Abstract
Background
Calcimimetic agents lower abnormal serum parathyroid hormone (PTH) levels in people who have chronic kidney disease (CKD), but the benefits and harms on patient‐level outcomes are uncertain. Since this review was first published in 2006 showing that evidence for calcimimetics was largely restricted to biochemical outcomes, additional studies have been conducted. This is an update of a review first published in 2006.
Objectives
To evaluate the benefits and harms of cinacalcet on patient‐level outcomes in adults with CKD.
Search methods
MEDLINE, EMBASE, CENTRAL and conference proceedings were searched for randomised controlled trials (RCTs) evaluating any calcimimetic against placebo or another agent in adults with CKD (persistent albuminuria > 30 mg/g with or without reduced glomerular filtration rate (GFR) (below 60 mL/min/1.73 m²)). We updated searches to 7 February 2013 including the Cochrane Renal Group's Specialised Register to complete this update.
Selection criteria
We included all RCTs of a calcimimetic administered to patients with CKD for the treatment of elevated serum PTH levels.
Data collection and analysis
Data were extracted on all relevant patient‐centred and surrogate outcomes. We summarised treatment estimates using random effects and expressed treatment effects as a risk ratio (RR) or mean difference (MD) with 95% confidence intervals (CI).
Main results
Eighteen studies (7446 participants) compared cinacalcet in addition to standard therapy with no treatment or placebo plus standard therapy. In adults with GFR category G5 (GFR below 15 mL/min/1.73 m²) treated with dialysis, routine cinacalcet treatment had little or no effect on all‐cause mortality (RR 0.97, 95% CI 0.89 to 1.05), imprecise effects on cardiovascular mortality (RR 0.67, 95% CI 0.16 to 2.87), and prevented surgical parathyroidectomy (RR 0.49, 95% CI 0.40 to 0.59) and hypercalcaemia (RR 0.23, 95% CI 0.05 to 0.97), but increased hypocalcaemia (RR 6.98, 95% CI 5.10 to 9.53), nausea (RR 2.02, 95% CI 1.45 to 2.81) and vomiting (RR 1.97, 95% CI 95% CI 1.73 to 2.24). Cinacalcet decreased serum PTH (MD ‐281.39 pg/mL, 95% CI ‐325.84 to ‐234.94) and calcium (MD ‐0.87 mg/dL, 95% CI ‐0.96 to ‐0.77) levels, but had little or no effect on serum phosphorous levels (MD ‐0.23 mg/dL, 95% CI ‐0.58 to 0.12).
Data were sparse for adults with GFR categories G3a to G4 (GFR 15 to 60 mL/min/1.73 m²) and kidney transplant recipients.
Overall, based on GRADE criteria, evidence for cinacalcet in adults with GFR category G5 treated with dialysis (mortality, parathyroidectomy, hypocalcaemia, and nausea) is of high or moderate quality. High quality evidence suggests "further research is very unlikely to change our confidence in the estimate of treatment effect" and moderate quality evidence is "further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate". Information for adults with less severe CKD GFR category G3a to G4 is of low or very low quality. This means that further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate
Authors' conclusions
Routine cinacalcet therapy reduced the need for parathyroidectomy in adults treated with dialysis and elevated PTH levels but does not improve all‐cause or cardiovascular mortality. Cinacalcet increases risks of nausea, vomiting and hypocalcaemia, suggesting harms may outweigh benefits in this population.
Plain language summary
Calcimimetics for secondary hyperparathyroidism in chronic kidney disease patients
Abnormal calcium and phosphorous levels in the blood and tissues occur in chronic kidney disease. These changes are linked to shorter survival and hardening of the arteries leading to heart disease. Standard therapy for abnormal calcium and other mineral levels includes dietary restrictions, phosphorous binders and vitamin D compounds. A newer treatment called cinacalcet showed promise for improving abnormal mineral levels but the effects of this drug on patient outcomes (the way patients feel function and survive) were unclear from early studies. We have updated an earlier review dated 2006 to include studies that assessed the effects of cinacalcet in about 7500 people with chronic kidney disease. While cinacalcet improves some blood abnormalities, it does not improve risk of death or heart disease in people treated with dialysis. In addition, people who take cinacalcet may experience increased nausea, vomiting and the need for blood tests to check blood calcium levels. The current research is high‐quality and means that additional new studies are unlikely to change our confidence in these results. Information for the use of cinacalcet in people with milder forms of kidney disease and those with a kidney transplant is insufficient to guide decision making.
Summary of findings
Summary of findings for the main comparison. Summary of findings for dialysis patients.
| Cinacalcet plus standard therapy versus placebo or standard therapy or both for patients with CKD and elevated PTH levels | |||||
| Patient or population: adults with CKD | |||||
|
Outcomes (median treatment duration) |
*Best estimate of control group risk | Relative effect (95% CI) | No of participants (studies) | Absolute effect per one year of treatment for 1000 treated (95%CI) | Quality of the evidence (GRADE) |
| GFR category G5 treated with dialysis | |||||
|
All‐cause mortality (8 months) |
200 per 1000 | RR 0.97 (0.89 to 95) | 6893 (14) | 6 fewer (22 fewer to 10 more) | ⊕⊕⊕⊕ high |
|
Parathyroidectomy (9 months) |
7 per 1000 | RR 0.49 (0.40 to 0.59) | 4893 (5) | 3 fewer (4 fewer to 3 fewer) | ⊕⊕⊕⊕ high |
|
Hypocalcaemia (7 months) |
10 per 1000 | RR 6.98 (5.10 to 9.53) | 6415 (12) | 60 more (41 more to 85 more) | ⊕⊕⊕⊕ high |
|
Nausea (7 months) |
150 per 1000 | RR 2.02 (1.45 to 2.81) | 6450 (12) | 153 more (68 more to 272 more) | ⊕⊕⊕ moderate |
| GFR category G3a‐G4 | |||||
|
All‐cause mortality (8 months) |
25 per 1000 | RR 0.29 (0.06 to 1.48) | 458 (2) | 18 fewer (23 fewer to 12 more) | ⊕⊕ low |
|
Parathyroidectomy (9 months) |
7 per 1000 | RR not estimable | 0 (0) | Not estimable | nil |
|
Hypocalcaemia (7 months) |
10 per 1000 | RR 31.9 (5.28 to 192.6) | 449 (2) | 310 more (43 more to 1910 more) | ⊕ very low |
|
Nausea (7 months) |
100 per 1000 | RR 2.26 (1.29 to 3.95) | 449 (2) | 126 more (29 more to 295 more) | ⊕⊕ low |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: Confidence interval; RR: Risk Ratio; Approximate absolute event rates of outcomes per year are derived from previously published cohort studies and registry data for the outcomes of all‐cause mortality (Weiner 2006) and parathyroidectomy (Kestenbaum 2004) or event rates in the control arm of contributing studies for outcomes of hypocalcaemia and nausea. Absolute numbers of people who had chronic kidney disease with mortality or parathyroidectomy events avoided or nausea or hypocalcaemia events caused per 1000 treated were calculated from the risk estimate for the outcome (and associated 95% confidence interval) obtained from meta‐analysis of placebo‐controlled studies together with the absolute population risk estimates. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
CKD ‐ chronic kidney disease; GFR ‐ glomerular filtration rate; PTH‐ parathyroid hormone
Background
Description of the condition
Abnormal calcium and phosphorous metabolism occurs with kidney failure and is associated with bone and vascular disease. In addition to causing reduced quality of life, these complications of chronic kidney disease (CKD) are associated with increased mortality and major cardiovascular events (Block 2004b; Ganesh 2001; Malluche 2004b; Marco 2003; Martin 2004; Stehman‐Breen 2004). Standard management of patients with CKD, particularly those with glomerular filtration rate (GFR) category G5 (KDIGO CKD 2013) includes treatment to control serum levels of calcium, phosphorous and parathyroid hormone (PTH) to prevent bone and soft‐tissue complications. Based on a number of association studies (Block 2004b; Ganesh 2001; Kestenbaum 2005; Marco 2003; Stevens 2004), including studies of bone histomorphometry (Hutchison 1993; Qi 1995; Wang 1995; Ziolkowska 2000), optimal ranges for serum phosphorous, calcium, the calcium by phosphorous product and PTH have been suggested (KHA‐CARI 2014; NKF 2003).
How the intervention might work
Specific management of elevated serum PTH levels in people with GFR categories G3a to G5 (estimated GFR below 60 mL/min/1.73 m²) may be accomplished by restriction of dietary phosphorous, calcium supplementation, or the use of vitamin D compounds or both (Albaaj 2003; Courant 1993). A novel class of drugs called calcimimetic agents have been developed to reduce PTH secretion and parathyroid cell proliferation, while decreasing levels of serum calcium, phosphorous and the calcium by phosphorous product (Mentaverri 2006; Mizobuchi 2007). Cinacalcet, a calcimimetic agent, was first approved in the United States in 2004 to lower elevated serum PTH levels in dialysis patients (FDA 2004). Cinacalcet mimics the action of calcium on calcium‐sensing receptors in the parathyroid gland to suppress PTH secretion. In an earlier version of this review that included eight studies (1,429 participants) published in late 2005 or earlier, cinacalcet markedly reduced serum levels of PTH (290 pg/mL), calcium (‐0.85 mg/dL) and phosphorous (‐0.29 mg/dL), that have all been shown to be associated with poorer outcomes in adults with CKD (Block 2004b)
Why it is important to do this review
However, while cinacalcet reduces biochemical parameters (serum PTH, calcium, and phosphorous), our earlier meta‐analysis found insufficient evidence for benefit on clinical outcomes. Despite this lack of evidence for patient‐level outcomes, cinacalcet has become the largest single drug cost for dialysis patients in the United States with annual prescribing costs of at least USD 260 million (USRDS 2012). A pooled analysis of four placebo‐controlled randomised controlled trials (RCTs) of cinacalcet in 2005 showed a large reduction in cardiovascular‐related hospitalisation which may have led to uncertainty for clinicians about the therapy benefits of cinacalcet treatment (Cunningham 2005a).
This systematic review now updates an earlier review that was performed at an early phase of calcimimetic usage, but one year after licensing by the FDA (FDA 2004). In the light of recent studies of cinacalcet, high prescribing costs in some regions and insufficient existing evidence for cinacalcet on patient‐level outcomes in an earlier review, we have updated the evidence summary for the benefits and harms of cinacalcet therapy in people with CKD to early 2013.
Objectives
To evaluate the benefits and harms of cinacalcet on patient‐level outcomes in adults with CKD.
Methods
Criteria for considering studies for this review
Types of studies
We included all RCTs of any calcimimetic agent (cinacalcet HCl (AMG‐073, Sensipar®), NPS R‐467 or NPS R‐568) administered to patients with CKD and elevated serum PTH levels.
Types of participants
Patients with CKD of any severity and elevated serum parathyroid levels.
Types of interventions
Any calcimimetic agent (e.g. cinacalcet HCl (AMG‐073, Sensipar®), NPS R‐467 or NPS R‐568).
Types of outcome measures
Primary outcomes
All‐cause mortality
Cardiovascular mortality
Parathyroidectomy
Fractures
Adverse events (hypocalcaemia, hypercalcaemia, nausea, vomiting, upper respiratory tract infection, dyspnoea, muscle weakness, headache, paraesthesia, abdominal pain, diarrhoea)
Secondary outcomes
At least 30% decrease in serum PTH level
Fractures
Mixed uraemic osteodystrophy
Bone histomorphometry
End of treatment PTH levels (any measure)
End of treatment serum calcium concentrations (mg/dL)
End of treatment serum phosphorous concentrations (mg/dL)
End of treatment calcium x phosphorous product (mg²/dL²)
Search methods for identification of studies
For this review update we searched EMBASE and the Cochrane Renal Group's Specialised Register (to 7 February 2013) through contact with the Trials' Search Co‐ordinator using search terms relevant to this review. The Cochrane Renal Group’s Specialised Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of renal‐related journals & the proceedings of major renal conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected renal‐journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal & ClinicalTrials.gov.
Studies contained in the Specialised Register were identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of the Cochrane Renal Group. Details of these strategies as well as a list of handsearched journals, conference proceedings and current awareness alerts are available in the 'Specialised Register' section of information about the Cochrane Renal Group.
See Appendix 1 for search terms used in strategies for this review.
Previous search strategies can be found in Strippoli 2006a.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that might have been relevant to the review. The titles and abstracts were screened independently by two or more authors, who discarded studies that were not applicable; however studies and reviews that might have included relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts and, if necessary the full text, of these studies to determine which studies satisfied the inclusion criteria.
Data extraction and management
Data extraction for population characteristics, interventions, non‐randomised co‐interventions, and risk of bias was carried out independently by two or more authors using standard data extraction forms in a purpose‐built database. Each author double‐checked data extraction and data entry independently and any discrepancies between authors were resolved through discussion.
The following data were extracted.
Population: category of CKD, mean age, proportion of men, baseline serum PTH level
Intervention: drug name, dosing strategy, target serum PTH level used, randomised and non‐randomised co‐interventions
Comparison: placebo or no treatment, randomised and non‐randomised co‐interventions
Outcomes: all‐cause mortality, cardiovascular mortality, parathyroidectomy, fracture, and treatment related adverse events
Study design: inclusion criteria; exclusion criteria, primary endpoint; duration of treatment, duration of follow‐up, number of participants, date of publication, number of centres, source of funding; study registration (for studies published after 2005); publication (full text publication, abstract publication, unpublished data); period of collection of clinical outcomes (total duration of follow‐up, specific phase(s) of follow‐up)
Risk of bias: sequence generation; allocation concealment; blinding of participants and investigators, blinding of outcome assessment, attrition, selective outcome reporting, other sources of bias (reporting only in conference proceedings, early termination of study, marked imbalance in baseline characteristics, sponsor on authorship or involved with data handling and analysis)
Data were cross checked between authors and discussed. Studies reported in non‐English language journals were translated before assessment. Where more than one publication of one study existed, reports were grouped together and the publication with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier versions, these data were used. Any discrepancy between published versions was highlighted. Any disagreements in data extraction were discussed with a third author.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011). The specific attributes of each risk of bias considered in the adjudication process are described in Appendix 2.
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study (detection bias)?
Participants and personnel
Outcome assessors
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
The estimate of effect of an experimental versus a control intervention on categorical outcomes (e.g. all‐cause mortality, one or more fractures, parathyroidectomy, one or more episodes of nausea) was analysed using the risk ratio (RR) measure and its 95% confidence interval (CI) for each study. Where proportions of participants experiencing an event were provided in the study reports only (instead of raw event data), we estimated the number of participants experiencing one or more events by multiplying the proportion affected by the sample size and contacted the authors or sponsors for additional information.
For continuous variables (end of treatment serum PTH, calcium, phosphorous, calcium x phosphorous), the mean difference (MD), and its 95% CI were calculated using the end of treatment values of the variable in the treatment and control groups.
Assessment of heterogeneity
We assessed for heterogeneity in summary effect estimates using the Cochran Q test and the I² test (Higgins 2003). We considered a P value < 0.10 to indicate significant heterogeneity.
Data synthesis
When appropriate and feasible, treatment effects were summarised using random effects meta‐analysis and results were expressed as a RR or MD and the 95% confidence interval.
We summarised the quality of the evidence together with absolute treatment effects based on estimated baseline risks by using the Grading of Recommendations Assessment, Development, and Evaluation guidelines (Guyatt 2008). To estimate the absolute number of people with CKD who avoided death or parathyroidectomy and incurred hypocalcaemia or nausea with calcimimetic therapy, the risk estimate and 95% CI were obtained from the corresponding meta‐analyses, together with the absolute population risk for people with each category of CKD derived from cohort studies and registry data for all‐cause mortality and parathyroidectomy (Kestenbaum 2004; USRDS 2012; Weiner 2006) and event rates in the control arm of meta‐analyses for hypocalcaemia and nausea.
Subgroup analysis and investigation of heterogeneity
We analysed data for all‐cause mortality, cardiovascular mortality, parathyroidectomy, hypocalcaemia, and nausea and vomiting within stratified analyses comprising adults with GFR category G5 treated with dialysis and GFR categories G33‐G4 (using the KDIGO nomenclature ‐ see Table 2 for more details) (KDIGO CKD 2013).
