Abstract
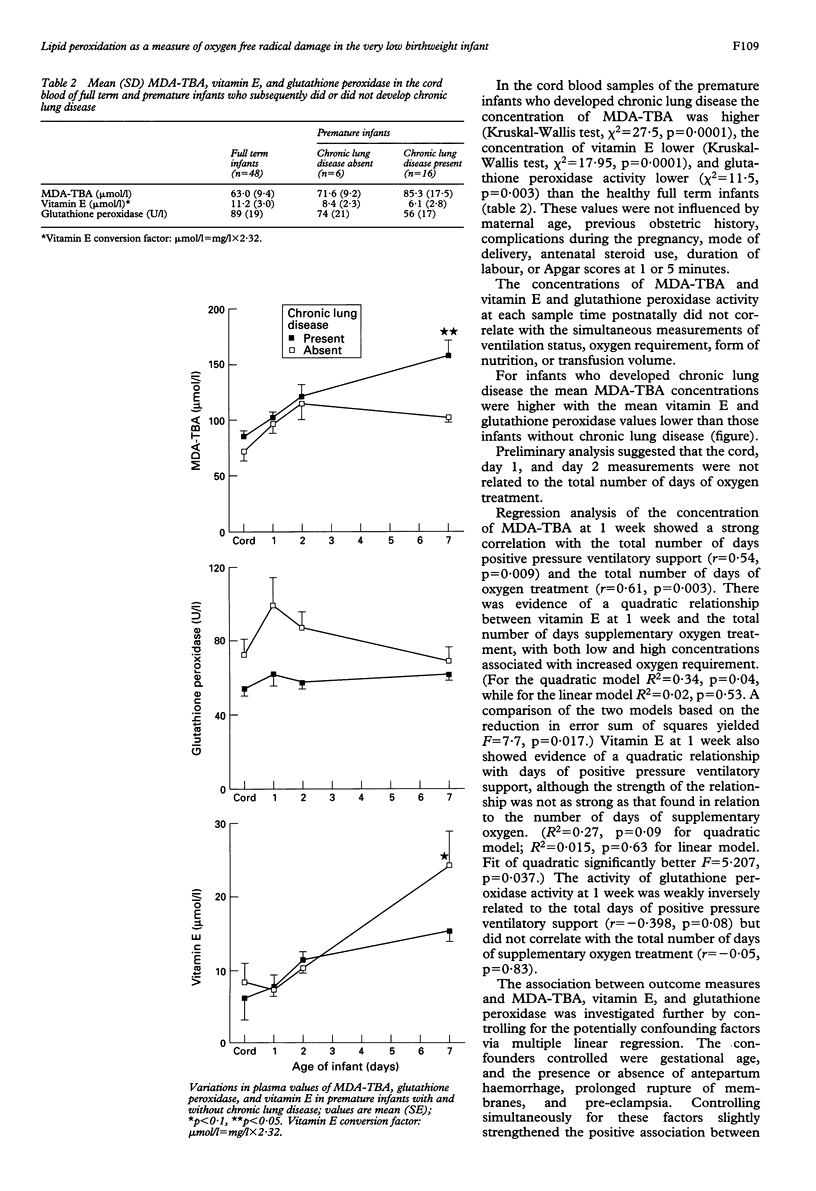
(ABSTRACTOxygen free radical mediated tissue injury is implicated as a major factor in the pathogenesis of the long term complications seen in the premature infant, and direct evidence of their role in the development of these long term problems is lacking. A prospective observational study of 78% of very low birthweight infants admitted to a level III neonatal intensive care unit in 1992 was undertaken to determine the relationship between lipid peroxidation products, antioxidant activity, and outcome. Lipid peroxidation (malondialdehyde-thiobarbituric acid, MDA-TBA) and antioxidant activity (vitamin E and glutathione peroxidase activity) were measured in 22 very low birthweight infants in the cord blood and the infant's blood at 24 hours, 48 hours, and 1 week of age and correlated with outcome measures. The normal range for these measures was established in the cord blood samples of 48 consecutive healthy full term infants. The concentration of MDA-TBA at 1 week correlated with the number of days of oxygen treatment and number of days of positive pressure ventilatory support. Controlling for gestational age and antenatal complications simultaneously the MDA-TBA concentration remained significantly associated with the number of days of oxygen treatment and the number of days of positive pressure ventilatory support. Glutathione peroxidase was low in the premature and full term infants consistent with the low concentrations of selenium known to be present in southern New Zealand. There was evidence of a quadratic relationship between vitamin E at 1 week and the total number of days of supplementary oxygen requirement, with both high and low values associated with increased oxygen requirement. This association, however, did not remain after controlling for gestational age and antenatal complications. These results support the role of oxygen free radicals in mediating tissue damage associated with the development of chronic lung disease in the premature infant.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amorde-Spalding K., D'Harlingue A. E., Phillips B. L., Byrne W. J., Cheng K. S., Cook N. E., Irias J. J. Tocopherol levels in infants < or = 1000 grams receiving MVI pediatric. Pediatrics. 1992 Dec;90(6):992–994. [PubMed] [Google Scholar]
- Ballagi-Pordány G., Richter J., Koltai M., Aranyi Z., Pogátsa G., Schaper W. Is malondialdehyde a marker of the effect of oxygen free radicals in rat heart tissue? Basic Res Cardiol. 1991 May-Jun;86(3):266–272. doi: 10.1007/BF02190606. [DOI] [PubMed] [Google Scholar]
- Bieri J. G., Tolliver T. J., Catignani G. L. Simultaneous determination of alpha-tocopherol and retinol in plasma or red cells by high pressure liquid chromatography. Am J Clin Nutr. 1979 Oct;32(10):2143–2149. doi: 10.1093/ajcn/32.10.2143. [DOI] [PubMed] [Google Scholar]
- Chen Y., Whitney P. L., Frank L. Negative regulation of antioxidant enzyme gene expression in the developing fetal rat lung by prenatal hormonal treatments. Pediatr Res. 1993 Feb;33(2):171–176. doi: 10.1203/00006450-199302000-00016. [DOI] [PubMed] [Google Scholar]
