Abstract
Purpose:
To determine differences in eye care utilization by frailty levels among Medicare beneficiaries with glaucoma
Design:
Retrospective cohort study
Subjects:
Medicare fee-for-service beneficiaries over 65 years with glaucoma, identified using International Classification of Diseases codes before 7/1/2014
Methods:
Using a validated claims-based frailty index (range: 0–1), beneficiaries were classified as non-/prefrail (0–0.19), mildly frail (0.20–0.29), and moderate-to-severely frail (≥0.30). Negative binomial regression analyses were used to estimate incident rate ratios (IRR) of eye care utilization by frailty levels between 7/1/2014 and 12/31/2016
Main outcome measures:
Current Procedural Terminology codes for eye examinations and eye care-related office visits; eye care-related inpatient and emergency department (ED) encounters; eye care-related nursing facility and home-visit encounters; visual field (VF) and retinal nerve fiber layer optical coherence tomography (RNFL OCT) tests; selective laser trabeculoplasties (SLT) and glaucoma surgeries
Results:
Among 76,260 Medicare beneficiaries with glaucoma, mean age was 78.9 (standard deviation 7.8), female beneficiaries constituted 60.5%, and 78.7% of beneficiaries self-identified as non-Hispanic White. Based on a claims-based frailty index, 79.5% of beneficiaries were non-/prefrail, 17.1% were mildly frail, and 3.4% were moderate-to-severely frail. Moderate-to-severely frail beneficiaries were less likely than non-/prefrail beneficiaries to have outpatient encounters (IRR 0.85, 95% Confidence Interval [CI] 0.83–0.88); VF tests (IRR 0.64, 95% CI 0.60–0.67); RNFL OCT tests (IRR 0.77, 95% CI 0.73–0.81); SLT (IRR 0.74, 95% CI 0.60–0.92); and glaucoma surgery (IRR 0.74, 95% CI 0.55–0.99), after adjusting for age, gender, glaucoma severity, race, and socioeconomic status. Compared to non-/prefrail beneficiaries, moderate-severely frail beneficiaries had higher rates of inpatient/ED encounters (IRR 5.03, 95% CI 2.36–10.71) and nursing facility/home-visit encounters (IRR 34.89, 95% CI 14.82–82.13).
Conclusions:
Compared to non-/prefrail Medicare beneficiaries with glaucoma, beneficiaries with moderate-to-severe frailty had lower rates of eye care utilization in the outpatient setting and higher rates of utilization in acute care settings. This suggests that frail patients may receive less disease monitoring and fewer interventions for their glaucoma management.
Keywords: Frailty, glaucoma, utilization, Medicare
Précis
Medicare beneficiaries with glaucoma and a higher claims-based frailty score utilize eye care services at a lower rate in the outpatient setting and at a higher rate in inpatient and acute settings.
Frailty, defined as an age-related decline in physiological function and reserve,1 has important implications for healthcare expenditure and patient morbidity and mortality.2,3 Frail older adults are at a higher risk of inpatient hospitalization, nursing home admission, disability, and premature mortality.4,5 While the causes of frailty are likely multifactorial, visual impairment is known to be associated with an increased risk for frailty among older adults.6–9 Although the exact mechanisms underlying the relationship between visual impairment and frailty are unknown, individuals with visual impairment face increased barriers to mobility and social support, which contributes to the deterioration of functional status. Visual impairment has also been proposed to share underlying pathologic pathways such as inflammation with a range of comorbidities associated with frailty, including cardiovascular disease and diabetes mellitus.7,10,11 Additionally, visual impairment has been shown to independently increase risk of other known causes of frailty, including depression and dementia.12,13 Therefore, further research investigating the relationship between causes of visual impairment and frailty is warranted.
Glaucoma, a chronic eye disease causing progressive visual field loss, is associated with increased morbidity, a high risk for vision-related disability, and difficulty performing activities of daily living, especially among older adults.14–16 However, little is known about the prevalence of frailty among older adults with glaucoma. Frailty may be an important factor in this population with potential implications for eye care utilization, including rates of disease monitoring, follow-up with ophthalmic providers, and disease intervention and procedures.
The current study uses a representative national 5% Medicare sample to describe the prevalence of frailty in beneficiaries with glaucoma, and to assess differences in eye care utilization in patients with moderate-to-severe frailty compared to those who are non-/prefrail. Understanding potential differences in eye care utilization among frail patients may help providers identify barriers to care for patients that need more intensive glaucoma management to prevent disease progression and worsening of vision-related morbidity and disability.