1. Current chronic kidney disease nomenclature used by KDIGO nomenclature.
| Prognosis of CKD by GFR and albuminuria categories: KDIGO 2012 |
Persistent albuminuria categories Description and range |
|||||
|
A1 Normal to mildly increased |
A2 Moderately increased |
A3 Severely increased |
||||
|
< 30 mg/g < 3 mg/mmol |
30 to 300 mg/g 3 to 30 mg/mmol |
> 300 mg/g > 30 mg/mmol |
||||
|
GFR categories (mL/min per 1.73 m²) Description and range |
G1 G2 |
Normal or high Mildly decreased |
> 90 60 to 89 |
Low | Moderate | High |
| G3a | Mild to moderately decreased | 45 to 59 | Moderate | High | Very high | |
| G3b | Moderate to severely decreased | 30 to 44 | High | Very high | ||
| G4 | Severely decreased | 15 to 29 | Very high | |||
| G5 | Kidney failure | < 15 | ||||
Description of the Kidney Disease: Improving Global Outcomes (KDIGO) nomenclature for chronic kidney disease used in this review (see the full KDIGO CKD 2013 for additional information).
GFR ‐ glomerular filtration rate
Sensitivity analysis
We conducted additional analyses excluding studies in which randomised co‐interventions strategies (vitamin D compounds) were not comparable between treatment arms.
Results
Description of studies
Results of the search
Initial search to November 2005
Our search for RCTs of calcimimetic interventions identified 186 records (see Figure 1 ‐ Study flow diagram). Of these, 148 were excluded after title and abstract review because they were clearly ineligible (non‐RCTs, RCTs of interventions not relevant to treatment of elevated PTH levels, not calcimimetic interventions, duplicate articles of the same study, or review articles). Of the remaining 38 potentially eligible studies (either full‐text or abstract publications), 30 were excluded because we could not confirm from the full‐text analysis or from contacting authors that they were RCTs, or that they were not a duplicate publication. Two attempts were made to contact all authors of the studies for clarifications of study designs and request supplemental data but we were not able to obtain some of the data nor to ascertain if some reports (presented in abstract form at the American Society of Nephrology (ASN) and the European Renal Association‐European Dialysis and Transplantation Association (ERA‐EDTA) meetings of years 2003 and 2004)) were subsets of other publications which had subsequently appeared as full‐text articles in scientific journals or were unique unpublished studies. These studies could therefore not be included and were listed under Characteristics of studies awaiting classification. We have subsequently reviewed these studies and ascertained all but Coburn 2003 are clearly duplicate reports of primary studies and these have been moved to the relevant studies.
1.
Study flow diagram.
Search update to February 2013
The updated search to February 7, 2013 is detailed in Figure 1.
We identified 78 records from the Cochrane Renal Group Specialised Register and 167 from EMBASE. After title and abstract review we excluded 134 reports (duplicate reports, not randomised, wrong population or intervention).
We screened 111 full‐text records and excluded 66 reports as they were not RCTs, did not investigate relevant interventions, or did not include the relevant population. Two ongoing studies from the original review could now be excluded as they are not randomised studies (CONTROL Study 2006; TARGET Study 2008) and three studies are awaiting classification.
The URL describing the report is no longer linked to the article and the authors could not be contacted to obtain additional information (UPen 2004a)
Two studies state they are subgroup analyses of three studies; however we were unable to determine which three studies (Drueke 2001a; Fournier 2004a).
Included studies
Initial review including search to November 2005
In the initial review publication we included eight studies (in eight publications) enrolling 1429 patients (Block 2004b; Goodman 2000; Goodman 2002; Harris 2004; Lindberg 2003; Lindberg 2005; Malluche 2008 (included as Malluche 2004 in original review); Quarles 2003a). These studies compared cinacalcet HCl (845 patients) to placebo (584 patients). Three of these studies reported cinacalcet HCl as AMG‐073. One study reported on the first‐generation calcimimetic R‐568. This drug has been withdrawn from clinical use because of poor bioavailability, variable serum concentrations and potential drug interactions caused by cytochrome P‐450 activity (Goodman 2000; Urena 2003).
In addition to these randomised interventions, patients received vitamin D for suppression of PTH and phosphate binders for management of hyperphosphataemia as co‐interventions in all studies in a non‐randomised fashion. There were no significant differences in the proportions of patients who were prescribed calcitriol, vitamin D analogues and phosphate binders as co‐interventions between the calcimimetic and placebo groups of the studies. Entry to some studies was restricted when patients had severely elevated PTH levels (e.g. iPTH > 800 pg/mL) while other studies stratified patients according to the severity of hyperparathyroidism. The mean age of patients enrolled in the studies ranged from 47 to 55 years. All patients had elevated PTH levels. On average, a higher proportion of males were enrolled in the studies (388 males compared to 220 females in the six studies that reported gender distribution). Follow‐up of the studies ranged from three to 26 weeks. All studies were supported by Amgen Inc., Thousand Oaks, CA, which holds the cinacalcet HCl patent. Of note, the largest report published (Goodman 2002) was based on the pooled results of two separate studies.
Updated review including search to February 2013
We included 10 additional studies in this review update (ACHIEVE Study 2008; ADVANCE Study 2010; Akiba 2008; Charytan 2005; Chonchol 2009; El Shafey 2011; EVOLVE study 2007; Fukagawa 2008; IMPACT SHPT Study 2012; OPTIMA Study 2008). One study from the original review (known as Malluche 2004) was updated using data from a newer publication report (Malluche 2008). Overall, the updated review included 18 studies comprising 7446 adults with CKD comparing a calcimimetic plus conventional therapy with placebo or no treatment with conventional therapy. We could include 17 studies in 7424 participants in the meta‐analyses. The characteristics of the included studies are described in Characteristics of included studies.
All included studies evaluated cinacalcet hydrochloride (referred to as R‐568 or AMG‐073 in the four earliest studies) (Goodman 2000; Goodman 2002; Quarles 2003a; Lindberg 2003). Cinacalcet in additional to conventional therapy (vitamin D compounds and phosphorous binding agents) was compared to placebo or conventional therapy or both in all studies. In three studies, the strategy for vitamin D therapy differed between treatment groups (ACHIEVE Study 2008; ADVANCE Study 2010; IMPACT SHPT Study 2012). The two earliest studies were short‐term evaluations of cinacalcet therapy (eight days (Goodman 2002) and 15 days (Goodman 2000)) in adults with GFR category G5 (treated with dialysis). Following these earliest studies of safety and biochemical efficacy, the first larger‐scale study of cinacalcet therapy was reported in 2004 in 741 adults with GFR category G5 (treated with dialysis) and measured treatment efficacy based on PTH concentrations (Block 2004a). Between 2004 and 2012, 11 additional studies were reported (ACHIEVE Study 2008; ADVANCE Study 2010; Akiba 2008; Charytan 2005; Chonchol 2009; El Shafey 2011; Fukagawa 2008; IMPACT SHPT Study 2012; Lindberg 2005; Malluche 2008; OPTIMA Study 2008) although none was powered to evaluate treatment effects on mortality. In late 2012, the Evaluation of Cinacalcet Therapy to Lower Cardiovascular Events (EVOLVE study 2007) in 3883 participants with GFR category G5 treated with dialysis was the first study specifically designed to evaluate calcimimetic therapy on a primary composite outcome of all‐cause mortality or non‐fatal cardiovascular event.
Cinacalcet therapy was given in the included studies generally at increasing doses (usually 30 to 180 mg/d) targeted to serum PTH concentrations. In one study, the cinacalcet dose prescribed was unclear (IMPACT SHPT Study 2012). Overall, 16 studies comprised 6988 participants with GFR category G5 treatment with dialysis (ACHIEVE Study 2008; ADVANCE Study 2010; Akiba 2008; Block 2004a; El Shafey 2011; EVOLVE study 2007; Fukagawa 2008; Goodman 2000; Goodman 2002; Harris 2004; IMPACT SHPT Study 2012; Lindberg 2003; Lindberg 2005; Malluche 2008; OPTIMA Study 2008; Quarles 2003a) and two studies comprised 458 participants with GFR category G3a to G5 (Charytan 2005; Chonchol 2009). Of studies in dialysis, 15 enrolled haemodialysis patients and one enrolled patients treated with either haemodialysis or peritoneal dialysis. Follow‐up duration was between eight days and 21.2 months (median: 6.5 months)
Excluded studies
We excluded studies as they were not evaluating a calcimimetic with or without standard therapy versus placebo or standard therapy or both, did not provide data for relevant outcomes, were not RCTs, or were not in the CKD population. The details of reasons for exclusions are provided in the table for Characteristics of excluded studies.
Risk of bias in included studies
Risk of bias in the included studies is summarised in Figure 2 (individual studies) and Figure 3 (overall summary).
2.
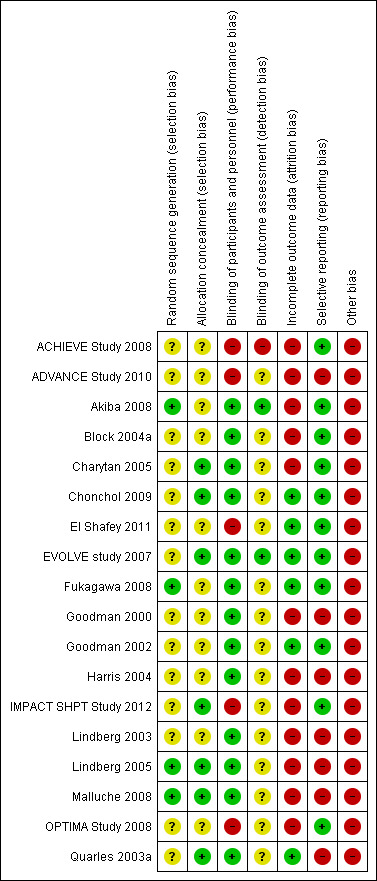
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
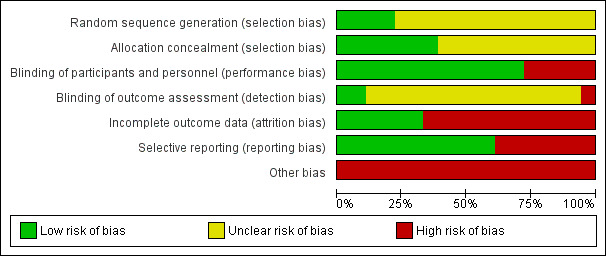
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Four studies reported adequate sequence generation (22%) (Akiba 2008; Fukagawa 2008; Lindberg 2005; Malluche 2008) and reporting on this item was unclear in the remainder. Allocation was adequately concealed in seven studies (39%) (Charytan 2005; Chonchol 2009; EVOLVE study 2007; IMPACT SHPT Study 2012; Lindberg 2005; Malluche 2008; Quarles 2003a) and unclear in the remainder.
Blinding
Participants and investigators were adequately blinded to treatment assignment in 13 studies (72%) (Akiba 2008; Block 2004a; Charytan 2005; Chonchol 2009; EVOLVE study 2007; Fukagawa 2008; Goodman 2000; Goodman 2002; Harris 2004; Lindberg 2003; Lindberg 2005; Malluche 2008; Quarles 2003a), and not blinded in five studies (ACHIEVE Study 2008; ADVANCE Study 2010; El Shafey 2011; IMPACT SHPT Study 2012; OPTIMA Study 2008). Outcome assessment was blinded in two studies (11%) (Akiba 2008; EVOLVE study 2007), not blinded in one study (ACHIEVE Study 2008), and unclear in the remainder.
Incomplete outcome data
Six studies were at low risk of attrition bias (33%) (Chonchol 2009; El Shafey 2011; EVOLVE study 2007; Fukagawa 2008; Goodman 2002; Quarles 2003a) and the remainder were considered at high risk.
Selective reporting
Eleven studies reported all expected outcomes (61%) including mortality, hypocalcaemia, and two or more of nausea, vomiting or diarrhoea (ACHIEVE Study 2008; Akiba 2008; Block 2004a; Charytan 2005; Chonchol 2009; El Shafey 2011; EVOLVE study 2007; Fukagawa 2008; Goodman 2002; IMPACT SHPT Study 2012; OPTIMA Study 2008).
Other potential sources of bias
One study described uneven treatment comparisons at baseline (El Shafey 2011). The study sponsor was on the authorship and/or involved in data collection, analysis and/or interpretation in 15 studies (ACHIEVE Study 2008; ADVANCE Study 2010; Block 2004a; Charytan 2005; Chonchol 2009; EVOLVE study 2007; Goodman 2000; Goodman 2002; Harris 2004; IMPACT SHPT Study 2012; Lindberg 2003; Lindberg 2005; Malluche 2008; OPTIMA Study 2008; Quarles 2003a). Of 10 studies reported since 2005, five (50%) reported evidence of study registration within a studies registry before publication (ADVANCE Study 2010; Chonchol 2009; EVOLVE study 2007; Fukagawa 2008; IMPACT SHPT Study 2012).
Effects of interventions
See: Table 1
Clinical outcomes
All‐cause and cardiovascular mortality
Compared to placebo or no treatment, cinacalcet had little or no effect on all‐cause mortality (Analysis 1.1.1 (14 studies, 6893 participants): RR 0.97, 95% CI 0.89 to 1.05; I² = 0%) in adults with GFR category G5 treated with dialysis and imprecise effects on all‐cause mortality in adults with GFR categories G3a to G4 (Analysis 1.1.2 (2 studies, 458 participants): RR 0.29, 95% CI 0.06 to 1.48; I² = 0%). There was no heterogeneity in treatment effects across studies in either subgroup and no statistical difference in treatment effects in the different stages of CKD.
1.1. Analysis.
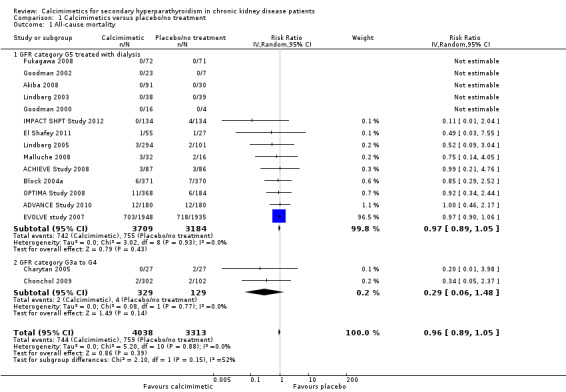
Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 1 All‐cause mortality.
Cinacalcet had uncertain effects on cardiovascular mortality for participants with GFR category G5 treated with dialysis (Analysis 1.2.1 (7 studies, 4542 participants): RR 0.67, 95% CI 0.16 to 2.87; I² = 37%) and GFR category G3a‐G4 (Analysis 1.2.2 (2 studies, 458 participants): RR 0.29, 95% CI 0.06 to 1.48; I² = 0%). There was no significant heterogeneity in treatment effects across studies in either subgroup and no statistical difference in treatment effects in the different stages of CKD.
1.2. Analysis.
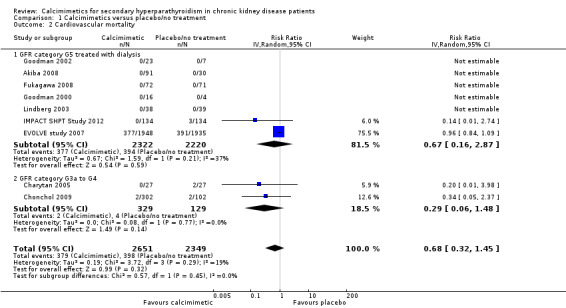
Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 2 Cardiovascular mortality.
Parathyroidectomy
Cinacalcet reduced the risk of parathyroidectomy (Analysis 1.3 (5 studies, 4893 participants): RR 0.49, 95% CI 0.40 to 0.59; I² = 0%) without evidence of heterogeneity in treatment estimates.