- Darlow B. A., Horwood L. J. Chronic lung disease in very low birthweight infants: a prospective population-based study. J Paediatr Child Health. 1992 Aug;28(4):301–305. doi: 10.1111/j.1440-1754.1992.tb02672.x. [DOI] [PubMed] [Google Scholar]
- Davidge S. T., Hubel C. A., Brayden R. D., Capeless E. C., McLaughlin M. K. Sera antioxidant activity in uncomplicated and preeclamptic pregnancies. Obstet Gynecol. 1992 Jun;79(6):897–901. [PubMed] [Google Scholar]
- Dolamore B. A., Brown J., Darlow B. A., George P. M., Sluis K. B., Winterbourn C. C. Selenium status of Christchurch infants and the effect of diet. N Z Med J. 1992 Apr 22;105(932):139–142. [PubMed] [Google Scholar]
- Draper H. H., Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–431. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- Engle W. A., Yoder M. C., Baurley J. L., Yu P. L. Vitamin E decreases superoxide anion production by polymorphonuclear leukocytes. Pediatr Res. 1988 Mar;23(3):245–248. doi: 10.1203/00006450-198803000-00001. [DOI] [PubMed] [Google Scholar]
- Frank L. Antioxidants, nutrition, and bronchopulmonary dysplasia. Clin Perinatol. 1992 Sep;19(3):541–562. [PubMed] [Google Scholar]
- Frank L., Lewis P. L., Sosenko I. R. Dexamethasone stimulation of fetal rat lung antioxidant enzyme activity in parallel with surfactant stimulation. Pediatrics. 1985 Mar;75(3):569–574. [PubMed] [Google Scholar]
- Frank L., Sosenko I. R. Prenatal development of lung antioxidant enzymes in four species. J Pediatr. 1987 Jan;110(1):106–110. doi: 10.1016/s0022-3476(87)80300-1. [DOI] [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Biology of disease: free radicals and tissue injury. Lab Invest. 1982 Nov;47(5):412–426. [PubMed] [Google Scholar]
- Huston R. K., Jelen B. J., Vidgoff J. Selenium supplementation in low-birthweight premature infants: relationship to trace metals and antioxidant enzymes. JPEN J Parenter Enteral Nutr. 1991 Sep-Oct;15(5):556–559. doi: 10.1177/0148607191015005556. [DOI] [PubMed] [Google Scholar]
- Jobe A. H. Pulmonary surfactant therapy. N Engl J Med. 1993 Mar 25;328(12):861–868. doi: 10.1056/NEJM199303253281208. [DOI] [PubMed] [Google Scholar]
- Kelly F. J., Rodgers W., Handel J., Smith S., Hall M. A. Time course of vitamin E repletion in the premature infant. Br J Nutr. 1990 May;63(3):631–638. doi: 10.1079/bjn19900149. [DOI] [PubMed] [Google Scholar]
- Kloner R. A., Przyklenk K., Whittaker P. Deleterious effects of oxygen radicals in ischemia/reperfusion. Resolved and unresolved issues. Circulation. 1989 Nov;80(5):1115–1127. doi: 10.1161/01.cir.80.5.1115. [DOI] [PubMed] [Google Scholar]
- Lindeman J. H., van Zoeren-Grobben D., Schrijver J., Speek A. J., Poorthuis B. J., Berger H. M. The total free radical trapping ability of cord blood plasma in preterm and term babies. Pediatr Res. 1989 Jul;26(1):20–24. doi: 10.1203/00006450-198907000-00008. [DOI] [PubMed] [Google Scholar]
