Abstract
Adults with Mild Cognitive Impairment (MCI) and Alzheimer’s disease (AD) have increased rates of Obstructive Sleep Apnea (OSA). Positive Airway Pressure (PAP) is the first-line treatment for OSA and may have potential benefits for slowing cognitive decline in these individuals. However, adherence is low in PAP users overall and those with cognitive impairment may have unique challenges. Furthermore, there has been little systematic study of the use of PAP or strategies to enhance PAP adherence among those with AD or MCI. The aim of this review is to examine existing observational, quasi-experimental and experimental studies of the effects of PAP on cognitive function. In addition, our goal was to gather evidence about the adherence rates, and support for PAP among adults with MCI and mild to moderate AD. Through searches of electronic databases (University of Utah Library, SAGE Publishing, PubMed, Wiley, EBSCO, Science Direct, ProQuest, and NCBI), we identified 11 articles that fit our study inclusion criteria. Synthesis of data was performed with a focus on cognitive outcomes of PAP interventions and adherence. Findings from the studies showed that multiple indices of memory improved with PAP use. Adherence in MCI and AD populations was largely comparable to adherence reported in general adult populations, but more research is needed to optimize systems for providing support for PAP users and caregivers. Results support PAP as a promising intervention in this population but more research is needed to make definitive conclusions about the relationship between PAP use and improved cognitive function. Furthermore, research is needed to determine if additional interventions are needed to support patients and caregivers.
Keywords: OSA, MCI, PAP, adherence, Alzheimer’s, sleep apnea
1. INTRODUCTION
Mild Cognitive Impairment (MCI) is characterized by decline in cognition (e.g., memory, executive functioning, language) but with preservation of daily function (e.g., driving, managing medications, handling finances, completing household chores). MCI is present among 12–18% of individuals over 60 years of age [1] and predicts a 7-fold increase in risk for development of Alzheimer’s Disease [2]. Emerging evidence demonstrates that OSA is common in individuals with MCI, with prevalence up to 40% [3] and that OSA may be linked mechanistically to the development of AD via tau and amyloid clearance [4]. Studies demonstrate that OSA may worsen symptoms of MCI [3,5]. Therefore, there is increasing interest in whether treating OSA can potentially stabilize or improve cognitive deficits seen in MCI and AD [6].
There are a growing number of studies that have examined positive airway pressure (PAP) treatment among individuals with MCI and AD [7]. However, little is known about adherence in this population. In studies conducted in the adult population with OSA and no MCI or AD diagnosis, PAP is effective at improving the symptoms of OSA, yet 46–83% of individuals are non-adherent to treatment, reducing its potential benefits [8,9]. Common reasons for OSA non-adherence in the general population include multiple factors, such as low motivation, low self-efficacy and claustrophobia [10]. Patients with OSA may experience additional challenges to PAP, related to problems with attention, memory and executive function needed to remember, set up and use PAP. In the broader medical literature, it is well known that cognitive decline affects patients’ ability to plan and execute treatments, such as remembering to take oral medications [11]. Because of these reasons, patients with cognitive decline and where relevant, their caregivers, may need additional resources/support to enhance treatment adherence and treatment outcomes. However, to date, there has been little systematic investigation of PAP adherence among individuals with MCI or AD or understanding of efforts to enhance PAP adherence in this population.
Therefore, the purpose of the current paper is to: 1) Summarize the research examining the effects of PAP adherence on cognitive function in populations with OSA and MCI/AD; 2) Examine PAP adherence among participants with MCI/AD; and 3) identify current practices for providing support for PAP for individuals with MCI/AD.
2. METHODS
2.1. Search Strategy
Our literature search began on July 1st, 2021 and completed on January 17th, 2023 and was updated again in June 2023. The authors used the following electronic databases to conduct the research: University of Utah Library, SAGE Publishing, PubMed, Wiley, EBSCO, Science Direct, ProQuest, and NCBI. The terms searched include “CPAP Adherence or CPAP usage”, “Obstructive Sleep Apnea or Sleep Apnea or OSA”, “Mild Cognitive Impairment or MCI or Mild Alzheimer’s Disease or Alzheimer’s Disease”, and “Sleep intervention or treatment or effect”, all commonly followed by “and” to connect the search terms. To identify additional articles, citations within other published reviews of similar topics were examined for relevant literature [3–5,7,12–28,29–38]. Each article was examined by a second reviewer to eliminate duplicates and ensure they fit within the criteria of the research.
2.2. Inclusion and Exclusion Criteria
Articles were included if they were peer-reviewed and studies met the following criteria: PAP interventions (including CPAP and auto-PAP), enrolled participants selected for aging-related cognitive decline, including MCI or AD who were diagnosed with obstructive sleep apnea, randomized and non-randomized quasi-experimental study designs, observational study designs and published in English.
2.3. Chart of Exclusion
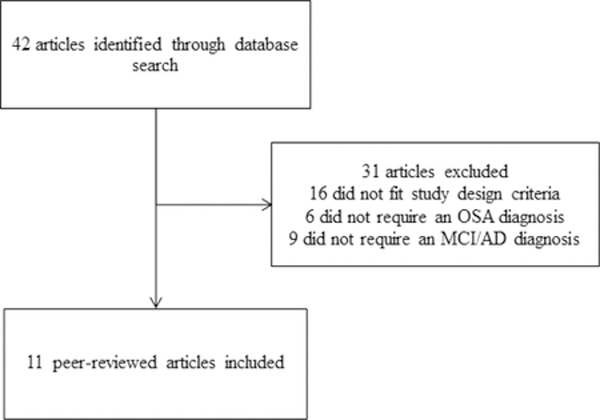
2.4. Data Extraction & Analysis
Data extracted from the articles include participant demographics, diagnosis and comorbidity status, study type, interventions, treatment dosage and control group, outcome measures, main findings, and PAP adherence. Synthesis of data was performed focusing on sleep interventions, differences in experimental groups and control groups, adherence, and outcomes.