1.3. Analysis.
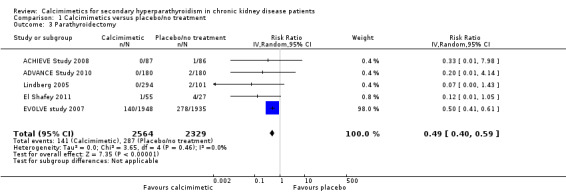
Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 3 Parathyroidectomy.
Fractures
The risk of one of more fractures was reported in extractable format in two studies. Cinacalcet had uncertain effects on risk of one or more fractures (Analysis 1.4 (2 studies, 3965 participants): RR 0.52, 95% CI 0.12 to 2.27) with significant heterogeneity in the treatment effect estimates of contributing studies (P = 0.05, I²; I² = 73%).
1.4. Analysis.
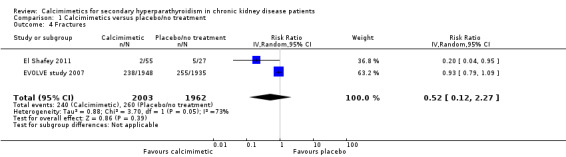
Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 4 Fractures.
Hypocalcaemia and hypercalcaemia
Definitions of hypocalcaemia and hypercalcaemia in the included studies are provided in Table 3. The cut‐off for the definition of hypocalcaemia generally ranged between 7.1 and 8.4 mg/dL and that of hypercalcaemia was 10.2 to 10.5 mg/dL. Cinacalcet increased hypocalcaemia in both adults with GFR category G5 treated with dialysis (Analysis 1.5.1 (12 studies, 6415 participants): RR 6.98, 95% CI 5.10 to 9.53; I² = 0%) and those with GFR category G3 to G4 (Analysis 1.5.2 (2 studies, 449 participants): RR 31.90, 95% CI 5.28 to 192.60; I² = 16%) without significant heterogeneity in treatment estimates between studies.
2. Definitions of parathyroid hormone target, and hypercalcaemia and hypocalcaemia endpoints.
| Study | Participants (treatment/control) | PTH level triggering reduction in cinacalcet dose | Calcium level triggering reduction in cinacalcet dose | Hypocalcaemia (study endpoint) | Hypercalcaemia (study endpoint) |
| ACHIEVE Study 2008 | 173 (87/86) | < 150 pg/mL | Symptoms of hypocalcaemia or < 7.5 mg/dL | < 8.4 mg/dL | > 10.2 mg/dL |
| ADVANCE Study 2010 | 360 (180/180) | ‐‐ | ‐‐ | Hypocalcaemia | Hypercalcaemia |
| Akiba 2008 | 121 (91/30) | ‐‐ | ‐‐ | Hypocalcaemia | ‐‐ |
| Block 2004a | 741 (371/370) | < 100 pg/mL | Symptoms of hypocalcaemia or < 7.8 mg/dL | Withdrawal due to hypocalcaemia | ‐‐ |
| Charytan 2005 | 54 (27/27) | ‐‐ | Dose‐related adverse event or < 7.8 mg/dL | < 8.4 mg/dL | ‐‐ |
| Chonchol 2009 | 404 (302/102) | PTH < 35 pg/mL for stage 3 and < 70 pg/mL for stage 4 | Symptoms of hypocalcaemia or < 7.5 mg/dL | < 7.5 mg/dL | ‐‐ |
| El Shafey 2011 | 82 (55/27) | < 92 pg/mL | Dose‐related adverse event or < 7.5 mg/dL | Hypocalcaemia | ‐‐ |
| EVOLVE study 2007 | 3883 (1948/1935) | < 150 pg/mL | < 7.5 mg/dL and/or symptoms of hypocalcaemia | < 8.0 mg/dL or < 7.5 mg/dL (unclear which threshold reported in study) | > 10.5 mg/dL |
| Fukagawa 2008 | 145 (72/73) | Investigators’ discretion or excessive decrease in PTH level | Investigators’ discretion or < 7.5 mg/dL | Hypocalcaemia | ‐‐ |
| Goodman 2000 | 21 (16/5) | ‐‐ | Symptoms of hypocalcaemia or ionised calcium < 4 mg/dL | Ionized calcium < 4 mg/dL | ‐‐ |
| Goodman 2002 | 30 (23/7) | ‐‐ | 8.0 mg/dL | < 8.0 mg/dL | ‐‐ |
| Harris 2004 | 23 (17/6) | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| IMPACT SHPT Study 2012 | 264 (134/134) | < 150 pg/mL | < 7.5 mg/dL | <8,.4 mg/dL | > 10.5 mg/dL |
| Lindberg 2003 | 78 (39/39) | < 100 pg/mL | Symptoms of hypocalcaemia or < 7.8 mg/dL | < 7.5 mg/dL | ‐‐ |
| Lindberg 2005 | 395 (294/101) | ‐‐ | Symptoms of hypocalcaemia or < 7.8 mg/dL | ‐‐ | ‐‐ |
| Malluche 2008 | 32 (19/13) | < 100 pg/mL | Symptoms of hypocalcaemia or < 7.8 mg/dL | ‐‐ | ‐‐ |
| OPTIMA Study 2008 | 552 (368/184) | < 150 pg/mL | < 8.0 mg/dL | < 7.5 mg/dL | ‐‐ |
| Quarles 2003a | 71 (36/35) | < 100 pg/mL | < 7.8 mg/dL | ‐‐ | ‐‐ |
PTH ‐ parathyroid hormone
1.5. Analysis.
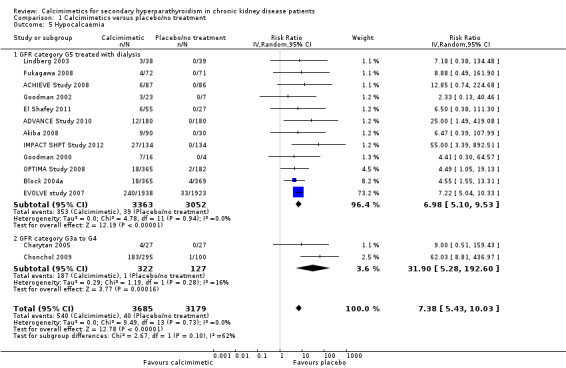
Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 5 Hypocalcaemia.
Cinacalcet reduced risks of one or more episodes of hypercalcaemia in adults with GFR category G5 treated with dialysis (Analysis 1.6 (4 studies, 4662 participants): RR 0.23, 95% CI 0.05 to 0.97) although there was significant heterogeneity in treatment estimates in the available studies (P = 0.005, I² = 77%).
1.6. Analysis.
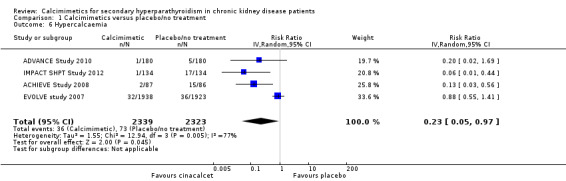
Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 6 Hypercalcaemia.
Nausea and vomiting
Cinacalcet increased nausea in participants with GFR category G5 treated with dialysis (Analysis 1.7.1 (12 studies, 6450 participants): RR 2.02, 95% CI 1.45 to 2.81; I² = 66%) and those with GFR category G3 to G4 (Analysis 1.7.2 (2 studies, 449 participants): RR 2.26, 95% CI 1.29 to 3.95; I² = 6%). There was no statistical evidence that treatment effects were different in the different categories of CKD but there was significant heterogeneity in treatment estimates in studies including dialysis patients (P < 0.0006; I² = 66%).
1.7. Analysis.
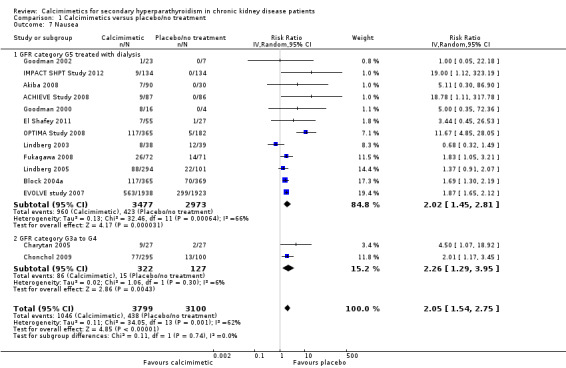
Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 7 Nausea.
Cinacalcet also increased vomiting in participants with GFR category G5 treated with dialysis (Analysis 1.8.1 (9 studies, 6323 participants): RR 1.97, 95% CI 1.73 to 2.24; I² = 3%) and those with GFR category G3 to G4 (Analysis 1.8.2 (1 study, 395 participants): RR 1.77, 95% CI 0.90 to 3.48). There was no statistical evidence that treatment effects were different in the different categories of CKD or significant heterogeneity in treatment estimates between studies.
1.8. Analysis.
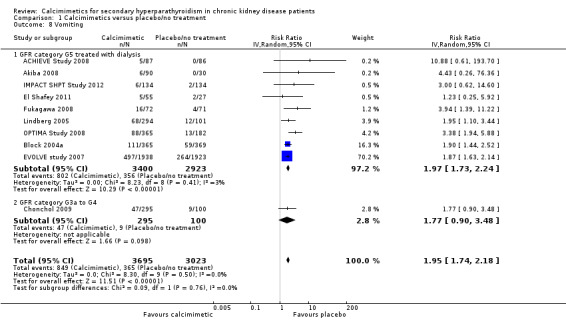
Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 8 Vomiting.
Other adverse events
Cinacalcet consistently increased diarrhoea in the available studies (Analysis 1.9 (8 studies, 5639 participants): RR 1.15, 95% CI 1.02 to 1.29; I² = 0%).
Cinacalcet had uncertain effects on abdominal pain (Analysis 1.10 (4 studies, 831 participants): RR 1.62, 95% CI 0.55 to 4.82) with significant heterogeneity in the treatment effect estimates of contributing studies (P = 0.02, I² = 70%)
Cinacalcet had uncertain effects on the risk of upper respiratory tract infection (Analysis 1.11 (4 studies, 1856 participants): RR 0.95, 95% CI 0.39 to 2.33) with statistically significant heterogeneity in estimated treatment effects between studies (P = 0.002, I² = 80%)
Cinacalcet had uncertain effects on asthenia (Analysis 1.12.1 (2 studies, 790 participants): RR 1.54, 95% CI 0.26 to 8.98) with statistically significant heterogeneity in the estimated treatment effects in available studies (P = 0.04, I² = 77%)
Cinacalcet increased muscle weakness (Analysis 1.12.2 (4 studies, 589 participants): RR 1.78, 95% CI 1.00 to 3.14; I² = 0%) without heterogeneity in treatment effects.
Cinacalcet had uncertain effects on dyspnoea (Analysis 1.13 (2 studies, 250 participants): RR 1.02, 95% CI 0.49 to 2.12; I² = 0%) without heterogeneity in treatment effects.
Cinacalcet had uncertain effects on headache (Analysis 1.14 (3 studies, 1115 participants): RR 1.11, 95% CI 0.65 to 1.91; I² = 25%) without significant heterogeneity in treatment effects.
1.9. Analysis.
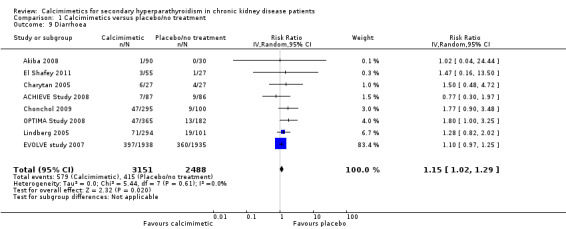
Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 9 Diarrhoea.
1.10. Analysis.
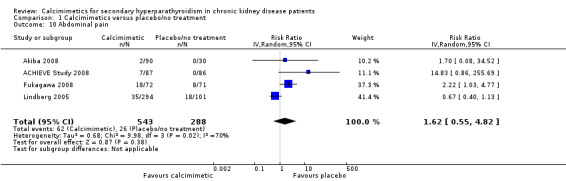
Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 10 Abdominal pain.
1.11. Analysis.
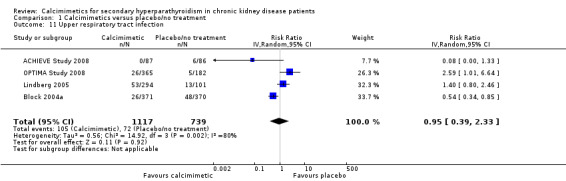
Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 11 Upper respiratory tract infection.
1.12. Analysis.
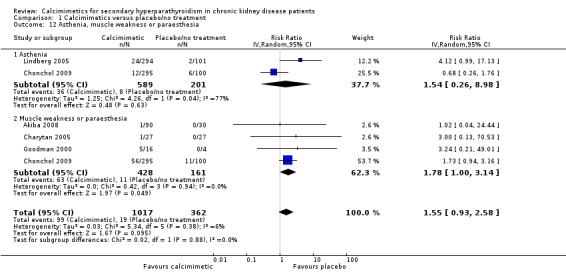
Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 12 Asthenia, muscle weakness or paraesthesia.
1.13. Analysis.
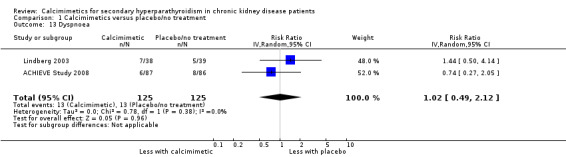
Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 13 Dyspnoea.
1.14. Analysis.
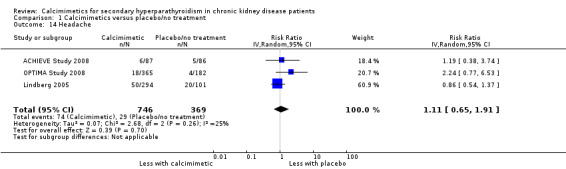
Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 14 Headache.
Biochemical parameters
Achieved serum PTH value target
Target serum PTH values are described in Table 3. Most PTH values targeted by cinacalcet therapy were a reduction of 30% or more from baseline or a reduction to below 250 pg/mL, 279 pg/mL or 300 pg/mL or a target value between 150 to 300 pg/mL. Cinacalcet increased the likelihood that serum PTH values were reduced to a target value (Analysis 1.15 (11 studies, 2853 participants): RR 3.06, 95% CI 1.89 to 4.98), although there was marked heterogeneity in the treatment estimates between studies (P < 0.00001, I² = 92%).
1.15. Analysis.
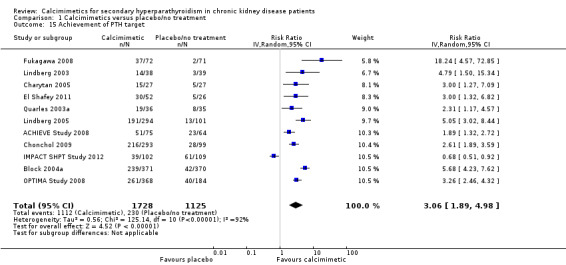
Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 15 Achievement of PTH target.
End of treatment serum PTH
Cinacalcet lowered serum PTH levels (Analysis 1.16 (7 studies, 1935 participants): MD ‐280.39 pg/mL, 95% CI ‐326.84 to ‐235.94) with moderate heterogeneity in the analysis (P = 0.16, I² = 34%).
1.16. Analysis.
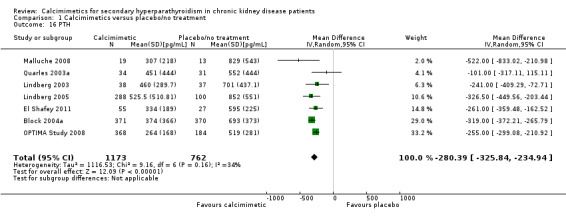
Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 16 PTH.
End of treatment serum calcium
Cinacalcet lowered end of treatment serum calcium levels (Analysis 1.17 (7 study, 1556 participants): MD ‐0.87 mg/dL, 95% CI ‐0.96 to ‐0.77; I² = 18%) without significant heterogeneity in the analysis.
1.17. Analysis.
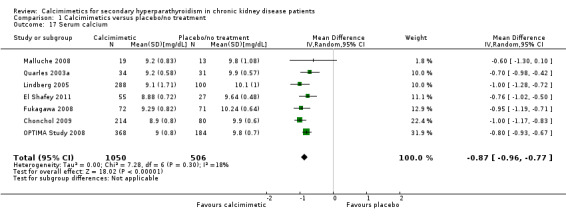
Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 17 Serum calcium.
