Tang G.Y. Development of soy milk Ampelopsis grossedentata composite beverage. Food Res. Dev. 2016;37:78–80. [Google Scholar]
- 24.Deng F.L. Master’s Thesis. Hubei College of Traditional Chinese Medicine; Wuhan, China: 2009. Pharmacological Study of Ampelopsis grossedentata Ginseng Capsules. [Google Scholar]
- 25.Li D.M., Li C.L., Wen Y. Development of Ampelopsis grossedentata compound bagged tea and its in vitro hypoglycemic effect. Mod. Food Sci. Technol. 2022;38:228–236. [Google Scholar]
- 26.Zheng C., Zeng J.H., Gu C.Q., Wei X.C. Development of health Ampelopsis grossedentata beverage. J. Guangzhou Univ. (Nat. Sci. Ed.) 2008;7:34–39. [Google Scholar]
- 27.Wang D.Y., Liu J.M., Zhang J.T., Zheng S.J. Research on the chemical composition of the Ampelopsis grossedentata. Subtrop. Plant Lett. 1998;2:39–44. [Google Scholar]
- 28.Wang D.Y., Xu S.Y. Isolation and structure determination of grossedentatasin. J. Zhangzhou Norm. Coll. (Nat. Sci. Ed.) 1996;2:62–64+67. [Google Scholar]
- 29.Wang D.Y. Isolation and structure determination of grossedentataside. J. Zhangzhou Norm. Coll. (Nat. Sci. Ed.) 1999;4:42–45. [Google Scholar]
- 30.Zhang Y.S., Yang W.L., Cui C. Study on the chemical composition of Ampelopsis grossedentata. Chin. Herb. Med. 2003;5:21–22. [Google Scholar]
- 31.He G.X., Pei G., Zhou T.D., Zhou X.X. Determination of total flavonoids and dihydromyricetin in Ampelopsis grossedentata. Chin. J. Tradit. Chin. Med. 2000;7:39–41. [PubMed] [Google Scholar]
- 32.Wang J., He L., Zheng N. Dihydromyricetin in Ampelosis grossedentata collected from different habitats. Chin. Tradit. Patent. Med. 2014;36:145–147. [Google Scholar]
- 33.Zhang J.Y., Chen Y., Luo H.Q. Recent update on the pharmacological effects and mechanisms of dihydromyricetin. Front. Pharmacol. 2018;9:1204. doi: 10.3389/fphar.2018.01204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo H.J., Liu Y., Liu J.B., Nong G., Liu W. Research progress on chemical composition and pharmacological activity of Ampelopsis grossedentata. Chin. Pat. Med. 2022;44:2595–2601. [Google Scholar]
- 35.Qin J.P., Xu X.Q., Li J.J. Study on the chemical composition of Guangxi Yao Ampelosis grossedentata. Nat. Prod. Res. Dev. 1997;4:41–43. [Google Scholar]
- 36.He G.X., Pei G., Du F.L., Ou Y.W., Li B. Study on the chemical composition of Ampelosis grossedentata. China Mod. Tradit. Chin. Med. 2007;12:11–13. [Google Scholar]
- 37.Zhang Y.S. Master’s Thesis. Hunan Agricultural University; Changsha, China: 2001. Isolation and Identification of Chemical Constituents of the Tea-Like Plant Ampelosis grossedentata and Study of Dihydromyricetin Extraction Technology. [Google Scholar]
- 38.Fu M., Li X.Y., Wang D.Y., Guo M.Q. Study of flavonoids in the leaves of Ampelosis grossedentata. Chin. J. Pharm. Sci. 2015;50:574–578. [Google Scholar]
- 39.Zhou T.D., Zhou X.X. Isolation, structural identification and pharmacological activity of dihydroflavonol from Ampelosis grossedentata. Chin. J. Pharmacol. 1996;8:10–13. [Google Scholar]
- 40.Bai X.X., Xia G.P., Zhao N.X., Dong H.L., Shao Z.Y., Han Y.M. Phenolic chemical composition of Ampelosis grossedentata from Zhangjiajie. Chin. Med. Mater. 2013;36:65–67. [PubMed] [Google Scholar]