3. RESULTS
3.1. Study and Participant Characteristics
A total of 11 articles met our inclusion criteria. Table 1 summarizes our data extraction and synthesis. Of the included articles, three were retrospective studies [39–41], four were quasi-experimental [42–45], one was a single-blind observational study [46] and three were randomized controlled trials (RCT) [47,48,49]. Six studies were conducted in the United States [40,43–45,47,48]. The other five were conducted in the following countries: China [42], one in France [46], Canada [39], Italy [41], and Australia [49]. The sample sizes ranged from 10 through 179 participants. The age for participants ranged from 40 to 92 years. Studies ranged in their demographic characteristics, but the majority of participants were White (59% to 90% White) and the percentage of men ranged from 47% to 89%. Six articles did not report racial demographics [39,41,42,46,47,49] and of those six, one did not report gender [47]. The method of classifying individuals as MCI or AD also differed across studies. For example, Zimmerman et al. enrolled participants who were “memory impaired” [43] defined as a T score at least one standard deviation below the mean (T score less than or equal to 40) on a cognitive evaluation conducted at baseline, whereas Wu et al. enrolled cognitively impaired participants [42] based on scores from neuropsychological tests administered at the inclusion phase. Costa et. al enrolled participants with cognitive impairment attributed to an underlying neurodegenerative or vascular etiology according to standard diagnostic guidelines published by the National Institute on Aging-Alzheimer’s Association (NIA-AA) and the National Institute of Neurological Disorders and Stroke in contribution with the Association Internationale pour la Recherche et l’Enseignement en Neurosciences (NINDS-AIREN) [40]. In addition to measuring cognitive function, Wu et al. [42] measured plasma C-reactive protein (CRP) concentration, and Cooke et al. [48] measured participants’ moods to determine potential contributors to PAP adherence. Table 1 describes the varying criteria for defining cognitive impairment across studies. Participants in five studies were diagnosed with mild to moderate OSA (AHI>=10) [43–45,47,48] and three studies enrolled patients with moderate to severe OSA (AHI >15) [42,46,49]. One study enrolled OSA participants with an AHI ≥5 with a significant oxygen desaturation of 88%, otherwise the inclusion was AHI>15 [39]. Another study enrolled OSA participants with an AHI 5–30 [40]. One study did not clearly state their OSA inclusion criteria [41]. There was also considerable variation with regard to length of treatment follow-up: two studies conducted follow-ups at approximately 3 years [40,46]. Four studies conducted follow-ups after one year [41,44,45,48]; one study conducted follow-ups after 6 months [42], while one study followed up between 2–12 months [40], and two others conducted follow-up assessments after 3 months [43,49]. The shortest follow-up time was three weeks [47].
Table 1.
Study Characteristics of Experimental Studies
| Author/Year | Diagnosis / OSA Criteria | N | Sample Characteristics | Study Type | PAP treatment Dosage and Duration | Control or comparison group | Assessment measures | Main Findings | Hours adherent; % adherent |
|---|---|---|---|---|---|---|---|---|---|
| Ancoli-Israel et al. (2008) [47] | Mild probable AD** / OSA (AHI >10) | 52 | PAP mean age 78.6±(6.8), sham PAP mean age 77.7±(7.7); N/A; N/A | Randomized controlled study | PAP for 6 weeks | Placebo PAP for 3 weeks and PAP or 3 weeks | Digit Span of WAIS-III (raw score), Digit Cancellation (total correct), Trails A (time to complete), DS and Symbol Search subtests of WAIS-III (raw score) , HVLT-R (total recall), Trails B (seconds to complete), WCST (conceptual level responses), SCWT (total words completed), FAS test & CF (animals) test (total words correct). | Composite neuropsychological scores improved in PAP group after three weeks. | The PAP group used their PAP for 5.8 hours a night for 73% of the nights. The PAP group used their PAP for 6.4 hours a night for 67% of the nights. The PAP group, when switched to therapeutic PAP, used it for a mean of 4.9 hours a night for 62% of the nights; 73%. |
| Cooke et. Al (2009)*** [48] | Mild to moderate AD diagnosis / OSA (AHI >=10) | 10 | 65–84 (mean age 75.7 years ±5.9); 9 of 10 were Caucasian; 3 female, 7 male (and 9 caregivers) | Quasi-experimental study | 13.3 months of PAP use. 6–21 months follow up period | Discontinue use of PAP after 6 weeks | MDRS, Digit Span of WAIS-III (raw score), Digit Cancellation tasks (total correct), Trails A (time to complete), DS and Symbol Search subtests of WAIS-III (raw score), HVLT-R (total recall), Trails B (seconds to complete), WCST (conceptual level responses), SCWT (total words completed), FAS test & CF (animals) test (total words correct). | Due to small sample size, there were no statistically significant differences in cognitive assessments showing improvement in executive function or psychomotor speed. | N/A; 50%. |
| Hoyos et. Al (2022) [49] | MCI / OSA (AHI>15) |
29 | 68.1 ±(7.6) mean age; 13 female, 16 male. | Randomized controlled crossover study | PAP for 12 weeks | No PAP use | Symbol digit modalities test (number of correct responses in 90 seconds), Trails A&B (time to complete), Stroop 3, RAVLT 1–5, A7, 75 (number of words, and % recall),Rey/Taylor Figure 3 (recall), Logical memory (immediate/delayed recall). | Medium effect size improvements in verbal and visual learning and memory in CPAP group. | 3.2; NA |
| Richards et. al (2019) [45] | Amnestic MCI* / OSA (AHI >10) | 59 | 55–89 (MCI+PAP mean age 67.4 ± 7.2), (MCI-PAP mean age 73.2 ± 8.6); 35 white; 24 female, 35 male | Quasi-experimental study | Participants with >= 4 hours mean PAP use for 1 year | Participants with <4 hours mean PAP use | HVLT-R (total recall), DS (age-adjusted total scaled score), MMSE, SCWT, Psychomotor Vigilance Task, E-Cog, ADCS-CGIC Scale. | PAP group improved in psychomotor / cognitive processing. Non-PAP users improved in attention. | N/A; 54% |
| Wang et. Al (2020) [44] | Amnestic MCI* / Mild OSA (AHI 10–14) | 17 | 55–89 (mean age=72.1±8.88years); 10 White, 7 other; 9 female, 8 male | Prospective quasi-experimental study | Participants with >= 4 hours mean PAP use for 1 year | Participants with <4 hours mean PAP use | HVLT-R (total recall), WAIS-R, DS (age adjusted total scale score), MoCA, E-Cog, ADCS-CGIC Scale, CDR Scale. | From baseline to one year, the PAP group showed an improvement in psychomotor / cognitive processing speed. | N/A; 47% |
| Wu et. Al (2016) [42] | Cognitive impairment / Moderate to severe OSA (AHI>15) | 178 - unclear how many were cognitively impaired out of the 178 sample. | 30–65 (mean age 46.0±10.4 years); N/A; 160 men | Quasi-experimental study | Moderate to severe OSA group was split into conservative treatment group and PAP group. Duration was 6 months | Behavioral measures (e.g., avoiding alcohol and supine sleep) and weight loss if BMI was>27 kg/m2. | MoCA, MMSE | MoCa scores improved in the PAP group after 6 months. | Article reported that all participants received treatment for >20 hours per week; N/A. |
| Zimmerman et. al (2006) [43] | “Memory impaired” = t score <=40 / OSA (AHI >10) | 179. 58 memory impaired out of 179 subjects (32 %). | 25–85 (mean age 48.1 ± 10.1); 88% white; 49 men, 9 women | Quasi-experimental study | Optimal PAP use (>6 hours per night) for 3 months | Mild (>2 hrs) and moderate (2–6 hrs) PAP users | HVLT-R (Delayed recall) | Optimal PAP users were more likely to have normalization of delayed memory compared to poor users after three months. | Optimal = 6.1–8.4 hours/night. Moderate = 2.3–5.9 hours/night. Poor = 0–1.9 hours/night. ; N/A |
Requires 5 criteria: (a) memory complaint, identified as a change from previous memory, verified by informant, (b) memory impairment 1.5 SDs below normal on the Logical Memory II Test, (c) not impaired general cognition (mini-mental state examination >= 24, (d) cognitive impairment without any repercussions on activities of daily living, (e) Clinical dementia rating (CDR) of 0–0.5.