End of treatment serum phosphorous
Cinacalcet had little or no effect on end of treatment serum phosphorous levels (Analysis 1.18 (8 studies, 2300 participants): MD ‐0.23 mg/dL, 95% CI ‐0.58 to 0.12) with marked heterogeneity in treatment effects between studies (P < 0.00001, I² = 88%).
1.18. Analysis.
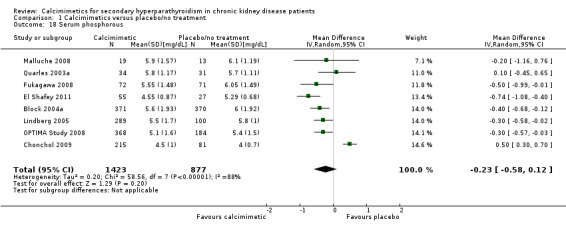
Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 18 Serum phosphorous.
End of treatment serum calcium by phosphorous product
Cinacalcet significantly lowered the serum calcium by phosphorous product (Analysis 1.19 (8 studies, 2395 participants): MD ‐5.25 mg²/dL², 95% CI ‐9.16 to ‐1.34) with marked heterogeneity in treatment effects between studies (P < 0.00001, I² = 91%).
1.19. Analysis.
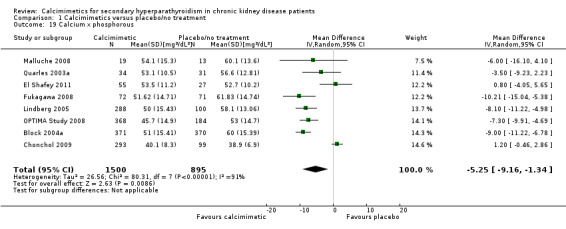
Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 19 Calcium x phosphorous.
Bone outcomes
Effects of calcimimetic therapy on bone structure and function was not consistently reported in available studies (Analysis 1.20; Analysis 1.21).
Sensitivity analyses
When we excluded the three studies in which randomised co‐intervention strategies for vitamin D compounds were not comparable between treatment arms, we observed similar treatment estimates in dialysis patients (all‐cause mortality: RR, 0.97, 95% CI 0.89 to 1.05; cardiovascular mortality: RR 0.95, 95% CI 0.84 to 1.08; hypocalcaemia: RR 6.72, 95% CI 4.88 to 9.25; nausea: RR 1.89, 95% CI 1.38 to 2.60; vomiting: RR 1.98 95% CI 1.71 to 2.30), although risks of hypercalcaemia became less certain (RR 0.88, 95% CI 0.55 to 1.41).
Absolute treatment effects
Estimated absolute treatment effects of cinacalcet (taking into consideration baseline event rates) combined with an overall grading of evidence quality are summarised in the Table 1. Overall, treating 1000 patients with GFR category G5 (treated with dialysis) with cinacalcet might be expected to prevent three experiencing parathyroidectomy, and 60 and 150 more to experience hypocalcaemia and nausea respectively, without altering mortality. In adults with less severe kidney disease (GFR category G3a to G5), treating 1000 patients for one year would result in approximately 300 and 120 experiencing hypocalcaemia and nausea respectively, without evidence for benefits on mortality or parathyroidectomy.
Discussion
Summary of main results
High‐ to moderate‐quality evidence for cinacalcet treatment in adults with GFR category G5 treated with dialysis and elevated PTH levels is available in 16 RCTs (6,988 participants). Routine cinacalcet therapy in dialysis patients at doses between 30 and 180 mg/d decreases serum PTH levels (281 pg/mL) and infrequently reduces surgical parathyroidectomy, but has little or no effect on total mortality, uncertain effects on cardiovascular‐related death, and is commonly associated with adverse events including nausea, vomiting, hypocalcaemia and diarrhoea. Evidence for adults with GFR category G3a to G4 is scant and generally low or very low quality. Because of lower absolute risks of parathyroidectomy in earlier stages of CKD, the benefits of cinacalcet identified by studies in dialysis populations are likely to be smaller if generalised to people who have less severe CKD. Data for adults with a functioning kidney transplant and those treated with peritoneal dialysis were largely absent. It should be noted that adverse treatment effects including nausea, vomiting and hypocalcaemia may have been transient and additional information about patient experiences of these outcomes would inform clinical decision‐making.
Although it is possible that routine cinacalcet prescribing has a beneficial effect on all‐cause mortality, consistent treatment effects across all available studies suggest that, at best, any benefit for mortality is likely to be small. Given that lag censoring analyses for outcomes (where data were censored six months after patients stopped using the study drug) were reported as prespecified secondary analyses in the EVOLVE study 2007 and suggested a potential benefit for cinacalcet on total mortality (hazard ratio 0.83, 95% CI 0.73 to 0.96), it might be argued that additional studies of cinacalcet are now needed or that cinacalcet lowers mortality. However, we suggest that these lag‐censoring approaches were secondary analyses only, and that existing evidence for all‐cause mortality is high‐quality according to GRADE criteria (Guyatt 2008). This means that additional studies are unlikely to change the treatment estimates we observed or our confidence in these estimates.
By contrast, evidence for GFR categories G3a to G4 (estimated GFR 15 to 60 mL/min/1.73 m²) was low or very low quality indicating that further data for this specific group of patients would be informative.
Overall completeness and applicability of evidence
Despite widespread adoption into clinical practice and approval by the FDA, the efficacy and tolerability of cinacalcet in available studies is now better understood and suggests routine cinacalcet therapy has little or no benefit for dialysis patients. Notably, all available studies of cinacalcet investigating cinacalcet used it as "routine" or "first‐line" therapy in patients with elevated serum PTH levels. The findings in these studies therefore do not assess the possibility that cinacalcet may improve patient outcomes when used as treatment of elevated PTH levels resistant to other therapy including vitamin D compounds and phosphorous binders. The National Health Service National Institute for Health and Clinical Excellence (NICE) Clinical practice guidelines have suggested that cinacalcet should be used when serum PTH levels are very high, other treatments have been ineffective and when surgical parathyroidectomy is contraindicated (NICE 2007). However, we advise caution in this clinical setting, as available studies for this approach to cinacalcet therapy are not available and, in particular, outcomes and adverse events comparing parathyroidectomy versus cinacalcet treatment are not available.
This updated review has provided additional evidence for patient‐level outcomes, beyond surrogate outcomes of efficacy that dominated earlier studies and our earlier review (Strippoli 2006a). A surrogate is a measurable outcome such as a laboratory or imaging test, which is responsive to the effect of an intervention (e.g. reduction of total cholesterol with statins) and is also causally associated with a clinically important outcome (e.g. reduction in all‐cause or cardiovascular mortality with statins). A valid surrogate end‐point therefore captures the full effect of an intervention but earlier in the causal chain of events (Bucher 1999; Psaty 1999; Temple 1999). Surrogate end points are used in preference to hard end points in RCTs because cost and sample size can be reduced and feasibility increased substantially. Compared with hard endpoints, surrogates allow for shorter study duration, and either occur more commonly or are continuous measures and so more sensitive to differences in treatment. In kidney disease, surrogates are commonly used in studies, and include dialysis adequacy, haemoglobin levels, left ventricular hypertrophy, and episodes of acute rejection, which have been the basis for the regulatory approval and clinical use of various drugs (Besarab 1998; Borrows 2004; Churchill 1997; McMahon 2004). However, not all surrogates are valid proxies of clinically important patient‐entered outcomes. In order for a surrogate to be valid, two criteria must be met. First, there must be a strong, independent and consistent association between the surrogate and the clinically important outcome, which comes from observational studies. For calcium, phosphorous and PTH this criterion has been met from a number of large‐scale cohort and cross sectional studies (Avram 1996; Ganesh 2001; Kestenbaum 2005; Stevens 2004). Second, and more importantly, for a surrogate to be valid there must also be evidence that using an intervention changes a surrogate (e.g. reduction of PTH with a calcimimetic) and results in an expected change in the patient‐based outcome distal to the surrogate in the same causal pathway for the disease in question (e.g. reduction of deaths with a calcimimetic). This criterion requires a RCT, which measures both the surrogate and the hard endpoint. In the available studies for cinacalcet, we have shown that despite large and clinically‐relevant improvements in serum PTH and serum calcium levels, mortality is not reduced and effects on cardiovascular mortality remain uncertain, suggesting that serum PTH and calcium are not sufficiently proven to be valid proxies of hard patient endpoints in studies of novel agents in CKD.
To date, cinacalcet has uncertain effects on fracture in CKD. The treatment effect we observed (RR 0.53) was similar in magnitude to, but less certain than the risk estimate measured in a pooled analysis of 4 similarly designed, RCTs of cinacalcet comprising 1184 participants with GFR category G5 treated with dialysis and serum PTH levels of 300 pg/mL or greater, in which the reported risk of fracture was 0.46 (95% CI 0.22 to 0.95) (Cunningham 2005a). It was unclear in that publication which studies were included in the analysis, and which included data for extended treatment in two studies that included only about half of the initially randomised participants. While it is possible that cinacalcet has beneficial effects on fracture, at present available evidence provides uncertain estimates of effect.
At this stage, data for cinacalcet in adults with earlier stages of CKD, peritoneal dialysis patients and those with a functioning kidney transplant are scarce or absent. There is currently no strong evidence to support the use of cinacalcet in these clinical settings.
Quality of the evidence
Overall, based on GRADE criteria considering risks of bias in individual studies consistency of the evidence between studies, directness of the evidence to clinical populations, precision of estimates and publication bias, (Table 1) evidence for cinacalcet in adults with GFR category G5 treated with dialysis (mortality, parathyroidectomy, hypocalcaemia, and nausea) is of high or moderate quality. High quality evidence suggests "further research is very unlikely to change our confidence in the estimate of treatment effect" and moderate quality evidence is "further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate".
Information for adults with less severe CKD GFR category G3a to G4 is of low or very low quality. This means that further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality evidence suggests any estimate of effect is very uncertain.
Potential biases in the review process
First, to illustrate the importance of prospective registration of studies recently adopted by all major biomedical journals and kidney journals, in order to ensure that all studies evaluating an intervention can be known and linked with publications to avoid publication bias (Simes 1986) and duplication bias (Egger 1998), we found it very difficult to link publications with specific studies. This was an issue with the first publication of this review in 2006 and continues with this update. While we believe we have eighteen unequivocally separate studies with unique data, we found numerous reports for which we believe data were duplicates of that already available in full‐text published reports. Duplicate reporting is known to be associated with an overestimation of true treatment effects and spurious precision if the studies are incorporated in meta‐analysis (Egger 1998). Prospective registration with a unique identification number for each study would avoid this; despite the recommendation for study registration, only 50% of the 10 included studies published after 2005 (when this requirement commenced) had clearly reported studies registration in the primary published report.
Second, sponsor involvement in authorship, data management and statistical analysis may influence study reporting and lead to a greater likelihood of positive results (Lexchin 2003). The sponsor played a role in the authorship or analysis of over 80% of available studies for cinacalcet suggesting a potential for over‐estimation of treatment benefits.
Third, the investigators of one large study (Block 2004a) combined the results of two separate but similar studies, a method used by the CLASS investigators that has been widely criticized (Juni 2003). When the cinacalcet group of one study is compared both with the placebo group of the same study (random allocation) but also the placebo group of the other study (non‐random allocation), outcome differences between the cinacalcet and placebo groups may be due to differences in study populations or co‐interventions; which are unknown and therefore cannot be adjusted for. Having identical study designs does not prevent these effects. Such study results would be better reported separately. Data could then be combined using meta‐analysis to provide a summary weighted estimate of the effects shown in the individual studies.
Agreements and disagreements with other studies or reviews
This review disagrees with the findings of a pooled analyses of four studies of cinacalcet reported in 2005 (Cunningham 2005a) comprising 1184 dialysis patients with an elevated serum PTH level. That study combined four studies without meta‐analysis and evaluated parathyroidectomy, fracture, hospitalisations and mortality to find marked reductions in the risk of parathyroidectomy (RR 0.07, 95% CI 0.01 to 0.55), fracture (RR 0.46, 95% CI 0.22 to 0.95) and cardiovascular hospitalisation (RR 0.61, 95% CI 0.43 to 0.86). These findings were in contrast to our meta‐analysis in 2006 which found no significant effects on patient‐based endpoints (Strippoli 2006a). Our current update which includes all available studies, indicates a similar but more uncertain effect on fracture, a smaller effect on parathyroidectomy, and could not provide estimates for hospitalisation.
Authors' conclusions
Implications for practice.
Evidence for benefits of routine cinacalcet treatment in adults with CKD and elevated PTH levels are limited to small absolute reductions in parathyroidectomy. Routine cinacalcet therapy in people with CKD does not appear warranted and benefits may be limited to preventing parathyroidectomy in the small number of patients for whom surgery is contraindicated. Nausea, vomiting and hypocalcaemia are commonly experienced by patients treated with cinacalcet.
Implications for research.
Additional studies of cinacalcet in adults with earlier stages of CKD, kidney transplant recipients and peritoneal dialysis patients would inform practice. Until then, widespread prescribing in these populations is not warranted.
Further studies in haemodialysis patients is unlikely to change the estimates of effect or our confidence in the current evidence
Studies comparing cinacalcet with surgical parathyroidectomy might be informative for patients who have elevated PTH levels resistant to standard therapies.
What's new
| Date | Event | Description |
|---|---|---|
| 11 June 2014 | New search has been performed | Methods updated, authorship change |
| 11 June 2014 | New citation required and conclusions have changed | New studies added |
| 7 February 2013 | Amended | Search updated |
History
Protocol first published: Issue 4, 2006 Review first published: Issue 4, 2006
| Date | Event | Description |
|---|---|---|
| 13 August 2009 | Amended | Contact details updated. |
| 13 May 2009 | Amended | Contact details updated. |
| 16 September 2008 | Amended | Converted to new review format. |
Notes
A systematic review and meta‐analysis that includes sequential meta‐analysis and meta‐regression of these data has been published in PLoS Medicine (Palmer 2013).
Acknowledgements
The authors wish to acknowledge the editorial and administrative support of Narelle Willis and Sandra Puckeridge. Ruth Mitchell, Linda Heslop and Gail Higgins, Trial Search Co‐ordinators of the Cochrane Renal Group, provided search strategies for this review.
