Abstract
Introduction
In the INBUILD trial in patients with progressive pulmonary fibrosis other than idiopathic pulmonary fibrosis (IPF), nintedanib slowed the rate of decline in forced vital capacity (FVC; mL/year) over 52 weeks compared with placebo. We assessed the efficacy of nintedanib across subgroups in the INBUILD trial by baseline characteristics.
Methods
We assessed the rate of decline in FVC over 52 weeks and time to progression of interstitial lung disease (ILD) (absolute decline from baseline in FVC % predicted > 10%) or death over the whole trial in subgroups based on sex, age, race, body mass index (BMI), time since diagnosis of ILD, FVC % predicted, diffusing capacity of the lungs for carbon monoxide (DLco) % predicted, composite physiologic index (CPI), GAP (gender, age, lung physiology) stage, use of anti-acid therapy and use of disease-modifying antirheumatic drugs (DMARDs) at baseline.
Results
The effect of nintedanib versus placebo on reducing the rate of decline in FVC over 52 weeks was consistent across the subgroups by baseline characteristics analysed. Interaction p values did not indicate heterogeneity in the treatment effect between these subgroups (p > 0.05). Over the whole trial (median follow-up time ∼19 months), progression of ILD or death occurred in similar or lower proportions of patients treated with nintedanib than placebo across the subgroups analysed, with no heterogeneity detected between the subgroups.
Conclusions
In the INBUILD trial, no heterogeneity was detected in the effect of nintedanib on reducing the rate of ILD progression across subgroups based on demographics, ILD severity or use of anti-acid therapy or DMARDs. These data support the use of nintedanib as a treatment for progressive pulmonary fibrosis.
Trial Registration Number
ClinicalTrials.gov Identifier: NCT02999178.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-023-02668-x.
Keywords: Nintedanib, Antifibrotic agents, Interstitial lung diseases, Pulmonary fibrosis, Demography
Key Summary Points
| Why carry out this study? |
| In the INBUILD trial in patients with progressive pulmonary fibrosis other than idiopathic pulmonary fibrosis, nintedanib slowed the rate of decline in forced vital capacity (FVC; mL/year) over 52 weeks compared with placebo |
| We investigated whether the effect of nintedanib on slowing the rate of decline in FVC was consistent across subgroups based on characteristics at baseline of the INBUILD trial |
| What was learned from the study? |
| No heterogeneity was detected in the effect of nintedanib on reducing the rate of decline in FVC across subgroups based on demographics, severity of interstitial lung disease, use of anti-acid therapy or use of disease-modifying antirheumatic drugs at baseline |
| These data support the use of nintedanib as a treatment for progressive pulmonary fibrosis |
Digital Features
This article is published with digital features, including a slide deck, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.24033384.
Introduction
A proportion of patients with interstitial lung diseases (ILDs) develop progressive disease, characterised by increasing fibrosis on high-resolution computed tomography (HRCT), worsening of lung function and symptoms, and early mortality [1–3]. It has been proposed to use the term “progressive pulmonary fibrosis” or “PPF” to describe such disease in individuals with an ILD other than idiopathic pulmonary fibrosis (IPF) [1]. Decline in forced vital capacity (FVC; mL/year) in patients with PPF is associated with an increased risk of mortality [4–9].
Nintedanib is a tyrosine kinase inhibitor that inhibits processes fundamental to the progression of lung fibrosis [10]. Nintedanib has been licensed for the treatment of IPF, fibrosing ILD associated with systemic sclerosis (SSc-ILD), and progressive fibrosing ILDs of any aetiology. In the randomised placebo-controlled INBUILD trial in patients with PPF (other than IPF), nintedanib reduced the rate of decline in FVC, with an adverse event profile characterised mainly by gastrointestinal events [11–13]. Previous studies have identified patient characteristics associated with a greater rate of ILD progression, including lower FVC, lower diffusing capacity of the lungs for carbon monoxide (DLco) and a usual interstitial pneumonia (UIP) pattern on HRCT [9, 14, 15]. We assessed the efficacy of nintedanib on slowing decline in FVC in the INBUILD trial in subgroups based on baseline characteristics.