Methods
This secondary analysis of Medicare claims data was approved by the Institutional Review Board/Ethics Committee of Hebrew SeniorLife Advarra and adhered to the tenets of the Declaration of Helsinki. A wavier of informed consent was obtained. A cohort of glaucoma patients was created using a 5% nationally representative random sample of Medicare beneficiaries from 01/01/2014 until 12/31/2016. Data used for this study consisted of institutional and non-institutional fee-for-service claims. Beneficiaries were included if they met the following criteria: at least one diagnosis code for glaucoma (International Classification of Diseases, Ninth Revision [ICD-9] codes 365.1–365.9 and Tenth Revision [ICD-10] codes H40.1-H40.9, H42) in the carrier file during the 6-month enrollment period between 01/01/2014 and 07/01/2014, and diagnosis of glaucoma in the Chronic Conditions Warehouse before 01/01/2014. Beneficiaries younger than 65 years of age, without continuous Part A and B enrollment, with hospice claims, and/or living in a nursing facility for more than 100 days during the 6-month enrollment period were excluded.
Frailty was assessed from Medicare claims data files using a claims-based frailty index (CFI) developed by Kim et al.17 In both a development and validation Medicare data set and in an independent cohort from the Health and Retirement Study, the CFI has been validated against reference standard clinical frailty assessments, physical performance, and health outcomes, and has outperformed the Charlson Comorbidity Index in predicting adverse health outcomes.17,18 The CFI estimates a deficit-accumulation frailty index using 52 ICD diagnosis variables, 25 Current Procedural Terminology (CPT) variables, and 16 Healthcare Common Procedure Coding System variables during the 6-month assessment period. The CFI scores range from 0 to 1 and classify individuals using the following cut-points: non-/prefrail 0–0.19, mildly frail 0.20–0.29, and moderate-severely frail 0.30–1.
Prior research has demonstrated that race and socioeconomic status (SES) are independent predictors of eye care utilization and thus were adjusted for in analyses.19,20 The following categories of race were defined in the cohort: Non-Hispanic White, Black/African American, Hispanic, Asian/Pacific Islander, and Other. SES was defined using Medicare enrollment-based low-income indicators, including dual eligibility for Medicare/Medicaid, eligibility for Part A/B state buy-in, and Part D limited income subsidies. To ensure adequate capture, only individuals meeting 2 or more of these indicators were classified as low SES. Glaucoma severity using ICD-9 and ICD-10 codes was also included to account for potential closer monitoring and intervention in patients with more severe disease. Additionally, medical comorbidities were adjusted for using a single numerical score defined by Gagne et al.21 This single numerical score combines measures of the Charlson Comorbidity Index and the Elixhauser Comorbidity Classification, and has performed better at predicting short- and long-term mortality than either individual index.
The following outcomes were derived from claims data using CPT codes: (1) outpatient encounters encompassing eye examinations and eye care-related office visits and consultations (CPT codes 92002, 92004, 92012, 92014, 92018, 92019 excluding post-operative code 99024 and evaluation and management codes 9201–5, 99211–5, 99241–5, 99354, 99355) (2) eye care-related inpatient and emergency department (ED) encounters (CPT codes 99221–3, 99231–3, 99251–5, 99281–5) (3) nursing facility and home-visit encounters (CPT codes 99301–9, 99310, 99315, 99231–3, 99251–5, 99281–5, 99301–9, 99310, 99315, 99318, 99325–7, 99334–7, 99342–5, 99347–50) (4) visual field (VF) (CPT codes 92081–3) and retinal nerve fiber layer optical coherence tomography (RNFL OCT) tests (CPT codes 92133, 92134) (5) duration, in days, in which any glaucoma medication was dispensed, and (6) selective laser trabeculoplasties (SLT) or glaucoma surgeries including trabeculectomy, aqueous shunt placement and minimally invasive glaucoma surgery with or without concurrent cataract extraction (CPT codes 66170, 66172. 66183, 0192T, 66180, 67255, 0191T, 0253T, 0191T, 0376T, 0474T, 0449T, 0450T, 66711, 65820, 66990, 66999, 66174, 66175, 66999, 66183, 66179, 66180). Utilization outcomes were assessed between July 1st, 2014 and December 31st, 2016.