Diagnosed according to the National Institute of Neurological and Communicative Disorders & Stroke-Alzheimer’s Disease and Related Disorders Association criteria.
Participants were enrolled in Ancoli-Israel et al., (2008) and this study included the 6–12 month follow-up.
3.2. Cognitive Assessments
Studies used a variety of cognitive assessment tools. Objective cognitive assessments included: Hopkins Verbal Learning Test – Revised (HVLT-R) [43–45,47,48], subtests from the Wechsler Adult Intelligence Scale-Revised or -III (WAIS-R/WAIS-III): Digit Symbol [44,45,47,48], Digit Span [47,48], and Symbol Search [47,48], Montreal Cognitive Assessment (MoCA)[39,42,44], Mini-Mental State Examination (MMSE) [39,41,42,45,46], Stroop Color and Word Test [45,47,48,49], Psychomotor Vigilance Task[45], Digit Cancellation tasks [47,48], Trail Making Test Part A [40,47,48,49], Trail Making Test Part B [40,47,48,49], 64-card version of the Wisconsin Card Sorting Test (WCST) [47,48], Letter fluency (FAS) and Category fluency (Animals) [47,48], Digit Symbol Modalities Test [50], Rey Auditory Verbal Learning Test [49], Rey–Osterrieth and Taylor Complex Figure Test [49], and Logical Memory immediate/delayed recall [49]. Self-reported or clinician-rated measures of cognition included: Everyday Cognition Scale (ECog) [29,30], Alzheimer’s Disease Cooperative Study - Clinical Global Impression of Change (ADCS-CGIC) Scale) [44,45], and Clinical Dementia Rating (CDR) Scale [40,43,47]. In addition to reviewing CDR scores, one study reviewed Annual Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) scores [40]. This modified CERAD included subsets of orientation, clock drawing, verbal fluency, phonemic fluency, Boston Naming Test, word list learning, recall and recognition, constructional praxis learning and recall, simple and complex visuospatial praxis (Rey–Osterrieth Complex Figure Test), simple calculations, and Trail Making Test Parts A and part B.
Most studies (8 out of 11) reported significant improvements in one or more cognitive outcome measures. Three studies reported improved scores in the HVLT-R, signifying an increase in learning and memory with sustained PAP use compared to non-adherent users [44,45,47]. Zimmerman et al. found that “optimal PAP users” (defined as using PAP >6 hours a night), had 7.9 times greater odds of experiencing normalization of delayed recall memory than poor PAP users who wore their PAPs for less than two hours a night [43]. Similarly, one study found that after six months of PAP use, MoCA scores significantly improved among participants with moderate to severe OSA, showing improvement in global cognition [42]. In one of the only randomized designs, Ancoli-Israel et al. observed that neuropsychological composite scores improved after three weeks of PAP use in a small sample of adults with mild AD [47]. Cooke et al. [48] examined the longer-term outcomes of participants from Ancoli-Israel et al. [47] and compared participants who continued to use PAP (n=5) to participants who discontinued use (n=5). Cooke et al. found improvements in executive function among the PAP group as shown by the effect size of the WCST (ES = 0.7), Stroop Color-Word Score (ES=−0.8), Trail Making Test Part B (ES=−0.3), and FAS (ES=−0.7), as well as positive effects on psychomotor speed with sustained PAP use [31]. Results were not statistically significant for this study due to the small sample size, but the effect sizes were moderate. Costa et al. performed multiple analyses with their participants test scores and found improvements in visuospatial and executive functioning in participants with good PAP adherence. Additionally, they found higher MoCa scores in participants with MCI and vascular MCI who were adherent to PAP compared to non-users and found improved MMSE scores among PAP users [39]. One study found alternative results among MMSE scores. Liguori et al. found a significant decrease in MMSE and an increase in CDR among their entire study sample, however, a mean change in follow-up scores found that MMSE scores did not differ among PAP users and non-PAP users, but there was a significant difference among CDR scores [41]. In the study conducted by Troussiere et al., the median annual MMSE decline was significantly slower in PAP users than non-PAP users [46]. Two studies did not find significant differences between PAP users and non-PAP users for Trails A and Trails B scores or other cognitive measures [40,49]. Hoyos et. al reported significance in some scales of the RAVLT as well as in the Rey-Osterrieth and Taylor Complex Figure Test 3 min recall score, which suggests this study observed improvements in verbal and visual learning and memory [49].