Methods
Trial Design
The design of the INBUILD trial (NCT02999178) has been described and the protocol is publicly available [11]. Briefly, patients had an ILD other than IPF with reticular abnormality with traction bronchiectasis (with or without honeycombing) of > 10% extent on HRCT, FVC ≥ 45% predicted and DLco ≥ 30% to < 80% predicted. Patients met criteria for ILD progression within the prior 24 months, based on worsening of FVC, abnormalities on HRCT, or symptoms, despite management deemed appropriate in clinical practice. Patients were randomised to receive nintedanib 150 mg twice daily or placebo, stratified by fibrotic pattern on HRCT (UIP-like fibrotic pattern or other fibrotic patterns [11]). The primary endpoint was the rate of decline in FVC over 52 weeks, assessed in two co-primary analysis populations: the overall population and patients with a UIP-like fibrotic pattern on HRCT. The first 52 weeks of the trial were followed by a period of variable duration during which patients continued to receive blinded treatment until all the patients had completed the trial. The final database lock took place after all patients had completed the follow-up visit or had entered the open-label extension study, INBUILD-ON (NCT03820726); the data available at this point are referred to as data from the whole trial [12]. The INBUILD trial protocol was approved by an Ethics Committee or Institutional Review Board at all the participating centres. The INBUILD trial was carried out in compliance with the protocol and with the principles of the Declaration of Helsinki and the Harmonised Tripartite Guideline for Good Clinical Practice of the International Conference on Harmonisation. The trial was approved by local authorities. All patients provided written informed consent prior to their participation.
Analyses
We assessed the rate of decline in FVC over 52 weeks in subgroups based on the following baseline characteristics: sex (male, female); age (< 65, ≥ 65 years); race (White, Asian, Black); body mass index (BMI) (< 25, ≥ 25 to < 30, ≥ 30 kg/m2); time since diagnosis of ILD (≤ 1, > 1 to ≤ 3, > 3 to ≤ 5, > 5 years); FVC % predicted (≤ 50, > 50 to ≤ 70, > 70 to ≤ 90, > 90); DLco % predicted [≤ and > median (43.3)]; composite physiologic index (CPI) [16] (≤ 45, > 45); GAP stage [17] (I, II or III); taking anti-acid therapy (yes, no); taking disease-modifying anti-rheumatic drugs (DMARDs) (yes, no). Anti-acid therapies were defined based on the WHO Drug Dictionary (version 19.MAR) [Anatomical Therapeutic Chemical codes for ‘antacids’ and ‘drugs for peptic ulcer and gastro-oesophageal reflux disease (GORD)’ and preferred name ‘drugs for acid related disorders’]. DMARDs were defined based on the WHO Drug Dictionary (version 19.MAR) standardised drug grouping excluding denosumab, plus baricitinib.
The rate of decline in FVC over 52 weeks was analysed using a random coefficient regression model (with random slopes and intercepts) including baseline FVC (mL) and HRCT pattern (UIP-like fibrotic pattern or other fibrotic patterns), and interaction terms for baseline-by-time, treatment-by-subgroup and treatment-by-subgroup-by-time. Interaction p values (based on F tests) were calculated as an indicator of the potential heterogeneity of the effect of nintedanib versus placebo across subgroups. In the same subgroups, we analysed the time to progression of ILD (defined as an absolute decline in FVC % predicted > 10%) or death over the whole trial, using Cox proportional hazards models (stratified by HRCT pattern) with terms for treatment to derive hazard ratios and 95% confidence intervals (CIs). Cox proportional hazards models with additional terms for subgroup and treatment-by-subgroup were used to calculate interaction p values. No adjustments for multiplicity were made. Analyses were performed using SAS version 9.4.
The analyses of the rate of decline in FVC over 52 weeks in subgroups by sex, age and race were pre-specified. The other analyses were conducted post hoc.
Results
A total of 663 patients were treated (332 with nintedanib, 331 with placebo). Their ILD diagnoses were hypersensitivity pneumonitis (26.1%), autoimmune disease-related ILDs (25.6%), idiopathic non-specific interstitial pneumonia (iNSIP) (18.9%), unclassifiable idiopathic interstitial pneumonia (IIP) (17.2%) and other fibrosing ILDs (12.2%) [11]. At baseline, 53.7% were male; 60.8% were aged ≥ 65 years; 73.6% were White; 28.4%, 36.6% and 35.0% had a BMI < 25, ≥ 25 to < 30 and ≥ 30 kg/m2, respectively; 20.2%, 34.3%, 19.8% and 25.7% had a time since diagnosis of ILD of ≤ 1, > 1 to ≤ 3, > 3 to ≤ 5 and > 5 years, respectively. Regarding disease severity, 11.3%, 47.4%, 31.5% and 9.8% of patients had FVC % predicted ≤ 50, > 50 to ≤ 70, > 70 to ≤ 90, > 90, respectively; 69.1% had CPI > 45; 55.7% were at GAP stage II or III. Anti-acid medication was taken by 57.3% of patients and DMARDs by 13.7% of patients. The baseline characteristics of the subgroups are summarised in Tables S1–S11 in the Supplementary Material.