Descriptive statistics were used to determine frequencies of eye care utilization in person-years by frailty group. Negative binomial regression was used to estimate the incidence rate ratio (IRR) and 95% confidence interval (CI) by frailty group, accounting for variable follow-up time. Models were adjusted for age, gender, race (for which Hispanic, Asian/Pacific Islander, and Other races were aggregated together as “Other” to ensure sufficient sample size), SES, and glaucoma severity. In our models, race appeared to be an independent predictor of underutilization of eye care, which has been demonstrated in prior studies.19 To further understand the association of race, frailty, and disparities in eye care utilization, we compared rates of utilization stratified by race and frailty group and adjusted for age, gender, glaucoma severity, and SES. In stratified analyses by race, individuals were classified as non-/prefrail (CFI 0–0.19) and frail (CFI ≥0.20) to ensure sufficient sample size. Given that frailty correlated positively with comorbidity burden, we further adjusted for the Gagne comorbidity score21 in a sensitivity analysis. Statistical analyses were performed in SAS version 9.4 and a 2-sided p-value <0.05 was considered statistically significant.
Results
There were 76,260 Medicare beneficiaries with glaucoma who met inclusion and exclusion criteria. The mean age was 78.9 (standard deviation [SD] 7.8), female beneficiaries constituted 60.5%, and 78.7% self-identified as non-Hispanic White (Table 1). Based on CFI scores, 79.5% of beneficiaries were non-/prefrail, 17.1% were mildly frail, and 3.4% were moderate-to-severely frail. Compared to non-/pre-frail beneficiaries, moderate-to-severely frail beneficiaries were more likely to be female (69.0% vs. 60.5%, p<0.0001), identify as Black/African American (16.7% vs. 12.9%, p<0.0001), meet two or more low-income indicators of low SES (26.9% vs. 11.9%, p<0.0001), and have greater comorbidity index scores (5.5 vs. 1.1, p<0.0001).
Table 1.
Cohort Characteristics. Sociodemographic and glaucoma disease characteristics of entire cohort and stratified by frailty group.
| Table 1: Cohort Characteristics | |||||
|---|---|---|---|---|---|
|
| |||||
| Baseline characteristics | Total | Non-frail or prefrail (CFIa 0–0.19) | Mildly frail (CFI 0.20–0.29) | Moderately and severely frail (CFI 0.30–1) | p-value |
|
| |||||