We also wish to acknowledge Drs Allison Tong and Grahame Elder who were authors on previous versions of this review.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of Bias Assessment Tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random) |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention | |
| Unclear: Insufficient information about the sequence generation process to permit judgement | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes) |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure | |
| Unclear: Randomisation stated but no information on method used is available | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon) |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias |
Data and analyses
Comparison 1. Calcimimetics versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 16 | 7351 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.89, 1.05] |
| 1.1 GFR category G5 treated with dialysis | 14 | 6893 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.89, 1.05] |
| 1.2 GFR category G3a to G4 | 2 | 458 | Risk Ratio (IV, Random, 95% CI) | 0.29 [0.06, 1.48] |
| 2 Cardiovascular mortality | 9 | 5000 | Risk Ratio (IV, Random, 95% CI) | 0.68 [0.32, 1.45] |
| 2.1 GFR category G5 treated with dialysis | 7 | 4542 | Risk Ratio (IV, Random, 95% CI) | 0.67 [0.16, 2.87] |
| 2.2 GFR category G3a to G4 | 2 | 458 | Risk Ratio (IV, Random, 95% CI) | 0.29 [0.06, 1.48] |
| 3 Parathyroidectomy | 5 | 4893 | Risk Ratio (IV, Random, 95% CI) | 0.49 [0.40, 0.59] |
| 4 Fractures | 2 | 3965 | Risk Ratio (IV, Random, 95% CI) | 0.52 [0.12, 2.27] |
| 5 Hypocalcaemia | 14 | 6864 | Risk Ratio (IV, Random, 95% CI) | 7.38 [5.43, 10.03] |
| 5.1 GFR category G5 treated with dialysis | 12 | 6415 | Risk Ratio (IV, Random, 95% CI) | 6.98 [5.10, 9.53] |
| 5.2 GFR category G3a to G4 | 2 | 449 | Risk Ratio (IV, Random, 95% CI) | 31.90 [5.28, 192.60] |
| 6 Hypercalcaemia | 4 | 4662 | Risk Ratio (IV, Random, 95% CI) | 0.23 [0.05, 0.97] |
| 7 Nausea | 14 | 6899 | Risk Ratio (IV, Random, 95% CI) | 2.05 [1.54, 2.75] |
| 7.1 GFR category G5 treated with dialysis | 12 | 6450 | Risk Ratio (IV, Random, 95% CI) | 2.02 [1.45, 2.81] |
| 7.2 GFR category G3a to G4 | 2 | 449 | Risk Ratio (IV, Random, 95% CI) | 2.26 [1.29, 3.95] |
| 8 Vomiting | 10 | 6718 | Risk Ratio (IV, Random, 95% CI) | 1.95 [1.74, 2.18] |
| 8.1 GFR category G5 treated with dialysis | 9 | 6323 | Risk Ratio (IV, Random, 95% CI) | 1.97 [1.73, 2.24] |
| 8.2 GFR category G3a to G4 | 1 | 395 | Risk Ratio (IV, Random, 95% CI) | 1.77 [0.90, 3.48] |
| 9 Diarrhoea | 8 | 5639 | Risk Ratio (IV, Random, 95% CI) | 1.15 [1.02, 1.29] |
| 10 Abdominal pain | 4 | 831 | Risk Ratio (IV, Random, 95% CI) | 1.62 [0.55, 4.82] |
| 11 Upper respiratory tract infection | 4 | 1856 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.39, 2.33] |
| 12 Asthenia, muscle weakness or paraesthesia | 5 | 1379 | Risk Ratio (IV, Random, 95% CI) | 1.55 [0.93, 2.58] |
| 12.1 Asthenia | 2 | 790 | Risk Ratio (IV, Random, 95% CI) | 1.54 [0.26, 8.98] |
| 12.2 Muscle weakness or paraesthesia | 4 | 589 | Risk Ratio (IV, Random, 95% CI) | 1.78 [1.00, 3.14] |
| 13 Dyspnoea | 2 | 250 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.49, 2.12] |
| 14 Headache | 3 | 1115 | Risk Ratio (IV, Random, 95% CI) | 1.11 [0.65, 1.91] |
| 15 Achievement of PTH target | 11 | 2853 | Risk Ratio (IV, Random, 95% CI) | 3.06 [1.89, 4.98] |
| 16 PTH | 7 | 1935 | Mean Difference (IV, Random, 95% CI) | ‐280.39 [‐325.84, ‐234.94] |
| 17 Serum calcium | 7 | 1556 | Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐0.96, ‐0.77] |
| 18 Serum phosphorous | 8 | 2300 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.58, 0.12] |
| 19 Calcium x phosphorous | 8 | 2395 | Mean Difference (IV, Random, 95% CI) | ‐5.25 [‐9.16, ‐1.34] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ACHIEVE Study 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: NS |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NS |
| Allocation concealment (selection bias) | Unclear risk | NS |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not used |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Lost to follow‐up 24% of the patients |
| Selective reporting (reporting bias) | Low risk | Data available for all included outcomes |
| Other bias | High risk | Sponsor on authorship |
ADVANCE Study 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NS |
| Allocation concealment (selection bias) | Unclear risk | NS |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label (http://clinicaltrials.gov/show/NCT00379899) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NS |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up 22.2 % patients |
| Selective reporting (reporting bias) | High risk | Not reported systematically (end of treatment calcium, phoshorous, PTH and adverse events) |
| Other bias | High risk | Sponsor on authorship |
Akiba 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computerised system |
| Allocation concealment (selection bias) | Unclear risk | NS |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Cinacalcet and placebo tablets were identical in appearance in order to maintain the double‐blind status of the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All laboratory determinations, except for hematological assessments, were performed at a central laboratory" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Per‐protocol analysis was used to analyse efficacy endpoints. 14.2% lost to follow‐up in the active arm. 10.7% lost to follow‐up in total |
| Selective reporting (reporting bias) | Low risk | All the prespecified outcomes were reported |
| Other bias | High risk | Authors are scientific advisors for sponsor |
Block 2004a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NS |
| Allocation concealment (selection bias) | Unclear risk | NS |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NS |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up 22% |
| Selective reporting (reporting bias) | Low risk | All the prespecified outcomes were reported |
| Other bias | High risk | Pooled data from 2 studies; statistical analyses and data interpretation by sponsor; data held by sponsor; editorial assistance from sponsor |
Charytan 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NS |
| Allocation concealment (selection bias) | Low risk | Centralised interactive voice‐response system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NS |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up 30% of patients |
| Selective reporting (reporting bias) | Low risk | All the prespecified outcomes were reported |
| Other bias | High risk | Sponsor on authorship |
Chonchol 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NS |
| Allocation concealment (selection bias) | Low risk | Interactive voice‐response system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NS |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up 3% of patients |
| Selective reporting (reporting bias) | Low risk | All the prespecified outcomes were reported |
| Other bias | High risk | Sponsor on authorship; sponsor involved in writing manuscript |
El Shafey 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NS |
| Allocation concealment (selection bias) | Unclear risk | NS |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NS |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up 5% of patients |
| Selective reporting (reporting bias) | Low risk | All the prespecified outcomes were reported |
| Other bias | High risk | Uneven comparisons |
EVOLVE study 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Generated by Amgen |
| Allocation concealment (selection bias) | Low risk | Interactive voice‐response system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up 7.9% of patients |
| Selective reporting (reporting bias) | Low risk | All the prespecified outcomes were reported |
| Other bias | High risk | Sponsor on authorship; sponsor involved in writing manuscript; sponsor held data and analysed data |
Fukagawa 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised computer system |
| Allocation concealment (selection bias) | Unclear risk | NS |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NS |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up 2.8% of patients |
| Selective reporting (reporting bias) | Low risk | All the prespecified outcomes were reported |
| Other bias | High risk | Study sponsored by Kirin Parma Co. LTD |
Goodman 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NS |
| Allocation concealment (selection bias) | Unclear risk | NS |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NS |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up 38% of patients |
| Selective reporting (reporting bias) | High risk | Not reported systematically (end of treatment calcium, phoshorous, PTH and adverse events) |
| Other bias | High risk | Sponsor on authorship |
Goodman 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: NS |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NS |
| Allocation concealment (selection bias) | Unclear risk | NS |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NS |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All the prespecified outcomes were reported |
| Other bias | High risk | Sponsor on authorship |
Harris 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NS |
| Allocation concealment (selection bias) | Unclear risk | NS |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NS |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up 59.1% of patients |
| Selective reporting (reporting bias) | High risk | Not reported systematically (end of treatment calcium, phosphorous, PTH and adverse events) |
| Other bias | High risk | Sponsor on authorship |
IMPACT SHPT Study 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: NS |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Generated by Clinical Statistics department of Abbott |
| Allocation concealment (selection bias) | Low risk | Interactive voice‐response system |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NS |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up 24.3% of patients |
| Selective reporting (reporting bias) | Low risk | All the prespecified outcomes were reported |
| Other bias | High risk | Sponsor on authorship; sponsor involved in writing manuscript; sponsor held data and analysed data |
Lindberg 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NS |
| Allocation concealment (selection bias) | Unclear risk | NS |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NS |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up 14.1% of patients |
| Selective reporting (reporting bias) | High risk | Not reported systematically (end of treatment calcium, phosphorous, PTH and adverse events) |
| Other bias | High risk | Sponsor on authorship |
Lindberg 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Programmatic algorithm |
| Allocation concealment (selection bias) | Low risk | Interactive voice‐response system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NS |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up 25.3% of patients |
| Selective reporting (reporting bias) | High risk | Not reported systematically (end of treatment calcium, phosphorous, PTH and adverse events) |
| Other bias | High risk | Sponsor on authorship |
Malluche 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Interactive voice‐response system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NS |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up 31.3% of patients |
| Selective reporting (reporting bias) | High risk | Not reported systematically (end of treatment calcium, posphorous, PTH and adverse events) |
| Other bias | High risk | Sponsor authorship |
OPTIMA Study 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NS |
| Allocation concealment (selection bias) | Unclear risk | NS |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NS |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up 21.9% of patients |
| Selective reporting (reporting bias) | Low risk | All the prespecified outcomes were reported |
| Other bias | High risk | Sponsor on authorship |
Quarles 2003a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NS |
| Allocation concealment (selection bias) | Low risk | Interactive voice‐response system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NS |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up 8.5% of patients |
| Selective reporting (reporting bias) | High risk | Not reported systematically (end of treatment calcium, phosphorous, PTH and adverse events) |
| Other bias | High risk | Sponsor on authorship |
BSAP ‐ bone‐specific alkaline phosphatase; Ca x P ‐ calcium‐phosphorous product; CAC ‐ coronary artery calcification score; DM ‐ diabetes mellitus; ESKD ‐ end‐stage kidney disease; GFR ‐ glomerular filtration rate; Hb ‐ haemoglobin; HCT ‐ haematocrit; HD ‐ haemodialysis; iPTH ‐ intact parathyroid hormone; ITT ‐ Intention‐to‐treat; MI ‐ myocardial infarction; NA ‐ not available; NS ‐ not stated; NTx ‐ cross‐linked N‐telopeptides of type I collagen; PTH ‐ parathyroid hormone; RCT ‐ randomised controlled trial; SHPT ‐ secondary hyperparathyroidism; TRACP ‐ tartrate‐resistant acid phosphatase
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Hilali 2011 | Not appropriate intervention |
| Coburn 2000a | Not appropriate outcome |
| CONTROL Study 2006 | Not RCT |
| Cunningham 2003 | Observational extension study |
| Cunningham 2005 | Observational extension study |
| de Francisco 2005 | Not RCT |
| Harris 2003 | Not appropriate outcome |
| Kaperonis 2012 | Not RCT |
| Moe 2003a | Not RCT |
| Moe 2005 | Not RCT |
| Moe 2005b | Not RCT |
| Padhi 2003 | Not CKD population |
| Pahl 1996 | Not intervention |
| Quarles 2003 | Not RCT |
| Schaefer 2008 | Not intervention |
| Sezer 2012 | Not intervention |
| TARGET Study 2008 | Not RCT |
CKD ‐ chronic kidney disease; RCT ‐ randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Coburn 2003.
| Methods | Randomised double‐blind placebo‐controlled study |
| Participants | Patients with CKD not treated with dialysis (GFR 15 to 50 mL/min) and intact PTH levels > 130 ng/mL |
| Interventions | Cinacalcet 30 to 180 mg/d titrated to obtain a ≥ 30% reduction in intact PTH levels |
| Outcomes | Mean change in PTH, per cent with reduced PTH ≥ 30%, calcium and phosphorous levels |
| Notes | Unclear whether an additional report of Charytan 2005 |
Drueke 2001a.
| Methods | Placebo controlled RCTs (3) |
| Participants | Dialysis patients Number: AMG‐073 (141); placebo (74) |
| Interventions | AMG‐073 (50 to 100 mg/d) |
| Outcomes | Ca x P; mean iPTH |
| Notes | Abstract only; states combined data of the first 12 weeks of 3 studies ‐ unable to determine which 3 studies |
Fournier 2004a.
| Methods | Placebo controlled RCTs (3) |
| Participants | Dialysis patients with iPTH ≥ 300 pg/mL Number: 955 |
| Interventions | Cinacalcet 30 to 180 mg/d |
| Outcomes | Ca x P; mean iPTH |
| Notes | Abstract only; states combined data of 3 studies ‐ unable to determine which 3 studies |
UPen 2004a.
| Methods | Randomised double‐blinded placebo controlled study |
| Participants | Participants with CKD and elevated serum parathyroid hormone levels |
| Interventions | AMG‐073 |
| Outcomes | Unclear |
| Notes | Reported as published by the University of Pennsylvania at http://renal2.med.upenn.edu/ but URL link broken and unable to obtain information about study to ascertain details and eligibility for this review |
Ca x P ‐ calcium phosphorous product; CKD ‐ chronic kidney diease; iPTH ‐ intact parathyroid hormone; RCT ‐ randomised controlled trial
Differences between protocol and review
The outcomes of cardiovascular mortality and one or more episodes of hypercalcaemia have been added to the review in the update to February 2013.
The study included in the review published in 2006 called Malluche 2004 has been updated to include data from a 2008 full text publication and renamed Malluche 2008.
Contributions of authors
Angela Ballinger: search screening, data extraction and analysis and input into writing of the review
Suetonia Palmer: design, conduct, data extraction and analysis, primary drafting and revisions of the review
Ionut Nistor: search screening, data extraction and analysis and input into writing of the review
Jonathan Craig: design, data analysis, writing the review
Giovanni Strippoli: design, conduct, data‐extraction and analysis, writing the review.
Sources of support
Internal sources
Cochrane Renal Group, Australia.
External sources
Suetonia Palmer receives a Fellowship from the Consorzio Mario Negri Sud from an unrestricted grant from Amgen Dompe, Italy.
Angela Ballinger completed this review as a University of Otago summer studentship project 2012/2013 supported by the Canterbury Medical Research Foundation, New Zealand.
Ionut Nistor was the recipient of a grant from European Renal Best Practice (ERBP) and the European Renal Association‐European Dialysis Transplantation Association (ERA‐EDTA), Other.
Declarations of interest
Angela Ballinger received a student stipend for a summer studentship 2012/2013 from the University of Otago to assist with completing this research.