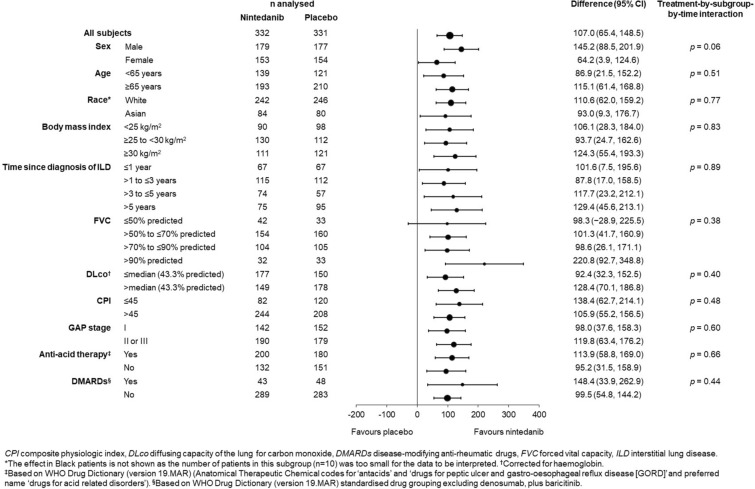
The effect of nintedanib versus placebo on reducing the rate of decline in FVC over 52 weeks was consistent across the subgroups based on baseline characteristics (p > 0.05 for all) (Fig. 1; Table S12 in the Supplementary Material).
Fig. 1.
Rate of decline in FVC (mL/year) over 52 weeks of the INBUILD trial in subgroups by baseline characteristics
The median follow-up time for progression of ILD or death was ∼19 months. Progression of ILD or death occurred in similar or lower proportions of patients treated with nintedanib than placebo across the subgroups analysed, with no heterogeneity detected across subgroups (p > 0.05 for all) (Table 1).
Table 1.
Time to ILD progression (absolute decline in FVC % predicted ≥ 10%) or death over the whole INBUILD trial in subgroups by baseline characteristics
| Baseline characteristic | n/N (%) with event | Hazard ratio (95% CI) |
Treatment-by-subgroup interaction p value |
|
|---|---|---|---|---|
| Nintedanib | Placebo | |||
| Sex | ||||
| Male | 76/179 (42.5) | 105/177 (59.3) | 0.62 (0.46, 0.84) | 0.66 |
| Female | 58/153 (37.9) | 76/154 (49.4) | 0.72 (0.51, 1.01) | |
| Age | ||||
| < 65 years | 49/139 (35.3) | 54/121 (44.6) | 0.74 (0.50, 1.09) | 0.58 |
| ≥ 65 years | 85/193 (44.0) | 127/210 (60.5) | 0.64 (0.49, 0.85) | |
| Racea | ||||
| White | 100/242 (41.3) | 137/246 (55.7) | 0.65 (0.50, 0.84) | 0.93 |
| Asian | 32/84 (38.1) | 41/80 (51.3) | 0.71 (0.45, 1.13) | |
| Body mass index (BMI) | ||||
| < 25 kg/m2 | 44/90 (48.9) | 59/98 (60.2) | 0.74 (0.50, 1.10) | 0.85 |
| ≥ 25 to < 30 kg/m2 | 48/130 (36.9) | 61/112 (54.5) | 0.65 (0.44, 0.94) | |
| ≥ 30 kg/m2 | 42/111 (37.8) | 61/121 (50.4) | 0.64 (0.43, 0.95) | |
| Time since diagnosis of ILD | ||||
| ≤ 1 year | 23/67 (34.3) | 41/67 (61.2) | 0.44 (0.26, 0.74) | 0.09 |
| > 1 to ≤ 3 years | 54/118 (45.8) | 59/112 (52.7) | 0.82 (0.56, 1.18) | |
| > 3 to ≤ 5 years | 24/73 (32.9) | 34/57 (59.6) | 0.50 (0.29, 0.84) | |
| > 5 years | 33/73 (45.2) | 47/95 (49.5) | 0.88 (0.56, 1.39) | |
| FVC % predicted | ||||
| ≤ 50% | 25/42 (59.5) | 20/33 (60.6) | 0.70 (0.38, 1.29) | 0.44 |
| > 50 to ≤ 70% | 55/154 (35.7) | 89/160 (55.6) | 0.57 (0.41, 0.80) | |
| > 70 to ≤ 90% | 41/104 (39.4) | 50/105 (47.6) | 0.80 (0.53, 1.21) | |
| > 90% | 13/32 (40.6) | 22/33 (66.7) | 0.51 (0.26, 1.03) | |
| DLco % predictedb | ||||
| ≤ median (43.3%) | 82/177 (46.3) | 92/150 (61.3) | 0.65 (0.48, 0.88) | 0.97 |
| > median (43.3%) | 51/149 (34.2) | 87/178 (48.9) | 0.66 (0.46, 0.93) | |
| CPI | ||||
| ≤ 45 | 25/82 (30.5) | 57/120 (47.5) | 0.61 (0.38, 0.98) | 0.77 |
| > 45 | 108/244 (44.3) | 122/208 (58.7) | 0.66 (0.51, 0.85) | |
| GAP stage | ||||
| I | 41/142 (28.9) | 68/152 (44.7) | 0.59 (0.40, 0.88) | 0.42 |
| II or III | 93/190 (48.9) | 113/179 (63.1) | 0.70 (0.53, 0.93) | |
| Taking anti-acid therapyc | ||||
| Yes | 88/201 (43.8) | 101/180 (56.1) | 0.72 (0.54, 0.96) | 0.34 |
| No | 46/131 (35.1) | 80/151 (53.0) | 0.58 (0.40, 0.83) | |
| DMARDsd | ||||
| Yes | 16/43 (37.2) | 31/48 (64.6) | 0.49 (0.26, 0.91) | 0.28 |
| No | 118/289 (40.8) | 150/283 (53.0) | 0.70 (0.55, 0.89) | |
CPI composite physiologic index, DLco diffusing capacity of the lung for carbon monoxide, DMARDs disease-modifying anti-rheumatic drugs, FVC forced vital capacity, GAP gender, age, lung physiology, ILD interstitial lung disease
aData on Black patients are not shown as the number of patients in this subgroup (n = 10) was too small for the data to be interpretedbCorrected for haemoglobin