3.3. PAP Adherence
All 11 articles reported adherence data, although there was considerable variability in how adherence was assessed and defined. Ten studies reported percentage of participants who used their PAP four or more hours per night, which varied from 35.6% to 73%. Four studies reported hours of adherence. In the study by Zimmerman et al. [43], 33% of participants wore their PAP from 6.1 to 8.4 hours, 43% of participants used from 2.3 to 5.9 hours, and 24% of participants wore their PAP 0 to 1.9 hours each night. Ancoli-Israel et al. reported that the PAP group used their PAP for 5.8 hours a night and used for 73% of the nights throughout the duration of the study [47]. Hoyos et. al reported an average of 3.2 hours of CPAP use per night with a standard deviation of 2.8 hours per night [49]. Skiba et al. reported a median PAP use of 3.88 hours, as 42 participants were PAP compliant (44%), 30 were non-compliant (31%), and 24 had no PAP use (25%) [40]. One study defined adherence as a weekly goal of 20 hours per week [42]. Most studies that used a cut-point used an average of 4 hours per night [39–41,44–46] as the criteria to define adherent vs. non-adherent use.
3.4. Support for PAP
Five out of the 11 studies provided information about efforts to support PAP use; however, studies varied greatly on the amount of detail reported about how patients were supported in using PAP, and support provision was rarely the primary focus of the investigations. Wang et al. reported providing staff-delivered education to their participants on OSA health risks, motivation, and ways to minimize barriers but did not report the number of sessions [44]. Wu et al. offered free telephone consultations to the patients by medical staff when needed [42]. Similarly, CPAP therapists followed up with participants at the one- and four-week mark with an in-person visit or phone call in the Hoyos et. al study [49]. Richards et al. [45] provided the most comprehensive support for participants, including phone and face-to-face interactions with trained project staff 12–14 hours over the year-long project, an educational video, and motivational interviewing by staff, where they reiterated participants’ health related goals and used a troubleshooting guideline to discuss participants concerns. Follow-up visits were conducted every three months to discuss PAP adherence, PAP hygiene, and for participants to share their experiences [45]. In the randomized study by Ancoli-Israel et al., participants and caregivers attended an orientation session, including hands-on instructions on using PAP [47]. The study by Cooke et al. reported long term follow-up of this trial, and the caregivers remained relevant to the evaluation. The caregivers in this study assisted participants by filling out questionnaires with them [48]. Additionally, caregivers in this study reported better sleep quality and stabilized psychopathology for their respective participants. [48]. Richards et al. included participants’ partners in the education sessions and follow-up phone calls [45]. In addition, their partners assisted with the use of the PAP and provided moral support. It is notable that these three studies were the only studies to include the caregiver, despite the prominent role of caregivers in both PAP and MCI [45,47,48]. None of the studies specifically examined the impact of support for PAP or behavioral interventions.
4. DISCUSSION
In this review, we examined studies that tested PAP use among patients with OSA and cognitive disorders, including MCI or AD. Overall, only 11 studies were identified that met inclusion criteria. Nevertheless, the limited studies reported suggest that PAP treatment may have potential for improving cognitive function in this population. Across studies, PAP users showed more cognitive improvement than suboptimal users over the follow-up period that ranged from 3 weeks to 3 years. Together, several studies showed improvement in memory and composite scores across several measures, for example: HVLT-R, MMSE, MoCA, and other neuropsychological scores with increased PAP use. This suggests potential for improvement in psychomotor speed, visuospatial function, executive function, recall, and attention. Despite these promising findings, more studies are needed and there is a need to improve methodological rigor including increasing the number of randomized studies, studies with larger sample sizes with more diverse participants and improving the standardization of cognitive criteria for study entry. Furthermore, although studies showed statistically significant improvement in cognition, they did not specify whether such improvements were clinically significant.
In terms of adherence, rates ranged from 35.6–73% but on average, about half of participants with MCI were adherent to PAP. Importantly, these rates, while low, are within a similar range of PAP adherence reported in the adult and older adult populations (30–60%) [51]. Due to differences in study design, rates cannot be truly compared across studies, and they also do not reflect a true prevalence of adherence because participants were not randomly assigned to PAP in most of the studies in our review. However, the finding that adherence rates are largely in the range of other adult samples without MCI/AD has important clinical implications as providers may be hesitant to prescribe PAP to individuals experiencing cognitive impairment, due to concerns about their ability to adhere to treatment. They also suggest there is an opportunity to improve PAP adherence among MCI/AD patients. Most studies defined moderate PAP use as ‘4 hours per night’ [39–41,44–46]. This may be due to a Medicare rule that states individuals must use their PAP for at least 4 hours a night in order to be compliant and have the cost of the PAP covered. It is important to consider that previous studies conducted in non-MCI/AD samples of adults have reported additional benefits of PAP use beyond 4 hours, and improvements in outcomes such as sleepiness and quality of life continued to improve up to 7 hours of use [52]. Therefore, if use is defined at 4 hours, it may underestimate the potential of PAP treatment among optimally adherent patients.
Our review also identified a gap in the understanding of how to support patients with MCI and AD in promoting PAP adherence. Although it is well-established that interventions such as education, Cognitive Behavioral Therapy (CBT), and motivational interviewing can improve PAP adherence [4], little is known about the effect of these interventions among patients with MCI/AD. We also specifically evaluated caregiver support among the studies in our review and found that only 3 studies formally included caregiver in the orientation or PAP set up session [45,47,48]. It is highly likely that caregivers were involved even if not specifically detailed in the articles (e.g., with the patient when he or she needed phone support). Although we cannot compare directly between studies, the Ancoli-Israel et al. study [47] had a robust caregiver support element to their study and reported the highest adherence of the studies review, which supports the importance of caregiver support. Out of the four observational studies summarized in this review, none of them reported having support for PAP [39–41,46]. Costa et al., who reported the lowest adherence rate among compliant PAP users (35.6%) out of all the studies, hypothesizes that their low adherence rates were likely due to the absence of a caregiver, but the authors did not provide details on how this was assessed [39]. Similarly, Skiba et al. reported increased PAP use among participants with amnestic MCI than non-amnestic MCI participants, which they attribute to these patients and family members being more motivated to slow progression of dementia [40]. In studies involving the general population, partner involvement has shown to play an integral role in PAP adherence [53–55]. Various types of involvement may influence PAP use, and partners provide strong incentive to continue treatment [55]. For patients with cognitive decline, additional instruction or reminders may be needed to improve adherence and including the caregiver/partner may be especially important to compensate for memory difficulties in the patient.