Suetonia Palmer: none known
Jonathan Craig: none known
Ionut Nistor: is a fellow of the Methods Support Team of European Renal Best Practice (ERBP), supported by a grant of the European Renal Association ‐ European Dialysis Transplantation Association (ERA‐EDTA)
Giovanni Strippoli: none known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
ACHIEVE Study 2008 {published data only}
- Charytan C, Corry D, Roppolo M, Ling X, Droge J, Fishbane S. Assessing the use of the calcimimetic cinacalcet with low dose vitamin D versus escalating doses of vitamin D alone in the treatment of secondary hyperparathyroidism (HPT) ‐ The ACHIEVE Study [abstract no: 39]. American Journal of Kidney Diseases 2007;49(4):A34. [CENTRAL: CN‐00615830] [Google Scholar]
- Charytan C, Wetmore J, Moustafa M, Martinez C, Stewart T, Ling X, et al. Efficacy of treatments targeting the calcium‐sensing receptor (CaR) and vitamin D receptor (VDR) in controlling parathyroid hormone (PTH) in hemodialysis (HD) patients: cinacalcet HCI plus low‐dose vitamin D vs flexible vitamin D alone [abstract no: SU‐PO812]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):764A. [Google Scholar]
- Fishbane S, Shapiro W, Corry D, Rappaport K, Vicks S, Ling X, et al. A comparison of cinacalcet HCI and low‐dose vitamin D vs escalating doses of vitamin D alone ‐ the ACHIEVE study [abstract no: PUB354]. Journal of the American Society of Nephrology 2006;17(Abstracts):891A. [CENTRAL: CN‐00615831] [Google Scholar]
- Fishbane S, Shapiro WB, Corry DB, Vicks SL, Roppolo M, Rappaport K, et al. Cinacalcet HCl and concurrent low‐dose vitamin D improves treatment of secondary hyperparathyroidism in dialysis patients compared with vitamin D alone: the ACHIEVE study results. Clinical Journal of The American Society of Nephrology: CJASN 2008;3(6):1718‐25. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbane S, Vicks S, Ling X, Turner S, Charytan C. Cinacalcet with low dose vitamin D provides improved control of parathyroid hormone, calcium, and calcium‐phosphorus product in hemodialysis patients with secondary hyperparathyroidism compared with vitamin D alone [abstract no: SaP383]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi363. [CENTRAL: CN‐00671779] [Google Scholar]
- Fishbane S, Wetmore J, Moustafa M, Martinez C, Ling X, Turner S, et al. Treatment with cinacalcet HC1 and concurrent low‐dose vitamin D improved management of secondary hyperparathyroidism (SHPT) compared with vitamin D alone [abstract no: SU‐FC100]. Journal of the American Society of Nephrology 2007;18(Abstracts):89A. [Google Scholar]
- Shireman TI, Almehmi A, Wetmore JB, Lu J, Pregenzer M, Quarles LD. Economic analysis of cinacalcet in combination with low‐dose vitamin D versus flexible‐dose vitamin D in treating secondary hyperparathyroidism in hemodialysis patients. American Journal of Kidney Diseases 2010;56(6):1108‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wetmore JB, Liu S, Krebill R, Menard R, Quarles D. Treatment with cinacalcet plus vitamin D results in lower FGF23 levels than vitamin D alone [abstract no: SA‐PO2779]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):739A. [Google Scholar]
- Wetmore JB, Liu S, Krebill R, Menard R, Quarles LD. Effects of cinacalcet and concurrent low‐dose vitamin D on FGF23 levels in ESRD. Clinical Journal of The American Society of Nephrology: CJASN 2010;5(1):110‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ADVANCE Study 2010 {published data only}
- Floege J, Raggi P, Block GA, Torres PU, Csiky B, Naso A, et al. Study design and subject baseline characteristics in the ADVANCE Study: effects of cinacalcet on vascular calcification in haemodialysis patients. Nephrology Dialysis Transplantation 2010;25(6):1916‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Floege J, Sprague S, Droge J, Banos A, Chertow G. ADVANCE: the effect of cinacalcet + low‐dose vitamin D on vascular calcification in hemodialysis patients ‐ methods [abstract no: 60]. American Journal of Kidney Diseases 2007;49(4):A39. [Google Scholar]
- Raggi P, Chertow GM, Torres PU, Csiky B, Naso A, Nossuli K, et al. The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low‐dose vitamin D on vascular calcification in patients on hemodialysis. Nephrology Dialysis Transplantation 2011;26(4):1327‐39. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Urena PA, Kopyt NP, Rodriguez M, Bridges IM, Dehmel B, Cooper K, et al. Efficacy of cinacalcet combined with low dose vitamin D in incident hemodialysis subjects with secondary hyperparathyroidism [abstract no: LB‐PO3145]. Journal of the American Society of Nephrology 2011;22(Abstracts):3B. [Google Scholar]
Akiba 2008 {published data only}
- Akiba T, Akizawa T, Tsukamoto Y, Uchida E, Iwasaki M, Koshikawa S, et al. Dose determination of cinacalcet hydrochloride in Japanese hemodialysis patients with secondary hyperparathyroidism. Therapeutic Apheresis & Dialysis 2008;12(2):117‐25. [18387159] [DOI] [PubMed] [Google Scholar]
- Akiba T, Akizawa T, Uchida E, Tsukamoto Y, Koshikawa S, KRN1493 Study Group. Randomized, double blind, placebo‐controlled, dose‐finding parallel study for KRN1493 (cinacalcet HC1) in Japanese hemodialysis patients [abstract no: F‐PO764]. Journal of the American Society of Nephrology 2005;16:503A. [Google Scholar]
- Akizawa T, Koshikawa S. Clinical study of cinacalcet in Japan. Therapeutic Apheresis & Dialysis 2008;12 Suppl 1:S13‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Block 2004a {published data only}
- Block GA, Martin KJ, Turner SA, Avram MM, Hercz G, Abu‐Alfa AK, et al. Phase 3 study results demonstrate efficacy and safety of the calcimimetic cinacalcet HCI in hemodialysis patients with secondary hyperparathyroidism (HPT) [abstract no: SA‐PO743]. Journal of the American Society of Nephrology 2003;14(Nov):461A. [CENTRAL: CN‐00644280] [Google Scholar]
- Block GA, Martin KJ, Francisco AL, Turner SA, Avram MM, Suranyi MG, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. New England Journal of Medicine 2004;350(15):1516‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Coyne DW, Stegman MH, Azad H, Joy MS, Mischel SF, Pokroy N, et al. Cinacalcet HCI controls secondary hyperparathyroidism (HPT) regardless of gender, race, age, and geography in patients with chronic kidney disease (CKD) receiving dialysis. [abstract no: SA‐PO754]. Journal of the American Society of Nephrology 2003;14(Nov):464A. [Google Scholar]
- Cunningham J, Chertow G, Goodman W, Danese M, Olson K, Klassen P, et al. The effect of cinacalcet HCl on parathyroidectomy, fracture, hospitalisation, and mortality in dialysis subjects with secondary hyperparathyroidism (HPT) [abstract]. 41st ERA‐EDTA Congress; 2004; May 15‐18; Lisbon (Portugal). 2004:219. [CN‐00509144]
- Cunningham J, Danese M, Olson K, Klassen P, Chertow GM. Effects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture, and health‐related quality of life in secondary hyperparathyroidism. Kidney international 2005;68(4):1793‐800. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Frazao JM, Holzer H, Stummvoll HK, Bahner U, Wilkie M, Zani V, et al. Cinacalcet (Mimpara©/Sensipar©) maintains achievement of NKF‐K/DOQI treatment targets for secondary hyperparathyroidism (HPT) in patients on dialysis [abstract no: SP209]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v89. [Google Scholar]
- Frazao JM, Messa P, Cunningham J, Evenepoel P, Shahapuni I, Urena P, et al. Early use of cinacalcet (Mimpara©/Sensipar©) in dialysis patients enables greatest achievement of NKF‐KDOQITM treatment targets for bone metabolism [abstract no: SO016]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv8. [Google Scholar]
- Frazao JM, Nicolini M, Torresgrossa JV, Kerr P, Jaeger PH, Sprague SM, et al. Cinacalcet HCl effectively reduces intact parathyroid hormone (iPTH) and Ca x P irrespective of the severity of secondary hyperparathyroidism (HPT) [abstract]. 41st ERA‐EDTA Congress; 2004; May 15‐18; Lisbon (Portugal). 2004:219.
- Goodman WG, Fadda GZ, Finkelstein FO Mittman N, Lien YH, LeBlanc M, et al. Cinacalcet HCl is an effective primary therapy for the management of secondary hyperparathyroidism [abstract]. Journal of the American Society of Nephrology 2003;14(Program & Abstracts):460A. [Google Scholar]
- Goodman WG, Francisco AL, Mittman N, Messa P, Lien YH, Leblanc M, et al. Cinacalcet HCI (SensiparTM) effectively manages secondary hyperparathyroidism (HPT) in new to dialysis patients [abstract no: PUB477]. Journal of the American Society of Nephrology 2004;15(Oct):863A. [CENTRAL: CN‐00644149] [Google Scholar]
- Goodman WG, Francisco AL, Moe SM, Cunningham J, Martin KJ, McCary LC, et al. Cinacalcet HCI (SensiparTM) is an effective treatment for secondary hyperparathyroidism (HPT) in CKD patients on dialysis [abstract no: F‐PO984]. Journal of the American Society of Nephrology 2004;15(Oct):280A. [CENTRAL: CN‐00644151] [Google Scholar]
- Lien YH, Silva AL, Whittman D. Effects of cinacalcet on bone mineral density in patients with secondary hyperparathyroidism. Nephrology Dialysis Transplantation 2005;20(6):1232‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lien YH, Silva AL, Whittman D. Effects of cinacalcet on bone mineral density in patients with secondary hyperparathyroidism [abstract no: W‐PO40084]. Nephrology 2005;10(Suppl 1):A301. [DOI] [PubMed] [Google Scholar]
- Martin KJ, Sherrard DJ, Nassar G, Campbell P, Curzi M, McCary KJ, et al. Control of bio‐intact parathyroid hormone (PTH) levels with the calcimimetic Cinacalcet HCl in hemodialysis patients with secondary hyperparathyroidism (HPT) [abstract no: SA‐PO747]. Journal of the American Society of Nephrology 2003;14(Nov):462A. [CENTRAL: CN‐00583599] [Google Scholar]
- Mittman N, Francisco ALM, Drueke T, Fadda GZ, Arruda JA, Abu‐Alfa AK, et al. Phase 3 studies demonstrate effective control of PTH and Ca x P in patients treated with cinacalcet HCI [abstract no: 73]. American Journal of Kidney Diseases 2004;43(4):A33. [Google Scholar]
- Moe SM, Chertow GM, Coburn JW, Quarles LD, Goodman WG, Block GA, et al. Achieving NKF‐K/DOQI bone metabolism and disease treatment goals with cinacalcet HCl. Kidney international 2005;67(2):760‐71. [CENTRAL: 15673327] [DOI] [PubMed] [Google Scholar]
- Sterrett JF, Strom J, Stummvoll HK, Bahner U, Disney A Soroka SD, et al. Long‐term treatment of secondary hyperparathyroidism (HPT) with cinacalcet (SensiparTM) in patients receiving dialysis [abstract]. Journal of the American Society of Nephrology 2004;15(Abstracts):275A. [CENTRAL: CN‐00583604] [Google Scholar]
- Sterrett JR, Strom J, Stummvoll HK, Bahner U, Disney A, Soroka SD, et al. Cinacalcet HCI (Sensipar/Mimpara) is an effective chronic therapy for hemodialysis patients with secondary hyperparathyroidism. Clinical Nephrology 2007;68(1):10‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Francisco A, Suranyi M, Cunningham J, Messa P, Locatelli F, Evenepoel P, et al. Greater achievement of NKF‐K/DOQI bone metabolism and disease targets with oral cinacalcet HCL in an EU/Australian phase 3 study in haemodialysis subjects [abstract]. 41st ERA‐EDTA Congress; 2004; May 15‐18; Lisbon (Portugal). 2004:319.
- Francisco AL, Suranyi M, Cunningham J, Messa P, Locatelli F, Evenepoel P, et al. Oral cinacalcet HCI (AMG 073) for the treatment of hemodialysis patients with secondary hyperparathyroidism (HPT): results of a European/Australian phase 3 study. [abstract no: SA‐PO742]. Journal of the American Society of Nephrology 2003;14(Nov):461A. [CENTRAL: CN‐00583675] [Google Scholar]
Charytan 2005 {published data only}
- Charytan C, Coburn JW, Chonchol M, Herman J, Lien YH, Liu W, et al. Cinacalcet hydrochloride is an effective treatment for secondary hyperparathyroidism in patients with CKD not receiving dialysis. American Journal of Kidney Diseases 2005;46(1):58‐67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Coburn JW, Charytan C, Chonchol M, Herman J, Lien YH, Liu W, et al. Cinacalcet HCl is an effective treatment for secondary hyperparathyroidism (HPT) in patients with chronic kidney disease (CKD) not yet receiving dialysis [abstract]. Journal of the American Society of Nephrology 2003;14(Program & Abstracts):460A. [DOI] [PubMed] [Google Scholar]
Chonchol 2009 {published data only}
- Chonchol M, Kopyt N, Herman J, Charytan C, Pischette V, Olson KA, et al. Cinacalcet HCI reduces parathyroid hormone (PTH) in patients with stage 3 and 4 chronic kidney disease (CKD) [abstract no: 18]. American Journal of Kidney Diseases 2004;43(4):A19. [CENTRAL: CN‐00602122] [Google Scholar]
- Chonchol M, Locatelli F, Abboud H, Charytan C, Francisco A, Jolly S, et al. A randomized, double‐blind, placebo‐controlled study of cinacalcet in CKD not on dialysis [abstract no: SA‐PO867]. Journal of the American Society of Nephrology 2007;18(Abstracts):533A. [CENTRAL: CN‐00583853] [Google Scholar]
- Chonchol M, Locatelli F, Abboud HE, Charytan C, Francisco AL, Jolly S, et al. A randomized, double‐blind, placebo‐controlled study to assess the efficacy and safety of cinacalcet HCl in participants with CKD not receiving dialysis. American Journal of Kidney Diseases 2009;53(2):197‐207. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
El Shafey 2011 {published data only}
- El‐Shafey EM, Alsahow AE, Alsaran K, Sabry AA, Atia M. Cinacalcet hydrochloride therapy for secondary hyperparathyroidism in hemodialysis patients. Therapeutic Apheresis & Dialysis 2011;15(6):547‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
EVOLVE study 2007 {published data only}
- Chertow G, Block G, Correa‐Rotter R, Drueke T, Floege J, Goodman W, et al. Evaluation of cinacalcet HCI therapy to lower cardiovascular events (EVOLVE) trial [abstract no: PUB359]. Journal of the American Society of Nephrology 2006;17(Abstracts):893A. [CENTRAL: CN‐00601998] [DOI] [PubMed] [Google Scholar]
- Chertow GM, Correa‐Rotter R, Block GA, Drueke TB, Floege J, Goodman WG, et al. Baseline characteristics of subjects enrolled in the Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) trial. Nephrology Dialysis Transplantation 2012;27(7):2872‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chertow GM, Pupim LB, Block GA, Correa‐Rotter R, Drueke TB, Floege J, et al. Evaluation of Cinacalcet Therapy to Lower Cardiovascular Events (EVOLVE): rationale and design overview. Clinical Journal of The American Society of Nephrology: CJASN 2007;2(5):898‐905. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Evolve Trial Investigators, Chertow GM, Block GA, Correa‐Rotter R, Drueke TB, Floege J, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. New England Journal of Medicine 2012;367(26):2482‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fukagawa 2008 {published data only}
- Akizawa T, Koshikawa S. Clinical study of cinacalcet in Japan. Therapeutic Apheresis & Dialysis 2008;12 Suppl 1:S13‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fukagawa M, Yumita S, Akizawa T, Tsukamoto Y, Uchida E, Iwasaki M, et al. Randomized, placebo‐controlled trial of cinacalcet (KRN1493) in Japanese dialysis patients with more severe hyperparathyroidism and longer dialysis vintage [abstract no: FP453]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi171. [Google Scholar]
- Fukagawa M, Yumita S, Akizawa T, Uchida E, Tsukamoto Y, Iwasaki M, et al. Cinacalcet (KRN1493) effectively decreases the serum intact PTH level with favorable control of the serum phosphorus and calcium levels in Japanese dialysis patients. Nephrology Dialysis Transplantation 2008;23(1):328‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Yumita S, Akizawa T, Uchida E, Tsukamoto Y, Koshikawa S, KRN1493 Study Group. A randomized, double blind, placebo‐controlled, 14 week study to assess the efficacy and safety of cinacalcet HCI in Japanese hemodialysis patients [abstract no: PUB348]. Journal of the American Society of Nephrology 2006;17(Abstracts):889A. [Google Scholar]
Goodman 2000 {published data only}
- Goodman WG, Frazao JM, Goodkin DA, Turner S, Liu W, Coburn JW. The calcimimetic, R‐568, lowers plasma parathyroid hormone levels in hemodialysis patients with secondary hyperparathyroidism [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):619A‐20A. [CENTRAL: CN‐00626084] [Google Scholar]
- Goodman WG, Frazao JM, Goodkin DA, Turner SA, Liu W, Coburn JW. A calcimimetic agent lowers plasma parathyroid hormone levels in patients with secondary hyperparathyroidism. Kidney International 2000;58(1):436‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Goodman 2002 {published data only}
- Goodman WG, Hladik GA, Turner SA, Blaisdell PW, Goodkin DA, Liu W, et al. Multiple doses of the calcimimetic AMG 073 reduce plasma parathyroid hormone in hemodialysis patients with secondary hyperparathyroidism (SHPT) [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):576A. [DOI] [PubMed] [Google Scholar]
- Goodman WG, Hladik GA, Turner SA, Blaisdell PW, Goodkin DA, Liu W, et al. The calcimimetic agent AMG 073 lowers plasma parathyroid hormone levels in hemodialysis patients with secondary hyperparathyroidism. Journal of the American Society of Nephrology 2002;13(4):1017‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Harris 2004 {published data only}
- Harris RZ, Padhi D, Marbury TC, Novelck RJ, Salfi M, Sullivan JT. Pharmacokinetics, pharmacodynamics and safety of cinacalcet hydrochloride in hemodialysis patients at doses up to 200 mg once daily. American Journal of Kidney Diseases 2004;44(6):1070‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
IMPACT SHPT Study 2012 {published data only}
- Ketteler M, Martin KJ, Cozzolino M, Goldsmith D, Sharma A, Khan S, et al. Paricalcitol versus cinacalcet plus low‐dose vitamin D for the treatment of secondary hyperparathyroidism in patients receiving haemodialysis: study design and baseline characteristics of the IMPACT SHPT study. Nephrology Dialysis Transplantation 2012;27(5):1942‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketteler M, Martin KJ, Wolf M, Amdahl M, Cozzolino M, Goldsmith D, et al. Paricalcitol versus cinacalcet plus low‐dose vitamin D therapy for the treatment of secondary hyperparathyroidism in patients receiving haemodialysis: results of the IMPACT SHPT study. Nephrology Dialysis Transplantation 2012;27(8):3270‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindberg 2003 {published data only}
- Lindberg JS, Moe SM, Goodman WG, Coburn JW, Sprague SM, Liu W, et al. The calcimimetic AMG 073 reduces parathyroid hormone and calcium x phosphorus in secondary hyperparathyroidism. Kidney International 2003;63(1):248‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lindberg JS, Moe SM, Goodman WG, Coburn JW, Turner SA, Blaisdell PW, et al. The calcimimetic AMG073 reduces parathyroid hormone (PTH), phosphorus (P), and calcium x phosphorus product (Ca x P) in patients with ESRD and secondary hyperparathyroidism (SHPT) [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):578A. [CENTRAL: CN‐00626121] [Google Scholar]
Lindberg 2005 {published data only}
- Coyne DW, Stegman MH, Azad H, Joy MS, Mischel SF, Pokroy N, et al. Cinacalcet HCI controls secondary hyperparathyroidism (HPT) regardless of gender, race, age, and geography in patients with chronic kidney disease (CKD) receiving dialysis. [abstract no: SA‐PO754]. Journal of the American Society of Nephrology 2003;14(Nov):464A. [Google Scholar]
- Cunningham J, Chertow G, Goodman W, Danese M, Olson K, Klassen P, et al. The effect of cinacalcet HCl on parathyroidectomy, fracture, hospitalisation, and mortality in dialysis subjects with secondary hyperparathyroidism (HPT) [abstract]. 41st ERA‐EDTA Congress; 2004; May 15‐18; Lisbon (Portugal). 2004:219. [CENTRAL: CN‐00509144]
- Cunningham J, Danese M, Olson K, Klassen P, Chertow GM. Effects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture, and health‐related quality of life in secondary hyperparathyroidism. Kidney international 2005;68(4):1793‐800. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Frazao JM, Holzer H, Stummvoll HK, Bahner U, Wilkie M, Zani V, et al. Cinacalcet (Mimpara©/Sensipar©) maintains achievement of NKF‐K/DOQI treatment targets for secondary hyperparathyroidism (HPT) in patients on dialysis [abstract no: SP209]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v89. [Google Scholar]
- Frazao JM, Messa P, Cunningham J, Evenepoel P, Shahapuni I, Urena P, et al. Early use of cinacalcet (Mimpara©/Sensipar©) in dialysis patients enables greatest achievement of NKF‐KDOQITM treatment targets for bone metabolism [abstract no: SO016]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv8. [Google Scholar]
- Frazao JM, Nicolini M, Torresgrossa JV, Kerr P, Jaeger PH, Sprague SM, et al. Cinacalcet HCl effectively reduces intact parathyroid hormone (iPTH) and Ca x P irrespective of the severity of secondary hyperparathyroidism (HPT). 41st ERA‐EDTA Congress; 2004; May 15‐18; Lisbon (Portugal). 2004:219.