| Sample size, n (%) | 76,260 (100.0) | 60,639 (79.5) | 13,006 (17.1) | 2,615 (3.4) | |
| Age, mean (SD) | 78.9 (7.8) | 78.3 (7.6) | 81.0 (7.7) | 82.6 (8.0) | <.0001b |
| Female, n (%) | 46,141 (60.5) | 36,023 (59.4) | 8,313 (63.9) | 1,805 (69.0) | <.0001c |
| Race, n (%) | |||||
| Non-Hispanic White |
60,012 (78.7) | 48,270 (79.6) | 9,797 (75.3) | 1,945 (74.4) | <.0001c |
| Black/ African American |
9,838 (12.9) | 7,475 (12.3) | 1,925 (14.8) | 438 (16.7) | |
| Hispanic | 4,089 (5.4) | 2,954 (4.9) | 958 (7.4) | 177 (6.8) | |
| Asian/Pacific Islander |
2,321 (3.0) | 1,940 (3.2) | 326 (2.5) | 55 (2.1) | |
| Full/partial dual eligibility for Medicare & Medicaid, n (%) | 9,051 (11.9) | 5,824 (9.6) | 2,523 (19.4) | 704 (26.9) | <.0001c |
| Part A/B state buy-in, n (%) | 8,765 (11.5) | 5,705 (9.4) | 2,409 (18.5) | 651 (24.9) | <.0001c |
| Full/partial Part D limited income subsidies (LIS), n (%) | 10,552 (13.8) | 6,934 (11.4) | 2,860 (22.0) | 758 (29.0) | <.0001c |
| Two or more low-income indicator (Dual eligibility, Part A/B buy-in, Part D LIS, n (%) | 9,083 (11.9) | 5,850 (9.6) | 2,529 (19.4) | 704 (26.9) | <.0001c |
| Combined comorbidity score, mean (SD) | 1.1 (2.2) | 0.5 (1.5) | 2.8 (2.5) | 5.5 (3.0) | <.0001b |
| Glaucoma severity, n (%) | |||||
| Indeterminate | 631 (0.8) | 461 (0.8) | 137 (1.1) | 33 (1.3) | <.0001c |
| Mild | 8,116 (10.6) | 6,757 (11.1) | 1,168 (9.0) | 191 (7.3) | |
| Moderate | 9,106 (11.9) | 7,336 (12.1) | 1,533 (11.8) | 237 (9.1) | |
| Severe | 6,822 (8.9) | 5,360 (8.8) | 1,214 (9.3) | 248 (9.5) | |
| Unspecified | 3,641 (4.8) | 2,837 (4.7) | 641 (4.9) | 163 (6.2) | |
| No severityrelated diagnosis code | 47,944 (62.9) | 37,888 (62.5) | 8,313 (63.9) | 1,743 (66.7) | |
Claims-based frailty index
Analysis of variance (ANOVA)
Chi-squared
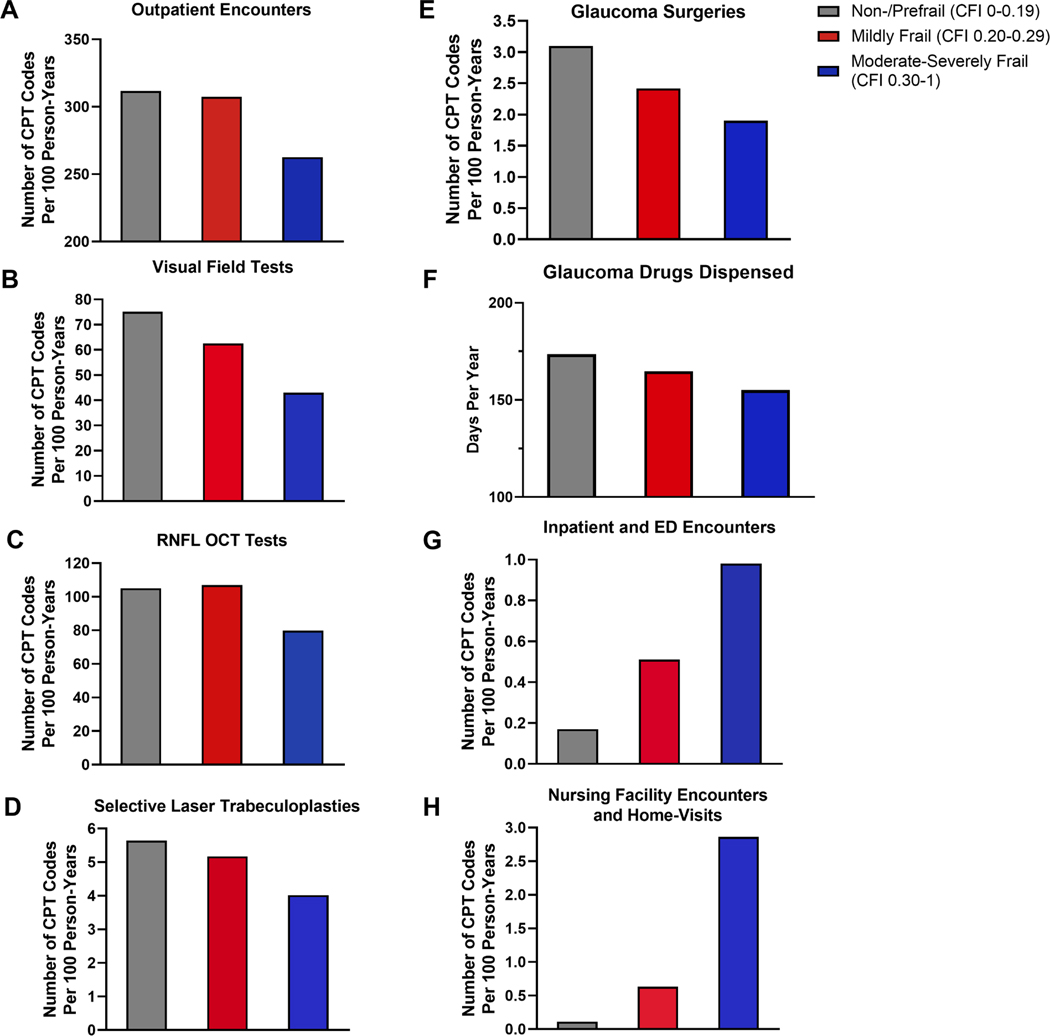
Figure 1 demonstrates the unadjusted rates per 100 person-years of utilization of eye care services. Beneficiaries with higher levels of frailty had lower utilization of outpatient encounters (non-/prefrail vs. moderate-to-severely frail: 311.7 vs. 262.6), VF tests (75.2 vs. 43.0), RNFL OCT tests (105.1 vs. 78.9), SLT (5.6 vs. 4.0), and glaucoma surgeries (3.1 vs. 1.9), and fewer days of glaucoma drugs dispensed (173.5 vs. 154.1). In contrast, moderate-to-severely frail beneficiaries had higher rates of inpatient and ED encounters (0.17 vs. 0.98) and nursing facility and home-visit encounters (0.11 vs. 2.86).