cBased on WHO Drug Dictionary [version 19.MAR) (Anatomical Therapeutic Chemical codes for ‘antacids’ and ‘drugs for peptic ulcer and gastro-oesophageal reflux disease (GORD)’ and preferred name ‘drugs for acid related disorders’]
dBased on WHO Drug Dictionary (version 19.MAR) standardised drug grouping excluding denosumab, plus baricitinib
Discussion
We used data from the INBUILD trial to assess the effect of nintedanib on slowing the progression of pulmonary fibrosis across subgroups based on baseline characteristics. No heterogeneity was detected in the effect of nintedanib on reducing the rate of FVC decline across subgroups based on demographics (age, sex, race, BMI), measures of disease severity (FVC, DLco, CPI, GAP stage, time since diagnosis of ILD), or use of anti-acid therapy or DMARDs at baseline. These findings are in line with the consistent effect of nintedanib across subgroups based on baseline characteristics observed in clinical trials in patients with IPF [18–25] and SSc-ILD [26–30]. Previous analyses of data from the INBUILD trial also found no heterogeneity in the effect of nintedanib across five subgroups by ILD diagnosis (hypersensitivity pneumonitis, autoimmune ILDs, iNSIP, unclassifiable IIP, other ILDs) [31] or among subgroups with ILD associated with rheumatoid arthritis, SSc, mixed connective tissue disease, or other autoimmune diseases [32]. Further, in a meta-analysis of data from the INBUILD trial, INPULSIS trials in patients with IPF and the SENSCIS trial in patients with SSc-ILD, there was no evidence of heterogeneity in the relative effect of nintedanib on reducing the rate of decline in FVC across these patient populations [33]. Combined data from these trials indicated that nintedanib reduced the rate of decline in FVC over 52 weeks by 51%.
Previous analyses of data from the INBUILD trial found no heterogeneity in the relative treatment effect of nintedanib between patients with a UIP-like fibrotic pattern versus other fibrotic patterns on HRCT [11, 32]. However, the absolute rate of decline in FVC was numerically greater in patients with a UIP-like fibrotic pattern than in those with other fibrotic patterns on HRCT [8, 11], consistent with previous studies showing that a UIP pattern on HRCT is associated with a higher risk of mortality in patients with progressive pulmonary fibrosis of varying aetiologies [4, 34–37].
Immunomodulatory medications have been shown to reduce ILD progression in specific populations of patients with autoimmune disease-related ILDs [38–42]. In our analyses, no heterogeneity was detected in the effect of nintedanib on reducing the rate of decline in FVC across subgroups based on the use of DMARDs at baseline in the overall INBUILD trial population, consistent with analyses in the subgroup of patients with autoimmune disease-related ILDs [32]. Similarly, previous analyses of data from the overall INBUILD trial population found no heterogeneity in the effect of nintedanib on slowing FVC decline between subgroups by use of glucocorticoids at baseline, or in analyses excluding patients taking restricted immunomodulatory medications at baseline or over 52 weeks of the trial [43]. Data from the INBUILD and SENSCIS trials suggest that the adverse event profile of nintedanib is also similar between patients who do and do not use immunomodulatory medications [28, 43]. For some patients with progressive pulmonary fibrosis associated with an autoimmune disease, combination therapy with immunomodulatory therapy and nintedanib may be the most appropriate treatment regimen for slowing ILD progression [1, 2, 44–46], but more research is needed to inform in which patients combination therapy should be used.