- Goodman WG, Fadda GZ, Finkelstein FO, Mittman N, Lien YH, LeBlanc M, et al. Cinacalcet HCl is an effective primary therapy for the management of secondary hyperparathyroidism [abstract]. Journal of the American Society of Nephrology 2003;14(Program & Abstracts):460A. [Google Scholar]
- Goodman WG, Francisco AL, Mittman N, Messa P, Lien YH, Leblanc M, et al. Cinacalcet HCI (SensiparTM) effectively manages secondary hyperparathyroidism (HPT) in new to dialysis patients [abstract no: PUB477]. Journal of the American Society of Nephrology 2004;15(Oct):863A. [CENTRAL: CN‐00644149] [Google Scholar]
- Goodman WG, Francisco AL, Moe SM, Cunningham J, Martin KJ, McCary LC, et al. Cinacalcet HCI (SensiparTM) is an effective treatment for secondary hyperparathyroidism (HPT) in CKD patients on dialysis [abstract no: F‐PO984]. Journal of the American Society of Nephrology 2004;15(Oct):280A. [CENTRAL: CN‐00644151] [Google Scholar]
- Lindberg JS, Culleton B, Wong G, Borah MF, Clark RV, Shapiro WB, et al. Cinacalcet HCl, an oral calcimimetic agent for the treatment of secondary hyperparathyroidism in hemodialysis and peritoneal dialysis: a randomized, double‐blind, multicenter study. Journal of the American Society of Nephrology 2005;16(3):800‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lindberg JS, Culleton B, Wong G, Borah MF, Clark RV, Shapiro WB, et al. Phase 3 experience with cinacalcet HCI in hemodialysis (HD) and peritoneal dialysis (PD) patients with secondary HPT [abstract no: SA‐PO752]. Journal of the American Society of Nephrology 2003;14(Nov):463A. [CENTRAL: CN‐00626029] [Google Scholar]
- Mittman N, Finkelstein F, Culleton B, Charytan C, Agarwal A, Albizem MA, et al. Cinacalcet HCI (SensiparTM) for the management of secondary hyperparathyroidism (HPT) in patients receiving peritoneal dialysis (PD) [abstract no: F‐PO985]. Journal of the American Society of Nephrology 2004;15(Oct):280A. [CENTRAL: CN‐00626108] [Google Scholar]
- Mittman N, Francisco AL, Drueke T, Fadda GZ, Arruda JA, Abu‐Alfa AK, et al. Phase 3 studies demonstrate effective control of PTH and Ca x P in patients treated with cinacalcet HCI [abstract no: 73]. American Journal of Kidney Diseases 2004;43(4):A33. [Google Scholar]
- Moe SM, Chertow GM, Coburn JW, Quarles LD, Goodman WG, Block GA, et al. Achieving NKF‐K/DOQI bone metabolism and disease treatment goals with cinacalcet HCl. Kidney international 2005;67(2):760‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Malluche 2008 {published data only}
- Cunningham J, Chertow G, Goodman W, Danese M, Olson K, Klassen P, et al. The effect of cinacalcet HCl on parathyroidectomy, fracture, hospitalisation, and mortality in dialysis subjects with secondary hyperparathyroidism (HPT) [abstract]. 41st ERA‐EDTA Congress; 2004; May 15‐18; Lisbon (Portugal). 2004:219. [CENTRAL: CN‐00509144]
- Cunningham J, Danese M, Olson K, Klassen P, Chertow GM. Effects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture, and health‐related quality of life in secondary hyperparathyroidism. Kidney international 2005;68(4):1793‐800. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Goodman WG, Francisco AL, Mittman N, Messa P, Lien YH, Leblanc M, et al. Cinacalcet HCI (SensiparTM) effectively manages secondary hyperparathyroidism (HPT) in new to dialysis patients [abstract no: PUB477]. Journal of the American Society of Nephrology 2004;15(Oct):863A. [CENTRAL: CN‐00644149] [Google Scholar]
- Goodman WG, Francisco AL, Moe SM, Cunningham J, Martin KJ, McCary LC, et al. Cinacalcet HCI (SensiparTM) is an effective treatment for secondary hyperparathyroidism (HPT) in CKD patients on dialysis [abstract no: F‐PO984]. Journal of the American Society of Nephrology 2004;15(Oct):280A. [CENTRAL: CN‐00644151] [Google Scholar]
- Malluche HH, Monier‐Faugere MC, Wang G, Fraza OJ, Charytan C, Coburn JW, et al. An assessment of cinacalcet HCl effects on bone histology in dialysis patients with secondary hyperparathyroidism. Clinical Nephrology 2008;69(4):269‐78. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Malluche HH, Monier‐Faugere MC, Wang G, Frazao JM, Charytan C, Coburn JW, et al. Cinacalcet HCl reduces bone turnover and bone marrow fibrosis in hemodialysis patients with secondary hyperparathyroidism (HPT) [abstract]. 41st ERA‐EDTA Congress; 2004; May 15‐18; Lisbon (Portugal). 2004:218‐9. [CENTRAL: CN‐00509338]
OPTIMA Study 2008 {published data only}
- Frazao JM, Braun J, Messa P, Dehmel B, Mattin C, Wilkie M. Is serum phosphorus control related to parathyroid hormone control in dialysis patients with secondary hyperparathyroidism?. BMC Nephrology 2012;13:76. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazao JM, Macario F, Yaqoob M, Bouman K, Braun J, Albertini B, et al. The Optima Study: earlier intervention of cinacalcet HCL (Sensipar©/Mimpara©) enables greater achievement of KDOQIT secondary hyperparathyroidism (SHPT) targets in dialysis patients [abstract no: 45]. American Journal of Kidney Diseases 2006;47(4):A30. [CENTRAL: CN‐00644220] [Google Scholar]
- Gonzalez MT, Hutchison AJ, Girndt M, Stahl‐Nilsson A, Zani V, Carter D, et al. Secondary hyperparathyroidism (HPT) in patients receiving peritoneal dialysis (PD) can be effectively managed with cinacalcet (Mimpara©/Sensipar©) [abstract no: SP359]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv134. [Google Scholar]
- Locatelli F, Macario F, Brink HS, Dhaene M, Pai P, Holzer H, et al. The OPTIMA study: efficacy of a cinacalcet (Mimpara©/Sensipar©) treatment algorithm to treat dialysis patients with elevated PTH and calcium‐phosphorus product (Ca x P) [abstract no: SP357]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv133. [CENTRAL: CN‐00644212] [Google Scholar]
- Messa P, Macario F, Yaqoob M, Bouman K, Braun J, Albertini B, et al. The OPTIMA study: assessing a new cinacalcet (Sensipar/Mimpara) treatment algorithm for secondary hyperparathyroidism. Clinical Journal of The American Society of Nephrology: CJASN 2008;3(1):36‐45. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messa P, Motellon JL, OPTIMA Study Group. Optimizing the use of cinacalcet (Mimpara©/Sensipar©) and vitamin D: interim results from the OPTIMA study [abstract no: F‐PO758]. Journal of the American Society of Nephrology 2005;16:501A‐2A. [CENTRAL: CN‐00644221] [Google Scholar]
- Messa P, Villa G, Braun J, Maduell F, Cruz J, Martin PY, et al. The OPTIMA study: lower doses of cinacalcet (Mimpara©/Sensipar©) are required to achieve KDOQITM secondary hyperparathyroidism (HPT) targets in dialysis patients with less severe disease [abstract no: MP324]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv407. [CENTRAL: CN‐00583842] [Google Scholar]
- Perrault L, Carter D, Molemans B, Maetzel A. Use of cinacalcet HCL (Mimpara©/Sensipar©) to control mineral metabolism in patients with end‐stage renal disease (ESRD) on dialysis complicated by secondary hyperparathyroidism (SHPT): a cost‐consequence analysis of cinacalcet HCL versus standard of care (SC) [abstract no: SP363]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv136. [Google Scholar]
- Wilkie M. A multi‐centre, randomised, open‐label study to compare the efficacy and safety of an oral calcimimetic agent (AMG 073) when two different vitamin D regimens are used in subjects with secondary hyperparathyroidism of end‐stage renal disease (ESRD). http://www.nihr.ac.uk/Profile/Pages/NRRResults.aspx?publication_id=N0059124801 (accessed 1 June 2014).