Figure 1.
Unadjusted rates of eye care utilization by frailty group. (A) Outpatient Encounters (B) Visual Field Tests (C) Retinal Nerve Fiber Layer Optical Coherence Tomography (RNFL OCT) Tests (D) Selective Laser Trabeculoplasties (E) Glaucoma Surgeries (F) Glaucoma Drugs Dispensed (G) Inpatient and Emergency Department (ED) Encounters (H) Nursing Facility Encounters and Home-Visits. Each individual chart represents utilization of a specific eye care service by non-/prefrail, mildly frail, and moderate-severely frail beneficiaries in number of Current Procedural Terminology codes per 100 person-years, and glaucoma drugs dispensed in days per year.
These associations were similar in negative binomial regression analyses without multivariable adjustment (Table 2) and with adjustment for age, gender, glaucoma severity, race, and SES (Table 3). After adjustment, relative to non-/prefrail beneficiaries, moderate-severely frail beneficiaries were less likely to have outpatient encounters (IRR 0.85, 95% CI 0.83–0.88); VF tests (IRR 0.64, 95% CI 0.60–0.67); RNFL OCT tests (IRR 0.77, 95% CI 0.73–0.81); SLT (IRR 0.74, 95% CI 0.60–0.92); glaucoma surgeries (IRR 0.74, 95% CI 0.55–0.99); and days of glaucoma drugs dispensed (IRR 0.88, 95% CI 0.81–0.96). The moderate-severely frail group also had a higher rate of inpatient and ED encounters (IRR 5.03, 95% CI 2.36–10.71) and nursing facility and home-visit encounters (IRR 34.89, 95% CI 14.82–82.13). After adjusting for comorbidity score in the sensitivity analysis, these trends remained largely consistent in that moderate-severely frail beneficiaries were less likely to utilize eye care services in outpatient settings and more likely in inpatient and acute settings (Supplemental Table 1, available at https://www.aaojournal.org).
Table 2.
Rates of eye care utilization by frailty group, unadjusted. Negative binomial regression results assessing incident rate ratio for each eye care utilization category by frailty group, unadjusted.
| Table 2: Rates of Eye Care Utilization by Frailty Group, Unadjusted | ||
|---|---|---|
|
| ||
| Outcomes | Incident Rate Ratio |
|
| Mildly frail (CFIa 0.20–0.29)b | Moderately and severely frail (CFI 0.30–1)b | |
|
| ||
| Outpatient eye examinations, office visits, consultations | 0.99 (0.97, 1.00) | 0.84 (0.82, 0.87) |
| Inpatient, emergency department encounters | 2.95 (1.98, 4.42) | 5.75 (2.64, 12.53) |
| Nursing facility encounters, home visits | 5.62 (3.35, 9.41) | 25.58 (9.34, 70.04) |
| Visual field tests | 0.83 (0.82, 0.85) | 0.57 (0.54, 0.60) |
| Retinal nerve fiber layer optical coherence tomography tests | 1.02 (1.00, 1.04) | 0.76 (0.72, 0.80) |
| Glaucoma drugs dispensed, days | 0.95 (0.91, 0.99) | 0.89 (0.82, 0.97) |
| Selective laser trabeculoplasties | 0.92 (0.85, 1.00) | 0.71 (0.58, 0.88) |
| Glaucoma surgeries | 0.78 (0.70, 0.87) | 0.61 (0.46, 0.82) |
Claims-based frailty index
Relative to non-/prefrail cohort (CFI 0–0.19)
Table 3.
Rates of eye care utilization by frailty group, adjusted. Negative binomial regression results assessing incident rate ratio for each eye care utilization category by frailty group, adjusted for age, gender, glaucoma severity, race, and socioeconomic status.