- 41.Wang D.Y., Zheng Z.Z., Xu S.Y., Zheng S.Z. Four new isoflavones from Ampelopsis grossedentata. J. Asian Nat. Prod. Res. 2002;4:303–308. doi: 10.1080/1028602021000049104. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y.F., Ying L., Sun D., Zhang S.K., Zhu Y.J., Xu P. Supercritical carbon dioxide extraction of bioactive compounds from Ampelopsis grossedentata stems: Process optimization and antioxidant activity. Int. J. Mol. Sci. 2011;12:6856–6870. doi: 10.3390/ijms12106856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan A.X., Huang X.M., Chen J. The chemical composition of explicit dental snake grapes. Chin. J. Tradit. Chin. Med. 1998;6:39–40+63. [Google Scholar]
- 44.Du Q.Z., Chen P., Jerz G., Winterhalter P. Preparative separation of flavonoid glycosides in leaves extract of Ampelopsis grossedentata using high-speed counter-current chromatography. J. Chromatogr. A. 2004;1040:147–149. doi: 10.1016/j.chroma.2004.03.062. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y., Zhou L.L., Li R., Wang Y. Study on the chemical composition of Ampelopsis grossedentata. Chin. Med. Mater. 2002;4:254–256. [PubMed] [Google Scholar]
- 46.Zhang Y.S., Yang W.L., Xiong H.P. Analysis of the chemical composition of volatile oil of Ampelopsis grossedentata. J. Hunan Agric. Univ. (Nat. Sci. Ed.) 2001;2:100–101. [Google Scholar]
- 47.Zhang S.X., Ao K.H., Zeng Q.H., Liu Y. Determination of chemical composition in volatile oil of Ampelopsis grossedentata by gas chromatography-mass spectrometry. China Brew. 2014;33:140–143. [Google Scholar]
- 48.Lai M.L., Yu J.P. Analysis of cyclic hydrocarbons in commercial Ampelopsis grossedentata by SPME-GC/MS. J. Mt. Agric. Biol. 2014;33:92–94. [Google Scholar]
- 49.Wang H.F., You X.Q. Gas chromatographic determination of aroma composition in Ampelopsis grossedentata. Nat. Prod. Res. Dev. 1996;4:47–50. [Google Scholar]
- 50.Zhou X.X., Zhou T.D., Tan C.S. The effect of dihydroyangenin bark on the contractile response of smooth muscle of rabbit thoracic aortic strips. Mod. Appl. Pharmacol. 1997;2:8–11+68. [Google Scholar]
- 51.Liu J.X., Zhou T.D. Experimental study on the effect of Yao Ampelopsis grossedentata on rabbit intestinal smooth muscle and detoxification. Chin. J. Ethn. Med. 1998;2:44–45. [Google Scholar]
- 52.Qin J.P., Tan J.N., Ou Y., Lu S.Y. Study on the method of determination of dihydroyangme barkin in Guangxi Yao Ampelopsis grossedentata. Chin. J. New Drugs. 2004;10:915–917. [Google Scholar]
- 53.Pan X.Y. Master’s Thesis. Dalian Ocean University; Dalian, China: 2019. Isolation and Identification of Polysaccharide from Ampelopsis grossedentata and Its Tablet Preparation. [Google Scholar]
- 54.Luo Z.Y., Cheng C., Li W., Wu M.C. Isolation and purification of water-soluble polysaccharides from Ampelopsis grossedentata. Food Sci. 2007;1:151–154. [Google Scholar]
- 55.Wang Y., Bian X., Park J., Ying L., Qian L., Xu P. Physicochemical properties, in vitro antioxidant activities and inhibitory potential against α-glucosidase of polysaccharides from Ampelopsis grossedentata leaves and stems. Molecules. 2011;16:7762–7772. doi: 10.3390/molecules16097762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zou Y.M., Fu M. Extraction process and content study of polysaccharides from Ampelopsis grossedentata. J. Huaihua Coll. 2011;30((Suppl. S1)):39–42. [Google Scholar]