Results of our review highlight the importance of conducting more research to understand the unique challenges of patients with cognitive disorders who are starting PAP and how to support patients with their treatment, including strategies to better incorporate and support the caregiver’s role in PAP treatment. These challenges may include difficulties using the equipment, due to lapses in memory or difficulty with executive function. In addition, older patients with PAP treatment are likely to have other medical treatments to attend to, including oral medication, blood pressure monitoring etc., which increase the burden of care for the patient and caregiver. Our group has recently developed a couples-based PAP adherence and sleep health intervention for patients with OSA and their partners, called We-PAP [53]. This intervention is based on the strong research supporting relationship quality and partner support for PAP [54,56], and awareness that partner sleep problems are interdependent with the patient [57]. For couples who have one partner who has OSA and cognitive decline, partner support may be even more impactful on adherence, to compensate for cognitive decline. Further, there is a critical need to develop sleep interventions that target a variety of sleep challenges beyond OSA, that are highly prevalent among individuals living with MCI and their partners/caregivers, as sleep problems can predict poorer quality of life in both partners and poorer treatment outcomes in the individual with MCI [55,58,59]. The results of this review demonstrate that understanding caregiver support for PAP is an understudied but important area for future research.
4.1. Limitations
Results of our review are limited by a small number of studies (only three randomized studies) and relatively short follow-up periods (3 weeks to 3 months). The generalizability of this review is also limited by the focus on MCI and AD, rather than a broader focus on other neurological disorders with potential cognitive impairment such as Parkinson’s Disease and stroke. Our team focused on patients with MCI or AD in this initial review because of the growing are of mechanistic research in this area and large body of research in AD caregiver research [60–62]; however, understanding the impact of PAP treatment and adherence among patients with other neurological disorders, as well as the potential mechanisms, is an important area of future research. In preparation for this submission, we also tested the search term “dementia” rather than Alzheimer’s disease or MCI and identified only two additional two articles on PAP use and stroke [63–64], which demonstrates an opportunity to conduct further research in vascular and other dementia populations. There were also limitations to the studies included in this review such as vague definitions of memory or cognitively impaired, rather than formal diagnoses (e.g., amnestic MCI, mild AD) and utilized global cognitive screening tests (e.g., MoCA, MMSE), rather than focused neuropsychological tests that could identify changes in specific cognitive processes. In addition, there was limited consideration of comorbidities, which are important because psychiatric and medical comorbidities such as depression and chronic pain are common in normal aging and patients with OSA but were rarely accounted for in the analyses. Although studies were conducted in a number of countries, most patients were largely non-Hispanic White or Asian, and there is limited data in Black or Hispanic populations. The strength of our review is that we identified promising areas of cognitive improvement and this review allowed us to highlight gaps in the literature that can drive future research priorities aimed at the questions of whether PAP can improve cognitive function in MCI/AD and how to best support patients with MCI/AD in using PAP.
5. CONCLUSION
A limited but growing number of studies have examined PAP treatment for patients with OSA and MCI, and studies suggest PAP is a promising intervention for slowing cognitive decline in this population. This review demonstrates that randomized controlled trials with standardized and comprehensive cognitive batteries are needed to determine the clinical utility of PAP treatment among individuals with MCI/ AD. Results also highlight the need for further research on how to optimally support patients with MCI and AD to promote optimal PAP adherence and whether such treatment may prevent or delay further cognitive decline.
Table 2.
Study Characteristics of Observational Studies
| Author/Year | Diagnosis / OSA Criteria | N | Sample Characteristics | Study Type | PAP treatment Dosage/Duration | Control or comparison group | Assessment measures | Main Findings | Hours adherent; % adherent |
|---|---|---|---|---|---|---|---|---|---|
| Costa et.al (2022) [39] | AD, Mild MCI, VaD, VaMC+/AHI>15 or an AHI>5 with a significant oxygen desaturation of 88% | 171 | (mean age=69.8 ± 10.6 years); 68.4% male | retrospective | PAP use 4 hours/night, for 7 days a week at follow-up. | <4 h of PAP use per night | MoCA, MMSE | Adherence >4h associated with improved MoCa and MMSE scores. There was inconclusive evidence of an association between PAP adherence and changes in other MoCA subdomain scores. | NA; 35.6% . |
| Liguori et. Al (2021) [41] | MCI or AD / and OSA | 24 | 65–84 (mean age = 74.8 years); 67% male. | retrospective | use PAP for ≥4 hours per night for >5 nights per week | <4 h of PAP use per night, or no use at all | MMSE, CDR | In the whole study sample, a significant decrease was found in MMSE scores, and a significant increase was found in CDR scores. Non-adherent PAP users showed a significantly higher mean change of CDR scores compared to PAP adherent patients. | NA; 50% |
| Skiba et. Al (2020) [40] | MCI / Mild to severe OSA (AHI 5–30) | 96 | 40–92 (mean age at MCI diagnosis=70.4 years); 66% Caucasian, 66% male. | retrospective | used PAP for average of >4 hours a night | <4 h of PAP use per night, or no use at all | CDR. Modified CERAD included the following sub-tests: orientation, clock drawing, verbal fluency, phonemic fluency, Boston naming, word list learning, recall and recognition, constructional praxis learning and recall, simple and complex visuospatial praxis (Rey–Osterrieth complex figure test), simple calculations, and Trail Making Test part A and part B. | No statistically significant differences in cognitive measures between the PAP and non-PAP group. | 3.88 hours; 44%. |
| Trousierre et. al (2014) [46] | Mild to moderate AD diagnosis / OSA (AHI >30) | 23 | (73.4 mean age in PAP group) 14 men, 9 women. | single blind, observational study | PAP use for more than 4 hours a night and for more than five nights per week, on average | <4 h of PAP use per night | MMSE | PAP adherence was associated with significantly slower MMSE decline over a three-year follow-up period. | NA; 60.9%. |
Diagnosed according to the National Institute on Aging-Alzheimer’s Association (NIA-AA) and the National Institute of Neurological Disorders and Stroke in contribution with the Association Internationale pour la Recherche et l’Enseignement en Neurosciences (NINDS-AIREN)
HIGHLIGHTS.