| Table 3: Rates of Eye Care Utilization by Frailty Group, Adjusted | ||||
|---|---|---|---|---|
|
| ||||
| Outcomes | Incident Rate Ratio |
|||
| Mildly frail (CFIa 0.20–0.29)b | Moderately and severely frail (CFI 0.30–1)b | Black/African Americanc | Otherd | |
|
| ||||
| Outpatient eye examinations, office visits, consultations | 0.99 (0.98, 1.00) | 0.85 (0.83, 0.88) | 0.93 (0.92, 0.95) | 1.03 (1.01, 1.04) |
| Inpatient, emergency department encounters | 2.38 (1.60, 3.56) | 5.03 (2.36, 10.71) | 2.12 (1.35, 3.34) | 1.29 (0.70, 2.38) |
| Nursing facility encounters, home visits | 5.65 (3.47, 9.21) | 34.89 (14.82, 82.13) | 1.60 (0.86, 2.96) | 1.07 (0.51, 2.22) |
| Visual field tests | 0.88 (0.86, 0.89) | 0.64 (0.60, 0.67) | 0.95 (0.94, 0.97) | 1.07 (1.05, 1.09) |
| Retinal nerve fiber layer optical coherence tomography tests | 1.02 (1.00, 1.04) | 0.77 (0.73, 0.81) | 0.80 (0.78, 0.82) | 0.97 (0.95, 1.00) |
| Glaucoma drugs dispensed, days | 0.94 (0.90, 0.98) | 0.88 (0.81, 0.96) | 0.98 (0.93, 1.03) | 0.97 (0.91, 1.03) |
| Selective laser trabeculoplasties | 0.93 (0.86, 1.01) | 0.74 (0.60, 0.92) | 0.97 (0.88, 1.06) | 1.03 (0.89, 1.19) |
| Glaucoma surgeries | 0.87 (0.78, 0.97) | 0.74 (0.55, 0.99) | 1.20 (1.08, 1.34) | 1.19 (1.07, 1.33) |
Claims-based frailty index
Relative to non-/prefrail cohort (CFI 0–0.19)
Relative to non-Hispanic White beneficiaries
Aggregate of Hispanic, Asian/Pacific Islander, and other beneficiaries, relative to non-Hispanic White beneficiaries
In stratified analyses, disparities in utilization by frailty group persisted by race (Table 4). Among non-Hispanic White beneficiaries, frail beneficiaries were less likely than non-frail beneficiaries to have outpatient encounters (IRR 0.97, 95% CI 0.96–0.99), VF tests (IRR 0.84, 95% CI 0.82–0.86) SLT (IRR 0.86, 95% CI 0.79–0.94), glaucoma surgeries (IRR 0.84, CI 0.75–0.95), and have fewer days of glaucoma drugs dispensed (IRR 0.94, 95% CI 0.90–0.98), and frail beneficiaries more likely to receive eye examinations during inpatient and ED encounters (IRR 2.24, 95% CI 1.45–3.47) and nursing facility and home-visit encounters (IRR 9.70, 95% CI 5.71–16.48). Similarly, among Black/African American beneficiaries, frail beneficiaries were less likely to have VF tests (IRR 0.87, 95% CI 0.83–0.91) and fewer days of glaucoma drugs dispensed (IRR 0.90, 95% CI 0.81–0.99), and more likely to have inpatient and ED encounters (IRR 5.31, 95% CI 2.23–12.67); and nursing facility and home-visit encounters (IRR 14.36, 95% CI 4.43–46.56).
Table 4.
Rates of eye care utilization by frailty group and stratified by race, adjusted. Negative binomial regression results assessing incident rate ratio for each utilization category for frail group, adjusted for age, gender, glaucoma severity and socioeconomic status, stratified by race.
| Table 4: Rates of Eye Care Utilization by Frailty Group and Stratified by Race, Adjusted | ||
|---|---|---|
|
| ||
| Incident Rate Ratio comparing frail (CFIa 0.2–1) vs non-/pre-frail (CFI <0.2) groups | ||
|
| ||
| Non-Hispanic White | Black/African American | |
|
| ||
| Outpatient eye examinations, office visits, consultations | 0.97 (0.96, 0.99) | 0.97 (0.94, 1.00) |
| Inpatient, emergency department encounters | 2.24 (1.45, 3.47) | 5.31 (2.23, 12.67) |
| Nursing facility encounters, home visits | 9.70 (5.71, 16.48) | 14.36 (4.43, 46.56) |
| Visual field tests | 0.84 (0.82, 0.86) | 0.87 (0.83, 0.91) |
| Retinal nerve fiber layer optical coherence tomography tests | 1.00 (0.98, 1.02) | 0.94 (0.89, 1.00) |
| Glaucoma drug dispensed, days | 0.94 (0.90, 0.98) | 0.90 (0.81, 0.99) |
| Selective laser trabeculoplasties | 0.86 (0.79, 0.94) | 1.01 (0.82, 1.23) |
| Glaucoma surgeries | 0.84 (0.75, 0.95) | 0.83 (0.65, 1.07) |
Claims-based frailty index
Next, we stratified by frailty to compare utilization of eye care services by race (Table 5). Racial disparities in outpatient eye care utilization persisted in both the non-frail group and frail group, but disparities in eye care utilization in inpatient and ED settings (IRR 0.41, 95% CI 0.05–3.66) and nursing facility and home-visit encounters (IRR 0.35, 95% CI 0.03–4.32) did not reach significance in the group of frail beneficiaries. Supplemental Figure 1 demonstrates the stratified rates per 100 person-years of utilization of eye care services, adjusted for age, gender, glaucoma severity, race, and SES (available at https://www.aaojournal.org).