- 57.Wu J., Zhao F.T., Fan K.J., Zhang J., Xu B.X., Wang Q.S., Tang T.T., Wang T.Y. Dihydromyricetin Inhibits Inflammation of Fibroblast-Like Synoviocytes through Regulation of Nuclear Factor-κB Signaling in Rats with Collagen-Induced Arthritis. J. Pharmacol. Exp. Ther. 2019;368:218–228. doi: 10.1124/jpet.118.253369. [DOI] [PubMed] [Google Scholar]
- 58.Wu J., Fan K.J., Wang Q.S., Xu B.X., Cai Q., Wang T.Y. Dihydromyricetin protects the knee joints of rats with collagen-induced arthritis by inhibition of NF-κB signaling and osteoclastic bone resorption. Food Funct. 2020;11:6251–6264. doi: 10.1039/D0FO00396D. [DOI] [PubMed] [Google Scholar]
- 59.Jia R., Ma J., Meng W., Wang N. Dihydromyricetin inhibits caerulin-induced TRAF3-p38 signaling activation and acute pancreatitis response. Biochem. Biophys. Res. Commun. 2018;503:1696–1702. doi: 10.1016/j.bbrc.2018.07.101. [DOI] [PubMed] [Google Scholar]
- 60.Wang R., Pi J., Su X., Liu J., Zeng X., Wong I., Huang L., Zhou H., Cai J., Li T., et al. Dihydromyricetin suppresses inflammatory responses in vitro and in vivo through inhibition of IKKβ activity in macrophages. Scanning. 2016;38:901–912. doi: 10.1002/sca.21339. [DOI] [PubMed] [Google Scholar]
- 61.Chen Y.L., Zhang Y.L., Dai Y.C., Tang Z.P. Systems pharmacology approach reveals the antiinflammatory effects of Ampelopsis grossedentata on dextran sodium sulfate-induced colitis. World J. Gastroenterol. 2018;24:1398–1409. doi: 10.3748/wjg.v24.i13.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao J., Liu B., Ning Z., Zhao R., Zhang A., Wu Q. Characterization and antioxidant activity of flavonoid-rich extracts from leaves of Ampelopsis grossedentata. J. Food Biochem. 2009;33:808–820. doi: 10.1111/j.1745-4514.2009.00253.x. [DOI] [Google Scholar]
- 63.Gao Q., Ma R., Chen L., Shi S., Cai P., Zhang S., Xiang H. Antioxidant profiling of vine tea (Ampelopsis grossedentata): Off-line coupling heart-cutting HSCCC with HPLC–DAD–QTOF-MS/MS. Food Chem. 2017;225:55–61. doi: 10.1016/j.foodchem.2016.11.122. [DOI] [PubMed] [Google Scholar]
- 64.Xie K., He X., Chen K., Chen J., Sakao K., Hou D.X. Antioxidant Properties of a Traditional Vine Tea, Ampelopsis grossedentata. Antioxidants. 2019;8:295. doi: 10.3390/antiox8080295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ou X.H., Ye Y., Huang Q.J., Liu H.G., Song Y.F. Study on antioxidant activity of Ampelopsis grossedentata. Nat. Prod. Res. Dev. 2013;25:245–248. [Google Scholar]
- 66.Ye L., Wang H., Duncan S.E., Eigel W.N., O’Keefe S.F. Antioxidant activities of Vine Tea (Ampelopsis grossedentata) extract and its major component dihydromyricetin in soybean oil and cooked ground beef. Food Chem. 2015;172:416–422. doi: 10.1016/j.foodchem.2014.09.090. [DOI] [PubMed] [Google Scholar]
- 67.Wang E.H., Qin Z.H., Yang L.S., Lu F.X., Qin T. Antioxidant activity evaluation of dihydromyricetin from Ampelopsis grossedentata on Guizhou traditional sausage. Food Sci. Technol. 2017;42:128–132. [Google Scholar]