PAP shows promise for slowing cognitive decline in populations with OSA and MCI or mild/moderate AD, largely with evidence from non-randomized studies.
Existing studies among individuals with MCI or mild/ moderate AD show PAP adherence rates are largely similar to rates reported in studies conducted among adults without cognitive impairment and about half of patients are non-adherent.
Randomized studies with larger and more racially and ethnically diverse samples are needed to identify causal relationships between CPAP treatment and cognition among more representative samples with MCI or mild/ moderate AD.
Acknowledgements
Funding for this project was provided R21AG067183–01A1S1 and R21AG067183–01A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Mild Cognitive Impairment (MCI) | Symptoms & Treatments | alz.org [Internet]. alz.org. Available from: https://www.alz.org/alzheimers-dementia/what-is-dementia/related_conditions/mild-cognitive-impairment
- 2.Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer’s disease. Lancet 2011;377(9770):1019–1031. [DOI] [PubMed] [Google Scholar]
- 3.Fernandes M, Placidi F, Mercuri NB, Liguori C. The Importance of diagnosing and the clinical potential of treating obstructive sleep apnea to delay mild cognitive impairment and alzheimer’s disease: A special focus on cognitive performance. Journal of Alzheimer 2021Jun.5;5(1):515–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrade AG, Bubu OM, Varga AW, Osorio RS. The relationship between obstructive sleep apnea and Alzheimer’s disease. Journal of Alzheimer 2018;640. [DOI] [PMC free article] [PubMed]
- 5.Vanek J, Prasko J, Genzor S, Ociskova M, Kantor K, Holubova M, Slepecky M, Nesnidal V, Kolek A, Sova M. Obstructive sleep apnea, depression and cognitive impairment. Sleep Medicine 2020Mar.23;72:50–8. [DOI] [PubMed] [Google Scholar]
- 6.Legault J, Thompson C, M- Ève Martineau-Dussault, André C, Baril A-A, Martinez Villar G, Carrier J, Gosselin N Obstructive sleep apnea and cognitive decline: A review of potential vulnerability and protective factors. Brain Sciences 2021May27;11(6):706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torossian M, Fiske SM, Jacelon CS. Sleep, mild cognitive impairment, and interventions for sleep improvement: An integrative review. Western Journal of Nursing Research 2021;41(11):1051–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: The challenge to effective treatment. Proceedings of the American Thoracic Society 2008;5(2):173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grewe FA, Bradicich M, Gaisl T, Roeder M, Thiel S, Sievi NA, Kohler M. Patterns of nightly CPAP usage in OSA patients with suboptimal treatment adherence. Sleep Medicine 2020Oct.1;74:109–15. [DOI] [PubMed] [Google Scholar]
- 10.Weaver TE. Best predictors of continuous positive airway pressure adherence. Sleep Medicine Clinics 2022;17(4):587–95. [DOI] [PubMed] [Google Scholar]
- 11.Smith D, Lovell J, Weller C, Kennedy B, Winbolt M, Young C, Ibrahim J. A systematic review of medication non-adherence in persons with dementia or cognitive impairment. PLOS ONE 2017;12(2). [DOI] [PMC free article] [PubMed]
- 12.Wang G, Goebel JR, Li C, Hallman HG, Gilford TM, Li W. Therapeutic effects of CPAP on cognitive impairments associated with OSA. Journal of Neurology 2019May16;267(10):2823–8. [DOI] [PubMed] [Google Scholar]
- 13.Mullins AE, Kam K, Parekh A, Bubu OM, Osorio RS, Varga AW. Obstructive sleep apnea and its treatment in aging: Effects on alzheimer’s disease Biomarkers, Cognition, Brain Structure and Neurophysiology. Neurobiology of Disease 2020Aug.18;145:105054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE . A systematic review of CPAP adherence across age groups: Clinical and empiric insights for developing CPAP adherence interventions. Sleep Medicine Reviews 2011Jun.8;15(6):343–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan X, Van Egmond L, Partinen M, Lange T, Benedict C. A narrative review of interventions for improving sleep and reducing circadian disruption in medical inpatients. Sleep Medicine 2019;59:42–0. [DOI] [PubMed] [Google Scholar]
- 16.Carruthers SP, Brunetti G, Rossell SL. Sleep disturbances and cognitive impairment in schizophrenia spectrum disorders: a systematic review and narrative synthesis. Sleep Medicine 2021;84:8–19. [DOI] [PubMed] [Google Scholar]
- 17.Cassidy-Eagle E, Siebern A, Unti L, Glassman J, O’Hara R. Cognitive behavioral treatment for insomnia in older adults with mild cognitive impairment in independent living facilities: A pilot study. Journal of Sleep Disorders and Medical Care 2018;1(1). [Google Scholar]
- 18.Petersen RC, Lopez O, Armstrong MJ, Getchius TS, Ganguli M, Gloss D, Gronseth GS, Marson D, Pringsheim T, Day GS, Sager M, Stevens J, Rae-Grant A. Practice guideline update summary: Mild cognitive impairment. Neurology 2017;90(3):126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landry GJ, Liu-Ambrose T. Buying time: a rationale for examining the use of circadian rhythm and sleep interventions to delay progression of mild cognitive impairment to Alzheimers disease. Frontiers in Aging Neuroscience 2014Dec.8;6(325). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bademli K, Lok N, Canbaz M, Lok S. Effects of physical activity program on cognitive function and sleep quality in elderly with mild cognitive impairment: A randomized controlled trial. Perspectives in Psychiatric Care 2018;55(3):401–8. [DOI] [PubMed] [Google Scholar]