Previous analyses of data from the INBUILD trial demonstrated that the adverse event profile of nintedanib was generally consistent across subgroups based on ILD diagnosis, age, sex, race and weight, but nausea, vomiting, liver enzyme elevations and dose reductions used to manage adverse events were more common among female than male patients [13, 31]. An analysis of pooled data from the INBUILD, INPULSIS and SENSCIS trials also showed that nausea, vomiting and hepatic adverse events, and dose reductions and treatment interruptions, were more frequent in female than male patients [47].
The strengths of our analyses include the randomised placebo-controlled trial design of the INBUILD trial and the standardised collection of data on FVC and adverse events. A limitation of our analyses is that the trial was not designed or powered to show a benefit of nintedanib in these subgroups of patients, and the interaction p values should be regarded as exploratory. The results obtained in small subgroups should in particular be interpreted with caution. The number of Black patients and the numbers of patients with specific comorbidities or taking specific comedications were too small for the data from these subgroups to be interpreted.
Conclusion
In the INBUILD trial, nintedanib had a consistent effect on reducing the rate of ILD progression across subgroups of patients with progressive pulmonary fibrosis based on demographics, measures of ILD severity, and use of comedications.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Medical Writing, Editorial, and Other Assistance
Writing assistance was provided by Elizabeth Ng and Wendy Morris of FleishmanHillard, London, UK, which was contracted and funded by BI. Boehringer Ingelheim was given the opportunity to review the article for medical and scientific accuracy as well as intellectual property considerations.
Authorship
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. The authors did not receive payment for the development of this manuscript.
Author Contributions
Martin Kolb, Kevin R Flaherty, Antje Prasse, Carlo Vancheri and Athol U Wells contributed to data acquisition. Data analysis was performed by Heiko Mueller. Martin Kolb, Kevin R Flaherty, Rafael S Silva, Antje Prasse, Carlo Vancheri, Heiko Mueller, Kamila Sroka-Saidi and Athol U Wells contributed to the interpretation of the data. Martin Kolb, Kevin R Flaherty, Rafael S Silva, Antje Prasse, Carlo Vancheri, Heiko Mueller, Kamila Sroka-Saidi and Athol U Wells commented on the manuscript and have approved the final version.
Funding
The INBUILD trial, and the subgroup analyses presented in this manuscript, were supported by Boehringer Ingelheim International GmbH (BI). Open access and rapid service fees were funded by BI.
Data Availability
To ensure independent interpretation of clinical study results and enable authors to fulfill their role and obligations under the ICMJE criteria, Boehringer Ingelheim grants all external authors access to relevant clinical study data. In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data after publication of the primary manuscript in a peer-reviewed journal, regulatory activities are complete and other criteria are met. Researchers should use https://vivli.org/to request access to study data and visit https://www.mystudywindow.com/msw/datasharing for further information.
Declarations
Conflict of Interest
Martin Kolb reports research funding from Boehringer Ingelheim (BI), Pieris, Roche; consulting fees from AbbVie, Algernon, Bellerophon, BI, Cipla, CSL Behring, Horizon, LabCorp, Roche, ShouTi, United Therapeutics; fees for speaking from BI, Novartis, Roche; fees for expert testimony from Roche; has participated on a data safety monitoring board or advisory board for United Therapeutics and LabCorp; and receives an allowance as Chief Editor of the European Respiratory Journal. Kevin R Flaherty reports grants paid to his institution from BI; royalties or licenses from UpToDate; consulting fees from Arrowhead, AstraZeneca, Bellerophon, BI, CSL Behring, Daewoong, DevPro, Dispersol, FibroGen, Horizon Therapeutics, Immunet, Insilico, Lupin, NeRRe Therapeutics, Pliant, Polarean, Pure Health, PureTech, Respivant, Roche/Genentech, Shionogi, Sun Pharmaceuticals, Trevi, United Therapeutics, Vicore. Rafael S Silva has nothing to report. Antje Prasse reports consulting fees from BI, CSL Behring, Novartis; fees for speaking from BI, Euroimmun, Gilead, Novartis; support for travel from BI; she holds a leadership or fiduciary role as a coordinator of the ILD group of the European Reference Network-lung. Carlo Vancheri reports grants paid to his institution from Roche; consulting fees from BI and Roche; fees for speaking from AstraZeneca, BI, GlaxoSmithKline, Glycocore, Menarini, Roche; payments for expert testimony and support for travel from BI and Roche; has participated on a data safety monitoring board or advisory board for BI, LabCorp, Roche; and his institution has received equipment, materials, drugs, or other services from BI, Biogen, Bristol Myers Squibb, FibroGen, Galapagos, Gilead, GlaxoSmithKline, Novartis, Pliant, Respivant Sciences, Roche, Sanofi. Heiko Mueller and Kamila Sroka-Saidi are employees of BI. Athol U Wells reports consulting fees from BI, Roche, Veracyte and fees for speaking from BI and Roche. Martin Kolb, Kevin R Flaherty and Athol U Wells were members of the INBUILD trial steering committee.