Table 5.
Rates of eye care utilization by racial group and stratified by frailty, adjusted. Negative binomial regression results assessing incident rate ratio for each utilization category by race, adjusted for age, gender, glaucoma severity and socioeconomic status, stratified by frailty group.
| Table 5: Rates of Eye Care Utilization by Race and Stratified by Frailty, Adjusted | ||
|---|---|---|
|
| ||
| Incident Rate Ratio comparing Black/African American vs. Non-Hispanic White | ||
|
| ||
| Non-Frail (CFIa <0.2) | Frail (CFI 0.2–1) | |
|
| ||
| Outpatient eye examinations, office visits, consultations | 0.93 (0.92, 0.95) | 0.92 (0.84, 0.99) |
| Inpatient, emergency department encounters | 2.67 (1.69, 4.22) | 0.41 (0.05, 3.66) |
| Nursing facility encounters, home visits | 2.03 (1.08, 3.80) | 0.35 (0.03, 4.32) |
| Visual field tests | 0.95 (0.94, 0.97) | 0.89 (0.77, 1.03) |
| Retinal nerve fiber layer optical coherence tomography tests | 0.80 (0.78, 0.82) | 0.71 (0.61, 0.84) |
| Glaucoma drug dispensed, days | 0.98 (0.93, 1.03) | 0.92 (0.74, 1.15) |
| Selective laser trabeculoplasties | 0.96 (0.87, 1.05) | 1.39 (0.81, 2.40) |
| Glaucoma surgeries | 1.21 (1.08, 1.34) | 0.85 (0.38, 1.89) |
Claims-based frailty index
Discussion
In a cohort of Medicare beneficiaries with glaucoma, we demonstrated that frail beneficiaries were less likely than non-/prefrail beneficiaries to undergo outpatient eye examinations and consultations, VF and RNFL OCT testing, selective laser trabeculoplasty procedures, and glaucoma surgeries, and have fewer days of glaucoma medications dispensed. Conversely, frail beneficiaries were more likely to receive eye examinations in inpatient, ED, nursing facility, and home-visit settings. These findings persisted among both White and Black frail beneficiaries, with further stratified analyses demonstrating that differences in utilization among frail patients could not be explained by race alone.
Notably, 20.5% of beneficiaries with glaucoma were frail in our study, similar to the overall frailty prevalence of 20.8% among the Medicare population.22 This suggests that frailty is an important factor in the care of older beneficiaries with glaucoma.
Our study found a lower rate of utilization of eye care services in the outpatient setting among frail Medicare beneficiaries with glaucoma, but a higher rate of eye-related encounters in more acute settings. Previous studies evaluating differences in healthcare utilization by frailty score in other clinical settings have identified higher healthcare costs and utilization among frail older adults.3,23 Frailty has been associated with higher total annual healthcare costs, both in the outpatient and inpatient settings, as well as increased utilization of homecare services. While our study is consistent in showing that frail beneficiaries received more eye care in inpatient, ED, home-visit, and nursing facility settings, we importantly found that frail beneficiaries with glaucoma were less likely to receive glaucoma testing and treatment in the outpatient setting. This is particularly significant given that glaucoma progression is primarily detected through ambulatory testing in outpatient clinics, and thus frail individuals with glaucoma may not be receiving sufficient monitoring and subsequent intervention to prevent further vision loss.
A possible explanation for this finding is that community-dwelling older adults with frailty may have greater comorbidity scores, as was observed in our study. Increased comorbidities may lead to more frequent hospitalizations and admissions to rehabilitation facilities, limiting the ability of frail patients to prioritize eye care visits. Indeed, frail older adults have been found to have a higher rate of adverse cardiovascular events, ED visits, inpatient hospitalizations, and ICU admissions compared to those who are non-frail.24,25 Additionally, frail individuals may have lower referral rates for outpatient glaucoma testing and interventions given provider perceptions of lower life expectancy or suboptimal risk-benefit for quality of life. However, while frailty and comorbidity are closely related, our sensitivity analysis showed that differences in eye care utilization among moderate-severely frail beneficiaries persisted after controlling for comorbidity scores, suggesting that frailty is independently associated with eye care utilization.