- 21.Burke SL, Hu T, Spadola CE, Li T, Naseh M, Burgess A, Cadet T. Mild cognitive impairment: associations with sleep disturbance, apolipoprotein e4, and sleep medications. Sleep Medicine 2018;52:168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavuoto MG, Kinsella GJ, Ong B, Pike KE, Nicholas CL. Naturalistic measurement of sleep in older adults with amnestic mild cognitive impairment: Anxiety symptoms do not explain sleep disturbance. Current Alzheimer Research 2019;16(3):233–42. [DOI] [PubMed] [Google Scholar]
- 23.Bakker JP, Wang R, Weng J, Aloia MS, Toth C, Morrical MG, Gleason KJ, Rueschman M, Dorsey C, Patel SR, Ware JH, Mittleman MA, Redline S. Motivational enhancement for increasing adherence to CPAP. Chest 2016;150(2):337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palm A, Midgren B, Theorell-Haglöw J, Ekström M, Ljunggren M, Janson C, Lindberg E. Factors influencing adherence to continuous positive airway pressure treatment in obstructive sleep apnea and mortality associated with treatment failure – a national registry-based cohort study. Sleep Medicine 2018;51:85–1. [DOI] [PubMed] [Google Scholar]
- 25.Igelström H, Åsenlöf P, Emtner M, Lindberg E. Improvement in obstructive sleep apnea after a tailored behavioural sleep medicine intervention targeting healthy eating and physical activity: a randomised controlled trial. Sleep and Breathing 2017;22(3):653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Georgoulis M, Yiannakouris N, Kechribari I, Lamprou K, Perraki E, Vagiakis E, Kontogianni MD. The effectiveness of a weight-loss Mediterranean diet/lifestyle intervention in the management of obstructive sleep apnea: Results of the “MIMOSA” randomized clinical trial. Clinical Nutrition 2021;40(3):850–9. [DOI] [PubMed] [Google Scholar]
- 27.Sánchez AI, Martínez P, Miró E, Bardwell WA, Buela-Casal G. CPAP and behavioral therapies in patients with obstructive sleep apnea: Effects on daytime sleepiness, mood, and cognitive function. Sleep Medicine Reviews 2009;13(3):223–3. [DOI] [PubMed] [Google Scholar]
- 28.Kang S-H, Yoon I-Y, Lee SD, Kim T. Effects of continuous positive airway pressure treatment on cognitive functions in the Korean elderly with obstructive sleep apnea. Sleep Medicine Research 2016;7(1):10–5. [Google Scholar]
- 29.Oliver C, Biswas B, Blackman J, Busse M, Butters A, Drew C, Gabb V, Harding S, Hoyos C, Kendrick A, Turner N, Coulthard E. A systematic review on adherence to continuous positive airway pressure (CPAP) treatment for obstructive sleep apnoea (OSA) in individuals with mild cognitive impairment and Alzheimer. Sleep Medicine 2022;100:239. [DOI] [PubMed] [Google Scholar]
- 30.Seda G, Matwiyoff G, Parrish JS. Effects of obstructive sleep apnea and CPAP on cognitive function. Current Neurology and Neuroscience Reports 2021May6;21(7). [DOI] [PubMed] [Google Scholar]
- 31.Jiang X, Wang Z, Hu N, Yang Y, Xiong R, Fu Z. Cognition effectiveness of continuous positive airway pressure treatment in obstructive sleep apnea syndrome patients with cognitive impairment: a meta-analysis. Experimental Brain Research 2021Sep.21;239(12):3537–52. [DOI] [PubMed] [Google Scholar]
- 32.Bubu OM, Andrade AG, Umasabor-Bubu OQ, Hogan MM, Turner AD, De Leon MJ, Ogedegbe G, Ayappa I, Jean-Louis GG, Jackson ML, Varga AW, Osorio RS. Obstructive sleep apnea, cognition and Alzheimer. Sleep Medicine Reviews 2020Apr.;50:101250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung Y, Silber M, Tippmann-Peikert M, St Louis E, Smith G, Ferman T, Knopman D, Petersen R, Boeve B. 1154 The effects of cpap on cognitive and functional measures in patients with mild cognitive impairment and alzheimer’s dementia. Sleep 2017;400:430–1. [Google Scholar]
- 34.McLellan B, Skiba V, Novikova M, Schultz L, Suneja A. 0953 Treatment of obstructive sleep apnea with positive airway pressure to improve cognitive function in mild cognitive impairment. Sleep 2019;420:383. [Google Scholar]
- 35.Blackman J, Swirski M, Clynes J, Harding S, Leng Y, Coulthard E. Pharmacological and non‐pharmacological interventions to enhance sleep in mild cognitive impairment and mild Alzheimer. Journal of Sleep Research 2021;30(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dunietz GL, Chervin RD, Burke JF, Conceicao AS, Braley TJ. Obstructive sleep apnea treatment and dementia risk in older adults. Sleep 2021;44(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunietz GL, Chervin RD, Burke JF, Braley TJ. Obstructive sleep apnea treatment disparities among older adults with neurological disorders. Sleep Health 2020;6(4):534–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai M, Li H, Huang C, Wang RY, Chuang L, Chen N, Liu C, Yang Y, Liu C, Hsu C, Cheng W, Lee L. Risk of Alzheimer. The Laryngoscope 2020;130(9):2292–8. [DOI] [PubMed] [Google Scholar]
- 39.Costa Y, Lim A, Thorpe K, Mitchell S, Masellis M, Lam B, Black S, Boulos M. Investigating changes in cognition associated with the use of CPAP in cognitive impairment and dementia: A Retrospective Study. Sleep Medicine 2022Dec.5;100:437–44. [DOI] [PubMed] [Google Scholar]
- 40.Skiba V, Novikova M, Suneja A, McLellan B, Schultz L. Use of positive airway pressure in mild cognitive impairment to delay progression to dementia. Journal of Clinical Sleep Medicine 2020Jun.15;16(6):863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liguori C, Cremascoli R, Maestri M, Fernandes M, Izzi F, Tognoni G, Scarpina F, Siciliano G, Mercuri NB, Priano L, Bonanni E, Placidi F. Obstructive sleep apnea syndrome and Alzheimer’s disease pathology: may continuous positive airway pressure treatment delay cognitive deterioration? Sleep and Breathing 2021Feb.22;25(4):2135–9. [DOI] [PubMed] [Google Scholar]
- 42.Wu SQ, Liao QC, Xu XX, Sun L, Wang J, Chen R. Effect of CPAP therapy on C-reactive protein and cognitive impairment in patients with obstructive sleep apnea hypopnea syndrome. Sleep and Breathing 2016;20(4):1185–92. [DOI] [PubMed] [Google Scholar]