Ethical Approval
The INBUILD trial protocol was approved by an Ethics Committee or Institutional Review Board at all the participating centres. The INBUILD trial was carried out in compliance with the protocol and with the principles of the Declaration of Helsinki and the Harmonised Tripartite Guideline for Good Clinical Practice of the International Conference on Harmonisation. The trial was approved by the local authorities. All patients provided written informed consent prior to their participation.
Footnotes
Prior Presentation. Some of these data were previously presented at the European Respiratory Society International Congress 2020 (7–9 September, virtual; presentation number 4576) and the American Thoracic Society International Conference 2020 (5–10 August, virtual; poster board number 709) and 2021 (14–19 May, virtual).
References
- 1.Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2022;205:e18–e47. doi: 10.1164/rccm.202202-0399ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajan SK, Cottin V, Dhar R, et al. Progressive pulmonary fibrosis: an expert group consensus statement. Eur Respir J. 2022:2103187. [DOI] [PMC free article] [PubMed]
- 3.Khor YH, Farooqi M, Hambly N, Kolb M, Ryerson CJ; Austin ILD Registry and CARE-PF Investigators. Patient characteristics and survival for progressive pulmonary fibrosis using different definitions. Am J Respir Crit Care Med. 2023;207:102–105. [DOI] [PMC free article] [PubMed]
- 4.Solomon JJ, Chung JH, Cosgrove GP, et al. Predictors of mortality in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J. 2016;47:588–596. doi: 10.1183/13993003.00357-2015. [DOI] [PubMed] [Google Scholar]
- 5.Gimenez A, Storrer K, Kuranishi L, et al. Change in FVC and survival in chronic fibrotic hypersensitivity pneumonitis. Thorax. 2018;73:391–392. doi: 10.1136/thoraxjnl-2017-210035. [DOI] [PubMed] [Google Scholar]
- 6.Volkmann ER, Tashkin DP, Sim M, et al. Short-term progression of interstitial lung disease in systemic sclerosis predicts long-term survival in two independent clinical trial cohorts. Ann Rheum Dis. 2019;78:122–130. doi: 10.1136/annrheumdis-2018-213708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krauss E, El-Guelai M, Pons-Kuehnemann J, et al. Clinical and functional characteristics of patients with unclassifiable interstitial lung disease (uILD): long-term follow-up data from European IPF Registry (eurIPFreg) J Clin Med. 2020;9:2499. doi: 10.3390/jcm9082499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown KK, Martinez FJ, Walsh SLF, et al. The natural history of progressive fibrosing interstitial lung diseases. Eur Respir J. 2020;55:2000085. doi: 10.1183/13993003.00085-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nasser M, Larrieu S, Si-Mohamed S, Ahmad K, Boussel L, Brevet M, et al. Progressive fibrosing interstitial lung disease: a clinical cohort (the PROGRESS study) Eur Respir J. 2021;57:2002718. doi: 10.1183/13993003.02718-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wollin L, Distler JHW, Redente EF, et al. Potential of nintedanib in treatment of progressive fibrosing interstitial lung diseases. Eur Respir J. 2019;54:1900161. doi: 10.1183/13993003.00161-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. 2019;381:1718–1727. doi: 10.1056/NEJMoa1908681. [DOI] [PubMed] [Google Scholar]
- 12.Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in progressive interstitial lung diseases: data from the whole INBUILD trial. Eur Respir J. 2022;59:2004538. doi: 10.1183/13993003.04538-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cottin V, Martinez FJ, Jenkins RG, et al. Safety and tolerability of nintedanib in patients with progressive fibrosing interstitial lung diseases: data from the randomized controlled INBUILD trial. Respir Res. 2022;23:85. doi: 10.1186/s12931-022-01974-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwon BS, Choe J, Chae EJ, Hwang HS, Kim YG, Song JW. Progressive fibrosing interstitial lung disease: prevalence and clinical outcome. Respir Res. 2021;22:282. doi: 10.1186/s12931-021-01879-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown KK, Inoue Y, Flaherty KR, et al. Predictors of mortality in subjects with progressive fibrosing interstitial lung diseases. Respirology. 2022;27:294–300. doi: 10.1111/resp.14231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wells AU, Desai SR, Rubens MB, et al. Idiopathic pulmonary fibrosis: a composite physiologic index derived from disease extent observed by computed tomography. Am J Respir Crit Care Med. 2003;167:962–969. doi: 10.1164/rccm.2111053. [DOI] [PubMed] [Google Scholar]