- Lockitch G., Jacobson B., Quigley G., Dison P., Pendray M. Selenium deficiency in low birth weight neonates: an unrecognized problem. J Pediatr. 1989 May;114(5):865–870. doi: 10.1016/s0022-3476(89)80154-4. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Muller D. P. Vitamin E therapy in retinopathy of prematurity. Eye (Lond) 1992;6(Pt 2):221–225. doi: 10.1038/eye.1992.43. [DOI] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979 Jun;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Paglia D. E., Valentine W. N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967 Jul;70(1):158–169. [PubMed] [Google Scholar]
- Phelps D. L. Neonatal oxygen toxicity--is it preventable? Pediatr Clin North Am. 1982 Oct;29(5):1233–1240. doi: 10.1016/s0031-3955(16)34257-2. [DOI] [PubMed] [Google Scholar]
- Phillips B., Franck L. S., Greene H. L. Vitamin E levels in premature infants during and after intravenous multivitamin supplementation. Pediatrics. 1987 Nov;80(5):680–683. [PubMed] [Google Scholar]
- Pitkänen O. M., Hallman M., Andersson S. M. Correlation of free oxygen radical-induced lipid peroxidation with outcome in very low birth weight infants. J Pediatr. 1990 May;116(5):760–764. doi: 10.1016/s0022-3476(05)82668-x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pierce M., Sosenko I. R., Frank L. Prenatal thyroid releasing hormone and thyroid releasing hormone plus dexamethasone lessen the survival of newborn rats during prolonged high O2 exposure. Pediatr Res. 1992 Oct;32(4):407–411. doi: 10.1203/00006450-199210000-00008. [DOI] [PubMed] [Google Scholar]
- Rosenfeld W., Evans H., Concepcion L., Jhaveri R., Schaeffer H., Friedman A. Prevention of bronchopulmonary dysplasia by administration of bovine superoxide dismutase in preterm infants with respiratory distress syndrome. J Pediatr. 1984 Nov;105(5):781–785. doi: 10.1016/s0022-3476(84)80307-8. [DOI] [PubMed] [Google Scholar]
- Sluis K. B., Darlow B. A., George P. M., Mogridge N., Dolamore B. A., Winterbourn C. C. Selenium and glutathione peroxidase levels in premature infants in a low selenium community (Christchurch, New Zealand). Pediatr Res. 1992 Aug;32(2):189–194. doi: 10.1203/00006450-199208000-00013. [DOI] [PubMed] [Google Scholar]
- Sullivan J. L., Newton R. B. Serum antioxidant activity in neonates. Arch Dis Child. 1988 Jul;63(7 Spec No):748–750. doi: 10.1136/adc.63.7_spec_no.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade C. R., Jackson P. G., Van Rij A. M. Quantitation of malondialdehyde (MDA) in plasma, by ion-pairing reverse phase high performance liquid chromatography. Biochem Med. 1985 Jun;33(3):291–296. doi: 10.1016/0006-2944(85)90003-1. [DOI] [PubMed] [Google Scholar]
- Wade C. R., van Rij A. M. Plasma thiobarbituric acid reactivity: reaction conditions and the role of iron, antioxidants and lipid peroxy radicals on the quantitation of plasma lipid peroxides. Life Sci. 1988;43(13):1085–1093. doi: 10.1016/0024-3205(88)90204-4. [DOI] [PubMed] [Google Scholar]
- Wilson D. C., Tubman R., Bell N., Halliday H. L., McMaster D. Plasma manganese, selenium and glutathione peroxidase levels in the mother and newborn infant. Early Hum Dev. 1991 Oct;26(3):223–226. doi: 10.1016/0378-3782(91)90162-v. [DOI] [PubMed] [Google Scholar]
- Wispe J. R., Roberts R. J. Molecular basis of pulmonary oxygen toxicity. Clin Perinatol. 1987 Sep;14(3):651–666. [PubMed] [Google Scholar]