- 43.Zimmerman ME, Arnedt JT, Stanchina M, Millman RP, Aloia MS. Normalization of memory performance and positive airway pressure adherence in memory-impaired patients with obstructive sleep apnea. Chest 2006;130(6):1772–8. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Cheng C, Moelter S, Fuentecilla JL, Kincheloe K, Lozano AJ, Carter P, Gooneratne N, Richards KC. One year of continuous positive airway pressure adherence improves cognition in older adults with mild apnea and mild cognitive impairment. Nursing Research 2020;69(2):157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richards KC, Gooneratne N, Dicicco B, Hanlon A, Moelter S, Onen F, Wang Y, Sawyer A, Weaver T, Lozano A, Carter P, Johnson J. CPAP adherence may slow 1‐year cognitive decline in older adults with mild cognitive impairment and apnea. Journal of the American Geriatrics Society 2019;67(3):558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Troussière A-C, Monaca Charley C, Salleron J, Richard F, Delbeuck X, Derambure P, Pasquier F, Bombois S. Treatment of sleep apnoea syndrome decreases cognitive decline in patients with Alzheimer. Journal of Neurology, Neurosurgery & Psychiatry 2014May14;85(12):1405–8. [DOI] [PubMed] [Google Scholar]
- 47.Ancoli-Israel S, Palmer BW, Cooke JR, Corey-Bloom J, Fiorentino L, Natarajan L, Liu L, Ayalon L, He F, Loredo JS. Cognitive effects of treating obstructive sleep apnea in alzheimer’s disease: A Randomized Controlled Study. Journal of the American Geriatrics Society 2008;56(11):2076–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cooke JR, Ayalon L, Palmer BW, Loredo JS, Corey-Bloom J, Natarajan L, Liu L, Ancoli-Israel S. Sustained use of CPAP slows deterioration of cognition, sleep, and mood in patients with Alzheimer. Journal of Clinical Sleep Medicine 2009;5(4):305–9. [PMC free article] [PubMed] [Google Scholar]
- 49.Hoyos CM, Cross NE, Terpening Z, D’Rozario AL, Yee BJ, LaMonica H, Marshall NS, Grunstein RR, Naismith SL. Continuous Positive Airway Pressure for Cognition in Sleep Apnea and Mild Cognitive Impairment: A Pilot Randomized Crossover Clinical Trial. American Journal of Respiratory and Critical Care Medicine 2022;205(12):1479–82. [DOI] [PubMed] [Google Scholar]
- 50.Rotenberg BW, Murariu D, Pang KP. Trends in CPAP adherence over twenty years of data collection: a flattened curve. Journal of Otolaryngology - Head & Neck Surgery 2016;45(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith A Symbol digit modalities test: Manual Los Angeles: Western Psychological Services. 1982;. [Google Scholar]
- 52.Weaver TE, Maislin G, Dinges DF, Bloxham T, George CFP, Greenberg H, Kader G, Mahowald M, Younger J, Pack AI. Relationship between hours of CPAP use and achieving normal levels of Sseepiness and daily functioning. Sleep 2007;30(6):711–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baron KG, Gilles A, Sundar KM, Baucom BRW, Duff K, Troxel WM (in press). Pilot and Feasibility Studies [DOI] [PMC free article] [PubMed]
- 54.Baron KG, Smith TW, Berg CA, Czajkowski LA, Gunn H, Jones CR. Spousal involvement in CPAP adherence among patients with obstructive sleep apnea. Sleep and Breathing 2010;15(3):525–34. [DOI] [PubMed] [Google Scholar]
- 55.McCurry SM, Pike KC, Vitiello MV, Logsdon RG, Teri L. Factors associated with concordance and variability of sleep quality in persons with Alzheimer. Sleep 2008;31(5):741–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baron KG, Smith TW, Czajkowski LA, Gunn HE, Jones CR. Relationship quality and CPAP adherence in patients with obstructive sleep apnea. Behavioral Sleep Medicine 2009;7(1):22–6. [DOI] [PubMed] [Google Scholar]
- 57.Troxel WM, Robles TF, Hall M, Buysse DJ. Marital quality and the marital bed: Examining the covariation between relationship quality and sleep. Sleep Medicine Reviews 2007;11(5):389–04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ye L, Malhotra A, Kayser K, Willis DG, Horowitz JA, Aloia MS, Weaver TE. Spousal involvement and CPAP adherence: A dyadic perspective. Sleep Medicine Reviews 2015;19:67–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beninati W, Harris CD, Herold DL, Shepard JW. The effect of snoring and obstructive sleep apnea on the sleep quality of bed partners. Mayo Clinic Proceedings 1999;74(10):955–8. [DOI] [PubMed] [Google Scholar]
- 60.Yeo BSY, Koh JH, Ng ACW, Loh S, See A, Seow DCC, Toh ST. The association of obstructive sleep apnea with blood and cerebrospinal fluid biomarkers of Alzheimer’s dementia - A systematic review and meta-analysis. Sleep Medicine Reviews 2023;70. [DOI] [PubMed]
- 61.Peter-Derex L, Yammine P, Bastuji H, Croisile B. Sleep and Alzheimer. Sleep Medicine Reviews 2015;19:29–8. [DOI] [PubMed] [Google Scholar]
- 62.Baron KG, Gilles A, Sundar KM, Baucom BRW, Duff K, Troxel W. Rationale and study protocol for We-PAP: a randomized pilot/feasibility trial of a couples-based intervention to promote PAP adherence and sleep health compared to an educational control. Pilot and Feasibility Studies 2022;8(1). [DOI] [PMC free article] [PubMed]
- 63.Aaronson JA, Hofman WF, Van Bennekom CA, Van Bezeij T, Van den Aardweg JG, Groet E, Kylstra WA, Schmand B. Effects of Continuous Positive Airway Pressure on Cognitive and Functional Outcome of Stroke Patients with Obstructive Sleep Apnea: A Randomized Controlled Trial. Journal of Clinical Sleep Medicine 2016;12(4):533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Costa YS, Lim AS, Thorpe KE, Colelli DR, Mitchell S, Masellis M, Lam B, Black SE, Boulos MI. Investigating changes in cognition associated with the use of CPAP in cognitive impairment and dementia: A retrospective study. Sleep Medicine 2023;101:437–44. [DOI] [PubMed] [Google Scholar]