- 17.Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156:684–691. doi: 10.7326/0003-4819-156-10-201205150-00004. [DOI] [PubMed] [Google Scholar]
- 18.Costabel U, Inoue Y, Richeldi L, et al. Efficacy of nintedanib in idiopathic pulmonary fibrosis across prespecified subgroups in INPULSIS. Am J Respir Crit Care Med. 2016;193:178–185. doi: 10.1164/rccm.201503-0562OC. [DOI] [PubMed] [Google Scholar]
- 19.Costabel U, Behr J, Crestani B, et al. Anti-acid therapy in idiopathic pulmonary fibrosis: insights from the INPULSIS trials. Respir Res. 2018;19:167. doi: 10.1186/s12931-018-0866-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taniguchi H, Xu Z, Azuma A, et al. Subgroup analysis of Asian patients in the INPULSIS trials of nintedanib in idiopathic pulmonary fibrosis. Respirology. 2016;21:1425–1430. doi: 10.1111/resp.12852. [DOI] [PubMed] [Google Scholar]
- 21.Kolb M, Richeldi L, Behr J, et al. Nintedanib in patients with idiopathic pulmonary fibrosis and preserved lung volume. Thorax. 2017;72:340–346. doi: 10.1136/thoraxjnl-2016-208710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cottin V, Azuma A, Raghu G, et al. Therapeutic effects of nintedanib are not influenced by emphysema in the INPULSIS trials. Eur Respir J. 2019;53:1801655. doi: 10.1183/13993003.01655-2018. [DOI] [PubMed] [Google Scholar]
- 23.Ryerson CJ, Kolb M, Richeldi L, et al. Effects of nintedanib in patients with idiopathic pulmonary fibrosis by GAP stage. ERJ Open Res. 2019;5:00127–2018. doi: 10.1183/23120541.00127-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jouneau S, Crestani B, Thibault R, et al. Analysis of body mass index, weight loss and progression of idiopathic pulmonary fibrosis. Respir Res. 2020;21:312. doi: 10.1186/s12931-020-01528-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glaspole I, Bonella F, Bargagli E, et al. Efficacy and safety of nintedanib in patients with idiopathic pulmonary fibrosis who are elderly or have comorbidities. Respir Res. 2021;22:125. doi: 10.1186/s12931-021-01695-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Distler O, Highland KB, Gahlemann M, et al. Nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med. 2019;380:2518–2528. doi: 10.1056/NEJMoa1903076. [DOI] [PubMed] [Google Scholar]
- 27.Azuma A, Chung L, Behera D, et al. Efficacy and safety of nintedanib in Asian patients with systemic sclerosis-associated interstitial lung disease: subgroup analysis of the SENSCIS trial. Respir Investig. 2021;59:252–259. doi: 10.1016/j.resinv.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Highland KB, Distler O, Kuwana M, et al. Efficacy and safety of nintedanib in patients with systemic sclerosis-associated interstitial lung disease treated with mycophenolate: a subgroup analysis of the SENSCIS trial. Lancet Respir Med. 2021;9:96–106. doi: 10.1016/S2213-2600(20)30330-1. [DOI] [PubMed] [Google Scholar]
- 29.Kuwana M, Allanore Y, Denton CP, et al. Nintedanib in patients with systemic sclerosis-associated interstitial lung disease: subgroup analyses by autoantibody status and modified Rodnan skin thickness score. Arthritis Rheumatol. 2022;74:518–526. doi: 10.1002/art.41965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Volkmann ER, Kreuter M, Hoffmann-Vold AM, et al. Dyspnoea and cough in patients with systemic sclerosis-associated interstitial lung disease in the SENSCIS trial. Rheumatology (Oxford) 2022;61:4397–4408. doi: 10.1093/rheumatology/keac091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wells AU, Flaherty KR, Brown KK, et al. Nintedanib in patients with progressive fibrosing interstitial lung diseases: subgroup analyses by interstitial lung disease diagnosis in the randomised, placebo-controlled INBUILD trial. Lancet Respir Med. 2020;8:453–460. doi: 10.1016/S2213-2600(20)30036-9. [DOI] [PubMed] [Google Scholar]