- Wilkie M, Pontoriero G, Macario F, Yaqoob M, Bouman K, Braun J, et al. Impact of vitamin D dose on biochemical parameters in patients with secondary hyperparathyroidism receiving cinacalcet. Nephron 2009;112(1):c41‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wilkie M, Salvadori M, Meester J, Jofre R, Koopman MG, Leidig FM, et al. The OPTIMA study: optimising the dose of vitamin D (vit D) in the presence of cinacalcet (Mimpara©/Sensipar©) to obtain maximum clinical benefit [abstract no: SP358]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv134. [CENTRAL: CN‐00583844] [Google Scholar]
Quarles 2003a {published data only}
- Quarles LD, Sherrard DJ, Adler S, Rosansky SJ, McCary LC, Liu W, et al. The calcimimetic AMG 073 as a potential treatment for secondary hyperparathyroidism of end‐stage renal disease. Journal of the American Society of Nephrology 2003;14(3):575‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Al‐Hilali 2011 {published data only}
- Al‐Hilali N, Hussain N, Kawy YA, Al‐Azmi M. A novel dose regimen of cinacalcet in the treatment of severe hyperparathyroidism in hemodialysis patients. Saudi Journal of Kidney Diseases & Transplantation 2011;22(3):448‐55. [MEDLINE: ] [PubMed] [Google Scholar]
Coburn 2000a {published data only}
- Coburn JW, Barri YM, Turner SA, Blaisdell PW, Goodkin DA, Liu W, et al. Single doses of the calcimimetic AMG 073 reduce parathyroid hormone levels in a dose dependent manner in hemodialysis patients with secondary hyperparathyroidism [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):573A‐4A. [CENTRAL: CN‐00550686] [DOI] [PubMed] [Google Scholar]
CONTROL Study 2006 {published data only}
- Chertow GM, Blumenthal S, Turner S, Roppolo M, Stern L, Chi EM, et al. Cinacalcet hydrochloride (Sensipar) in hemodialysis patients on active vitamin D derivatives with controlled PTH and elevated calcium x phosphate. Clinical Journal of The American Society of Nephrology: CJASN 2006;1(2):305‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Reed J, Keightley G, Blumenthal S, Bradley C, LaBrecque J, Turner S, et al. The CONTROL STUDY: enhanced achievement of NKF‐K/DOQI bone metabolism and disease targets using cinacalcet HCL (Sensipar) [abstract]. Journal of the American Society of Nephrology 2004;15(Abstracts):280A. [Google Scholar]
Cunningham 2003 {published data only}
- Cunningham J, Holzer H, Reichel H, Braun J, Hawley C, Urena P, et al. Sustained, long‐term reductions in parathyroid hormone (iPTH) after 3 years of treatment with calcimimetic cinacalcet HCl in patients with secondary hyperparathyroidism (SPTH) [abstract no: M479]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):150‐1. [CENTRAL: CN‐00583594] [Google Scholar]
Cunningham 2005 {published data only}
- Cunningham J, Urena P, Reichel H, Holzer H, Drueke T, Zani V, et al. Long term efficacy of cinacalcet in secondary hyperparathyroidism (HPT) of end stage renal disease (ESRD) [abstract no: SP210]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v89. [Google Scholar]
de Francisco 2005 {published data only}
- Francisco A, Evenepoel P, Brink HS, Mellotte G, Barata JD, Zani V, et al. Cinacalcet (Mimpara©/Sensipar©) effectively reduces intact parathyroid hormone (iPTH) and serum calcium (CA) regardless of serum calcium level in patients with secondary hyperparathyroidism (HPT) [abstract no: MO30]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v197. [Google Scholar]
Harris 2003 {published data only}
- Harris R, Padhi D, Salfi M, Yates W, Simiens MA, Sulivan JT. Pharmacokinetics (PK), pharmacodynamics (PD), and safety of daily administration of cinacalcet HCI up to 300 mg in chronic kidney disease (CKD) patients on hemodialysis (HD) [abstract no: SA‐PO746]. Journal of the American Society of Nephrology 2003;14(Nov):462A. [CENTRAL: CN‐00583298] [Google Scholar]
Kaperonis 2012 {published data only}
- Kaperonis N, Kourvelou C, Sgantzos A, Nastou D, Ntatsis G, Ziakka S, et al. Cinacalcet vs. paricalcitol in hemodialysis patients [abstract]. Nephrology Dialysis Transplantation 2012;27(Suppl 2):ii497‐8. [Google Scholar]
Moe 2003a {published data only}
- Moe SM, Sprague SM, Cunningham J, Drueke T, Adler S, Rosansky SJ, et al. Long‐term treatment of secondary hyperparathyroidism (HPT) with the calcimimetic cinacalcet HCI [abstract no: SA‐PO753]. Journal of the American Society of Nephrology 2003;14:463A‐4A. [Google Scholar]
Moe 2005 {published data only}
- Belozeroff V, Goodman W, Ren L, Kalantar‐Zadeh K. Cinacalcet lowers serum alkaline phosphatase (alkPhos) in maintenance hemodialysis (MHD) patients [abstract no: SA‐PO698]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):495A‐6A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushinsky DA, Avram MM, Abu‐Alfa AK, Borah MF, Shapiro WB, Olson KA, et al. Characterization of the effects of cinacalcet HC1 (Sensipar©/Mimpara©) on serum calcium in dialysis patients with secondary hyperparathyroidism (HPT) [abstract no: F‐PO756]. Journal of the American Society of Nephrology 2005;16:501A. [CENTRAL: CN‐00583862] [Google Scholar]
- Moe SM, Coburn JW, Quarles LD, Goodman WG, Chertow GM, Block GA, et al. Achievement of proposed NKF‐K/DOQI bone metabolism and disease targets: treatment with cinacalcet HCl in dialysis patients with uncontrolled secondary hyperparathyroidism (HPT) [abstract no: SU‐FC217]. Journal of the American Society of Nephrology 2003;14(Nov):48A. [Google Scholar]
- Moe SM, Goodman WG, Cunningham J, Drueke T, Adler S, Rosansky SJ, et al. Cinacalcet HC1 sustains long‐term control of secondary hyperparathyroidism (HPT) [abstract no: F‐PO755]. Journal of the American Society of Nephrology 2005;16:501A. [Google Scholar]
Moe 2005b {published data only}
- Moe SM, Cunningham J, Bommer J, Adler S, Rosansky SJ, Urena‐Torres P, et al. Long‐term treatment of secondary hyperparathyroidism with the calcimimetic cinacalcet HCl. Nephrology Dialysis Transplantation 2005;20(10):2186‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Padhi 2003 {published data only}
- Padhi D, Harris R, Salfi M, Yates W, Hansen J, Flynn J, et al. Cinacalcet HCI absorption in study subjects is not affected by coadministration of medications commonly prescribed to chronic kidney disease (CKD) patients (pantoprazole, sevelamer HCI, and calcium carbonate). [abstract no: SA‐PO744]. Journal of the American Society of Nephrology 2003;14:461A. [Google Scholar]
Pahl 1996 {published data only}
- Pahl M, Jara A, Bover J, Rodriguez M, Felsenfeld AJ. The set point of calcium and the reduction of parathyroid hormone in hemodialysis patients. Kidney international 1996;49(1):226‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Quarles 2003 {published data only}
- Quarles LD, Zeig S, Spiegel DM, Silver MR, Vokes TJ, Torregrosa JV, et al. Cinacalcet HCl controls secondary hyperparathyroidism (HPT) in dialysis patients regardless of disease severity [abstract]. Journal of the American Society of Nephrology 2003;14(Program & Abstracts):463A. [Google Scholar]
Schaefer 2008 {published data only}
- Schaefer RM, Bover J, Dellanna F, Sanz D, Asensio C, Sanchez GM, et al. Efficacy of cinacalcet administered with the first meal after dialysis: the SENSOR Study. Clinical Nephrology 2008;70(2):126‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schaefer RM, Bover J, Kleophas W, Sanz D, Asensio C, Alonso JL, et al. The SENSOR study: a study to evaluate the efficacy of administering cinacalcet (Mimpara©/Sensipar©) with the first meal after dialysis [abstract no: MO003]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv288. [CENTRAL: CN‐00671819] [Google Scholar]
Sezer 2012 {published data only}
- Sezer S, Tutal E, Bal Z, Ozelsancak R, Demirci BG, Torun D, et al. Treatment of secondary hyperparathyroidism in maintenance hemodialysis patients: a randomised clinical trial comparing paricalcitol, calcitriol and cinacalcet [abstract]. Nephrology Dialysis Transplantation 2012;27(Suppl 2):ii509‐10. [Google Scholar]
TARGET Study 2008 {published data only}
- Block GA, Zeig S, Sugihara J, Chertow GM, Chi EM, Turner SA, et al. Combined therapy with cinacalcet and low doses of vitamin D sterols in patients with moderate to severe secondary hyperparathyroidism. Nephrology Dialysis Transplantation 2008;23(7):2311‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chertow GM, Corpier C, Zieg S, Stegman M, Lynn R, Abu‐Alfa AK, et al. Preliminary results from TARGET: treatment strategies to achieve recommended K/DOQI goals in ESRD patients on Cinacalcet [abstract]. Journal of the American Society of Nephrology 2004;15(Abstracts):863A. [Google Scholar]
- Chertow GM, Lu ZJ, Xu X, Knight TG, Goodman WG, Bushinsky DA, et al. Self‐reported symptoms in patients on hemodialysis with moderate to severe secondary hyperparathyroidism receiving combined therapy with cinacalcet and low‐dose vitamin D sterols. Hemodialysis International 2012;16(2):188‐97. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Coburn 2003 {published data only}
- Coburn JW, Charytan C, Chonchol M, Herman J, Lien YH, Liu W, et al. Cinacalcet HCl is an effective treatment for secondary hyperparathyroidism (HPT) in patients with chronic kidney diseases (CKD) not receiving dialysis [abstract]. Journal of the American Society of Nephrology 2003;14(Program & Abstracts):460A. [DOI] [PubMed] [Google Scholar]
Drueke 2001a {published data only}
- Drueke T, Cunningham J, Goodman WG, Horl WH, Braun J, Chen MG, et al. Short‐term treatment of secondary hyperparathyroidism (SHPT) with the calcimimetic agent AMG 073 [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):764A. [CENTRAL: CN‐00550690] [Google Scholar]
Fournier 2004a {published data only}
- Fournier A, Pontoiero G, Walker R, Martin‐Malo A, Wilkie M, Coyne DW, et al. Cinacalcet HCL reduces parathyroid hormone (PTH) and calcium‐phosphate product (Ca x P) regardless of concurrent changes in vitamin D administration [abstract]. 41st ERA‐EDTA Congress; 2004; May 15‐18; Lisbon (Portugal). 2004:319. [CENTRAL: CN‐00509198]
UPen 2004a {published data only}
- University of Pennsylvania, Renal Electrolyte, Hypertension Division. 2004. A randomized, double‐blind, placebo‐controlled study to assess the safety and efficacy of a calcimimetic agent (AMG 073) in subjects with secondary hyperparathyroidism of chronic renal insufficiency. http://www.uphs.upenn.edu/renal/research/clinical_research_chronic_kidney.html (accessed 2 June 2014).
Additional references
Albaaj 2003
- Albaaj F, Hutchison A. Hyperphosphataemia in renal failure: causes, consequences and current management. Drugs 2003;63(6):577‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Avram 1996
- Avram MM, Sreedhara R, Avram DK, Muchnick RA, Fein P. Enrollment parathyroid hormone level is a new marker of survival in hemodialysis and peritoneal dialysis therapy for uremia. American Journal of Kidney Diseases 1996;28(6):924‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Besarab 1998
- Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, et al. The effects of normal, as compared with low, hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. New England Journal of Medicine 1998;339(9):584‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Block 2004b
- Block GA, Klassen PS, Lazarus LM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality and morbidity in maintenance hemodialysis. Journal of the American Society of Nephrology 2004;15(8):2208‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Borrows 2004
- Borrows R, Loucaidou M, Tromp J, Cairns T, Griffith M, Hakim N, et al. Steroid sparing with tacrolimus and mycophenolate mofetil in renal transplantation. American Journal of Transplantation 2004;4(11):1845‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bucher 1999
- Bucher HC, Guyatt GH, Cook DJ, Holbrook A, McAlister FA. Users' guides to the medical literature: XIX. Applying clinical trial results. A. How to use an article measuring the effect of an intervention on surrogate end points. Evidence‐Based Medicine Working Group. JAMA 1999;282(8):771‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Churchill 1997
- Churchill DN, Thorpe KE, Vonesh EF, Keshaviah PR. Lower probability of patient survival with continuous peritoneal dialysis in the united states compared with Canada. Canada‐USA (CANUSA) Peritoneal Dialysis Study Group. Journal of the American Society of Nephrology 1997;8(6):965‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Courant 1993
- Courant O, Letessier E, Moutel MG, Hamy A, Paineau J, Visset J. Surgical treatment of secondary hyperparathyroidism in chronic kidney failure. Results of total parathyroidectomy with parathyroid autotransplantation. Journal de Chirurgie 1993;130(8‐9):327‐34. [MEDLINE: ] [PubMed] [Google Scholar]
Cunningham 2005a
- Cunningham J, Danese M, Olson K, Klassen P, Chertow GM. Effects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture, and health‐related quality of life in secondary hyperparathyroidism. Kidney international 2005;68(4):1793‐800. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Egger 1998
- Egger M, Smith GD. Bias in location and selection of studies. BMJ 1998;316(7124):61‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
FDA 2004
- US Food, Drug Administration. Sensipar (cinacalcet HCl) tablets. http://www.fda.gov/safety/medwatch/safetyinformation/ucm194427.htm (accessed 2 June 2014).
Ganesh 2001
- Ganesh SK, Stack AG, Levin NW, Hulbert‐Shearon T, Port FK. Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patient. Journal of the American Society of Nephrology 2001;12(10):2131‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman A D, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hutchison 1993
- Hutchison AJ, Whitehouse RW, Boulton HF, Adams JE, Mawer EB, Freemont TJ, et al. Correlation of bone histology with parathyroid hormone, vitamin D3, and radiology in end‐stage renal disease. Kidney international 1995;26(5):622‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Juni 2003
- Juni P, Sterchi R, Dieppe P. Systematic review of celecoxib for osteoarthritis and rheumatoid arthritis. Problems compromise review's validity. BMJ 2003;326(7384):334. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
KDIGO CKD 2013
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney International ‐ Supplement 2013;3:1‐150. [Google Scholar]
Kestenbaum 2004
- Kestenbaum B, Seliger SL, Gillen DL, Wasse H, Young B, Sherrard DJ, et al. Parathyroidectomy rates among United States dialysis patients: 1990‐1999. Kidney International 2004;65(1):282‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kestenbaum 2005
- Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. Journal of the American Society of Nephrology 2005;16(2):520‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
KHA‐CARI 2014
- KHA‐CARI guidelines. http://www.cari.org.au/ (accessed 2 June 2014).
Lexchin 2003
- Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ 2003;326(7400):1167‐70. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Malluche 2004b
- Malluche HH, Mawad H, Monier‐Faugere MC. The importance of bone health in end‐stage renal disease: out of the frying pan, into the fire?. Nephrology Dialysis Transplantation 2004;19 Suppl 1:i9‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marco 2003
- Marco MP, Craver L, Betriu A, Belart M, Fibla J, Fernandez E. Higher impact of mineral metabolism on cardiovascular mortality in a European hemodialysis population. Kidney International ‐ Supplement 2003;85:S111‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Martin 2004
- Martin KJ, Olgaard K, Coburn JW, Coen GM, Fukagawa M, Langman C, et al. Diagnosis, assessment and treatment of bone turnover abnormalities in renal osteodystrophy. American Journal of Kidney Diseases 2004;43(3):558‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McMahon 2004
- McMahon LP, Roger SD, Levin A, SLIMHEART Investigators Group. Development, prevention and potential reversal of left ventricular hypertrophy in chronic kidney disease. Journal of the American Society of Nephrology 2004;15(6):1640‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mentaverri 2006
- Mentaverri R, Yano S, Chattopadhyay N, Petit L, Kifor O, Kamel S, et al. The calcium sensing receptor is directly involved in both osteoclast differentiation and apoptosis. FASEB Journal 2006;20(14):2562‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mizobuchi 2007
- Mizobuchi M, Ogata H, Hatamura I, Saji F, Koiwa F, Kinugasa E, et al. Activation of calcium‐sensing receptor accelerates apoptosis in hyperplastic parathyroid cells. Biochemical & Biophysical Research Communications 2007;362(1):11‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NICE 2007
- NICE: National Institute for Health and Care Excellence. TA117 Hyperparathyroidism ‐ cinacalcet guidance (24 January 2007). http://guidance.nice.org.uk/TA117/Guidance/ (accessed 2 June 2014).
NKF 2003
- National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. American Journal of Kidney Diseases 2003;42(4 Suppl 3):S1‐210. [MEDLINE: ] [PubMed] [Google Scholar]
Psaty 1999
- Psaty BM, Weiss NS, Furberg CD, Koepsell TD, Siscovick DS, Rosendaal FR, et al. Surrogate end points, health outcomes, and the drug‐approval process for the treatment of risk factors for cardiovascular disease. JAMA 1999;282(8):786‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Qi 1995
- Qi Q, Monier‐Faugere MC, Geng Z, Malluche HH. Predictive value of serum parathyroid hormone levels for bone turnover in patients on chronic maintenance dialysis. American Journal of Kidney Diseases 1995;26(4):622‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Simes 1986
- Simes RJ. Publication bias: the case for an international registry of clinical trials. Journal of Clinical Oncology 1986;4(10):1529‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stehman‐Breen 2004
- Stehman‐Breen C. Osteoporosis and chronic kidney disease. Seminars in Nephrology 2004;24(1):78‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stevens 2004
- Stevens LA, Djurdjev O, Cardew S, Cameron EC, Levin A. Calcium, phosphate, and parathyroid hormone levels in combination and as a function of dialysis duration predict mortality: evidence for the complexity of the association between mineral metabolism and outcomes. Journal of the American Society of Nephrology 2004;15(3):770‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Temple 1999
- Temple R. Are surrogate markers adequate to assess cardiovascular disease drugs?. JAMA 1999;282(8):790‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Urena 2003
- Urena P, Frazao JM. Calcimimetic agents: review and perspectives. Kidney International ‐ Supplement 2003;85:S91‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
USRDS 2012
- Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Herzog C, et al. United States Renal Data System 2012 annual data report: atlas of chronic kidney disease and end‐stage renal disease in the United States. American Journal of Kidney Diseases 2013;61(1 Suppl 1):e1‐e480. [DOI] [PubMed] [Google Scholar]
Wang 1995
- Wang M, Hercz G, Sherrard DJ, Maloney NA, Segre GV, Pei Y. Relationship between intact 1‐84 parathyroid hormone and bone histomorphometric parameters in dialysis patients without aluminum toxicity. American Journal of Kidney Diseases 1995;26(5):836‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Weiner 2006
- Weiner DE, Tabatabai S, Tighiouart H, Elsayed E, Bansal N, Griffith J, et al. Cardiovascular outcomes and all‐cause mortality: exploring the interaction between CKD and cardiovascular disease. American Journal of Kidney Diseases 2006;48(3):392‐401. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ziolkowska 2000
- Ziolkowska H, Paniczyk‐Tomaszewska M, Debinski A, Polowiec Z, Sawicki A, Sieniawska M. Bone biopsy results and serum bone turnover parameters in uremic children. Acta Paediatrica 2000;89(6):666‐71. [MEDLINE: ] [PubMed] [Google Scholar]
References to other published versions of this review
Palmer 2013
- Palmer SC, Nistor I, Craig JC, Pellegrini F, Messa P, Tonelli M, et al. Cinacalcet in patients with chronic kidney disease: a cumulative meta‐analysis of randomized controlled trials. PLoS Medicine 2013;10(4):e1001436. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Strippoli 2006a
- Strippoli GF, Tong A, Palmer SC, Elder G, Craig JC. Calcimimetics for secondary hyperparathyroidism in chronic kidney disease patients. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD006254] [DOI] [PubMed] [Google Scholar]
Strippoli 2006b
- Strippoli GF, Palmer S, Tong A, Elder G, Messa P, Craig JC. Meta‐analysis of biochemical and patient‐level effects of calcimimetic therapy. American Journal of Kidney Diseases 2006;47(5):715‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]