- 68.Xu Y., Wang W.Z., Zhou Y.F. A study of Ampelopsis grossedentata flavonoids improving blood lipid levels in rats by influencing the AMPK signaling pathway. Chongqing Med. 2022;51:1626–1630+1637. [Google Scholar]
- 69.Ran L., Wang X., Lang H., Xu J., Wang J., Liu H., Mi M., Qin Y. Ampelopsis grossedentata supplementation effectively ameliorates the glycemic control in patients with type 2 diabetes mellitus. Eur. J. Clin. Nutr. 2019;73:776–782. doi: 10.1038/s41430-018-0282-z. [DOI] [PubMed] [Google Scholar]
- 70.Xiang J., Lv Q., Yi F., Song Y., Le L., Jiang B., Xu L., Xiao P. Dietary Supplementation of Vine Tea Ameliorates Glucose and Lipid Metabolic Disorder via Akt Signaling Pathway in Diabetic Rats. Molecules. 2019;24:1866. doi: 10.3390/molecules24101866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liao Y.P., Wang S., An F.X., Ge Z.W., Lan Y., Zhang Z. Study on the hypotensive effect of Ampelopsis grossedentata. China Mod. Drug Appl. 2013;7:229–230. [Google Scholar]
- 72.Fan L., Qu X., Yi T., Peng Y., Jiang M., Miao J., Xiao P. Metabolomics of the Protective Effect of Ampelopsis grossedentata and Its Major Active Compound Dihydromyricetin on the Liver of High-Fat Diet Hamster. Evid. Based Complement. Altern. Med. 2020;2020:3472578. doi: 10.1155/2020/3472578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ma E.X., Li S.Y., Cheng X.Y., Wang W.N., Wang Y., Wang Y., Li J.C. Study on the anti-hyperuricemia and renal function protection effect of total flavonoids of Ampelopsis grossedentata. Chin. Pharmacol. Clin. 2021;37:80–85. [Google Scholar]
- 74.Wu S.H., Yu H.J., Yu T., Zheng Z.G., Ke D., Zhao L. Study on the hypo-ureic acid effect of Ampelopsis grossedentata extract. Food Ind. Sci. Technol. 2021;42:350–355. [Google Scholar]
- 75.Shimizu Y., Sakurada T., Matsuoka S., Yui K., Hosoi T. Vine Tea (Ampelopsis grossedentata) Extract Suppresses Postprandial Uric Acid Levels via Both Inhibition of Xanthine Oxidase and Facilitation of Uric Acid Excretion. Curr. Dev. Nutr. 2020;4((Suppl. S2)):474. doi: 10.1093/cdn/nzaa045_107. [DOI] [Google Scholar]
- 76.Tong H., Zhang X., Tan L., Jin R., Huang S., Li X. Multitarget and promising role of dihydromyricetin in the treatment of metabolic diseases. Eur. J. Pharmacol. 2020;870:172888. doi: 10.1016/j.ejphar.2019.172888. [DOI] [PubMed] [Google Scholar]
- 77.Chen S., Yu J.P. Experimental study on the anti-inflammatory and antibacterial effects of total flavonoids of Ampelopsis grossedentata. J. Guiyang Coll. Tradit. Chin. Med. 2013;35:1–3. [Google Scholar]
- 78.Zhang Q.Y., Li R., Zeng G.F., Liu B., Liu J., Shu Y., Liu Z.K., Qiu Z.D., Wang D.J., Miao H.L., et al. Dihydromyricetin inhibits migration and invasion of hepatoma cells through regulation of MMP-9 expression. World J. Gastroenterol. 2014;20:10082–10093. doi: 10.3748/wjg.v20.i29.10082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Y., Kumar P.S., Tan S., Huang C., Xiang Z., Qiu J., Tan X., Luo J., He M. Anticancer and antibacterial flavonoids from the callus of Ampelopsis grossedentata; a new weapon to mitigate the proliferation of cancer cells and bacteria. RSC Adv. 2022;12:24130–24138. doi: 10.1039/D2RA03437A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li X., Yang Z.S., Cai W.W., Deng Y., Chen L., Tan S.L. Dihydromyricetin Inhibits Tumor Growth and Epithelial-Mesenchymal Transition through regulating miR-455-3p in Cholangiocarcinoma. J. Cancer. 