- 32.Matteson EL, Kelly C, Distler JHW, et al. Nintedanib in patients with autoimmune disease-related progressive fibrosing interstitial lung diseases: subgroup analysis of the INBUILD trial. Arthritis Rheumatol. 2022;74:1039–1047. doi: 10.1002/art.42075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonella F, Cottin V, Valenzuela C, et al. Meta-analysis of effect of nintedanib on reducing FVC decline across interstitial lung diseases. Adv Ther. 2022;39:3392–3402. doi: 10.1007/s12325-022-02145-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adegunsoye A, Oldham JM, Bellam SK, et al. Computed tomography honeycombing identifies a progressive fibrotic phenotype with increased mortality across diverse interstitial lung diseases. Ann Am Thorac Soc. 2019;16:580–588. doi: 10.1513/AnnalsATS.201807-443OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim HC, Lee JS, Lee EY, et al. Risk prediction model in rheumatoid arthritis-associated interstitial lung disease. Respirology. 2020;25:1257–1264. doi: 10.1111/resp.13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walsh SLF, Sverzellati N, Devaraj A, et al. Connective tissue disease related fibrotic lung disease: high resolution computed tomographic and pulmonary function indices as prognostic determinants. Thorax. 2014;69:216–222. doi: 10.1136/thoraxjnl-2013-203843. [DOI] [PubMed] [Google Scholar]
- 37.Oldham JM, Lee CT, Wu Z, et al. Lung function trajectory in progressive fibrosing interstitial lung disease. Eur Respir J. 2022;59:2101396. doi: 10.1183/13993003.01396-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tashkin DP, Elashoff R, Clements PJ, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354:2655–2666. doi: 10.1056/NEJMoa055120. [DOI] [PubMed] [Google Scholar]
- 39.Tashkin DP, Roth MD, Clements PJ, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med. 2016;4:708–719. doi: 10.1016/S2213-2600(16)30152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ebata S, Yoshizaki A, Oba K, et al. Safety and efficacy of rituximab in systemic sclerosis (DESIRES): a double-blind, investigator-initiated, randomised, placebo-controlled trial. Lancet Rheumatol. 2021;3:E489–497. doi: 10.1016/S2665-9913(21)00107-7. [DOI] [PubMed] [Google Scholar]
- 41.Roofeh D, Lin CJF, Goldin J, et al. Tocilizumab prevents progression of early systemic sclerosis-associated interstitial lung disease. Arthritis Rheumatol. 2021;73:1301–1310. doi: 10.1002/art.41668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maher TM, Tudor VA, Saunders P, et al. Rituximab versus intravenous cyclophosphamide in patients with connective tissue disease-associated interstitial lung disease in the UK (RECITAL): a double-blind, double-dummy, randomised, controlled, phase 2b trial. Lancet Respir Med. 2023;11:45–54. doi: 10.1016/S2213-2600(22)00359-9. [DOI] [PubMed] [Google Scholar]
- 43.Cottin V, Richeldi L, Rosas I, et al. Nintedanib and immunomodulatory therapies in progressive fibrosing interstitial lung diseases. Respir Res. 2021;22:84. doi: 10.1186/s12931-021-01668-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoffmann-Vold AM, Maher TM, Philpot EE, et al. The identification and management of interstitial lung disease in systemic sclerosis: evidence-based European consensus statements. Lancet Rheumatol. 2020;2:E71–83. doi: 10.1016/S2665-9913(19)30144-4. [DOI] [PubMed] [Google Scholar]
- 45.Khanna D, Lescoat A, Roofeh D, et al. Systemic sclerosis-associated interstitial lung disease: how to incorporate two Food and Drug Administration-approved therapies in clinical practice. Arthritis Rheumatol. 2022;74:13–27. doi: 10.1002/art.41933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rahaghi FF, Hsu VM, Kaner RJ, et al. Expert consensus on the management of systemic sclerosis-associated interstitial lung disease. Respir Res. 2023;24:6. doi: 10.1186/s12931-022-02292-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoffmann-Vold AM, Volkmann ER, Allanore Y, et al. Safety and tolerability of nintedanib in patients with interstitial lung diseases in subgroups by sex: a post-hoc analysis of pooled data from four randomised controlled trials. Lancet Rheumatol. 2022;4:E679–E687. doi: 10.1016/S2665-9913(22)00215-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
To ensure independent interpretation of clinical study results and enable authors to fulfill their role and obligations under the ICMJE criteria, Boehringer Ingelheim grants all external authors access to relevant clinical study data. In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data after publication of the primary manuscript in a peer-reviewed journal, regulatory activities are complete and other criteria are met. Researchers should use https://vivli.org/to request access to study data and visit https://www.mystudywindow.com/msw/datasharing for further information.