2021;12:6058–6070. doi: 10.7150/jca.61311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ye L., Yin G., Jiang M., Tu B., Li Z., Wang Y. Dihydromyricetin Exhibits Antitumor Activity in Nasopharyngeal Cancer Cell Through Antagonizing Wnt/β-catenin Signaling. Integr. Cancer Ther. 2021;20:1534735421991217. doi: 10.1177/1534735421991217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zuo Y., Lu Y., Xu Q., Sun D., Liang X., Li X., Li Y. Inhibitory effect of dihydromyricetin on the proliferation of JAR cells and its mechanism of action. Oncol. Lett. 2020;20:357–363. doi: 10.3892/ol.2020.11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang F., Chen X., Yuan D., Yi Y., Luo Y. Golgi reassembly and stacking protein 65 downregulation is required for the anti-cancer effect of dihydromyricetin on human ovarian cancer cells. PLoS ONE. 2019;14:e0225450. doi: 10.1371/journal.pone.0225450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu J., Xiao Z., Li H., Zhu N., Gu J., Wang W., Liu C., Wang W., Qin L. Present Status, Challenges, and Prospects of Dihydromyricetin in the Battle against Cancer. Cancers. 2022;14:3487. doi: 10.3390/cancers14143487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liang H., He K., Li T., Cui S., Tang M., Kang S., Ma W., Song L. Mechanism and antibacterial activity of vine tea extract and dihydromyricetin against Staphylococcus aureus. Sci. Rep. 2020;10:21416. doi: 10.1038/s41598-020-78379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xiao X.N., Wang F., Yuan Y.T., Liu J., Liu Y.Z., Yi X. Antibacterial Activity and Mode of Action of Dihydromyricetin from Ampelopsis grossedentata Leaves against Food-Borne Bacteria. Molecules. 2019;24:2831. doi: 10.3390/molecules24152831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhan X.S., Liu J.X., Zhu Z., Tang J.J., Zhang J.B., Yin S.H., Zhang M.Q. In vitro inhibition of two aquatic pathogenic bacteria by Ampelopsis grossedentata powder and dihydromyricetin. Fish. Res. 2022;44:162–168. [Google Scholar]
- 88.Umair M., Sultana T., Zhu X.Y., Senan A.M., Saqib J., Labiba K., Muhammad A., Mian A.M., Dhama K., Al-Areqi N.A., et al. LC-ESI-QTOF/MS characterization of antimicrobial compounds with their action mode extracted from vine tea (Ampelopsis grossedentata) leaves. Food Sci. Nutr. 2022;10:422–435. doi: 10.1002/fsn3.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhong Z.X., Zhou G.F., Chen X.F., Qin J.P. Long-term toxicity test of total flavonoids of Guangxi Ampelopsis grossedentata. Shizhen Natl. Med. 2003;4:193–195. [Google Scholar]
- 90.Chen Y.Q. Ph.D. Thesis. Huazhong Agricultural University; Wuhan, China: 2007. Extraction and Isolation of Flavonoids and Dihydromyricetin from Garcinia Cambogia, Its Hypolipidemic Effect and Safety Evaluation of Ampelopsis grossedentata. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Source databases for these publications include the Science Citation Index (SCI), SCI Expanded, and the Chinese Science Citation Database.