In addition to receiving fewer outpatient visits and less glaucoma testing, moderate-severely frail Medicare beneficiaries with glaucoma were found to have lower rate ratios of glaucoma laser and surgery compared to non-/prefrail beneficiaries. This could be due to decreased monitoring and subsequently missed opportunities for surgical intervention to prevent further glaucoma progression. Another explanation may be that frail individuals are less likely to present for follow-up, reducing the likelihood that they undergo these procedures. Glaucoma specialists may also be reluctant to recommend laser procedures or surgery due to more potential postoperative complications in frail older adults. Prior studies have found that frailty is associated with an increased risk of both minor and major postoperative complications, surgical readmission (including after elective outpatient surgery), and mortality.26–29 Older adults with frailty have also been shown to have increased postoperative home care needs.23 Therefore, in patients with frailty and perceived lower life expectancy, ophthalmic providers may believe that the risks of surgery outweigh the potential benefits, most importantly the preservation of vision. While providers may believe that there is little benefit to sight-saving surgery in frail older patients with limited life expectancy, failure to prevent vision loss in this population has been associated with an increased risk of falls and injuries, leading to further complications, as well as greater neuropsychiatric morbidity.30–33
Given these findings, novel solutions may be needed to improve access and disease monitoring and management for older adults with glaucoma and frailty. One promising modality may be hybrid telemedicine. In this model of care, patients may present for brief in-person imaging and testing, which can include VF and RNFL OCT testing, followed by a subsequent virtual visit with their glaucoma specialist.34,35 Indeed, one recent study found that hybrid telemedicine was able to provide safe and high quality care for patients with nonurgent ophthalmic disease, including glaucoma.36 Hybrid telemedicine can also be instituted at point-of-care settings such as primary care clinics, which can reduce time, travel, and cost barriers for frail patients who may already have a high burden of medical visits. Additionally, there several home-based measurement devices such as at-home tonometry and OCT in development, which may be utilized in conjunction with telemedicine to improve access to glaucoma care and disease monitoring in the future.
In our study, a higher proportion of Black beneficiaries were classified as frail compared to non-Hispanic White beneficiaries. These findings are consistent with prior research showing greater functional impairment among Black patients compared to White patients, even after controlling for income and education.37 Data from the National Health and Aging Trends study have also shown a higher risk of frailty among Black individuals compared to White individuals, a disparity that persisted after controlling for BMI, income, and a number of chronic medical conditions.38 While previous work has shown that Black race predicts underutilization of eye care in outpatient settings and overutilization in acute settings,19 our results show that differences in utilization by frailty group largely persisted irrespective of race. Conversely, although the analysis that stratifies by frailty to compare eye care utilization by race did not reach significance due to low power, there appears to be a relationship between race and eye care utilization in the non-frail group. However, some of these associations lose significance when comparing Black and White beneficiaries within the frailty group, particularly in regard to eye care utilization in inpatient, ED, nursing facility and home-visit settings. Combined, these results suggest that race and frailty are independent predictors of eye care utilization and that key differences in utilization within the frail cohort are not entirely driven by race.
This study has several important strengths. We used a large, representative cohort of Medicare beneficiaries and a validated claims-based frailty index to measure frailty, allowing us to detect differences in eye care utilization among frail older adults with glaucoma. Furthermore, outcomes were measured over an 18-month period, allowing for sufficient measurement of utilization patterns. Several limitations of this study are associated with inherent deficiencies that exist in claims-based data. ICD-9 and ICD-10 codes for glaucoma may have reduced accuracy when compared with standard clinical criteria, decreasing our ability to capture all Medicare beneficiaries with glaucoma in our cohort. Further, since Medicare does not provide SES classification, ascertainment of SES among beneficiaries may not be completely accurate given our use of surrogate indicators of low SES. Outcomes may also be systematically under- or over-reported due to inaccuracies in CPT coding for utilization outcomes. Additionally, certain covariates, such as glaucoma severity, were not available for all beneficiaries and thus could not be fully captured in analyses.
In conclusion, Medicare beneficiaries with glaucoma and a higher claims-based frailty score were found to utilize eye care services at a lower rate in the outpatient setting and at a higher rate in inpatient and acute settings. Furthermore, frail older adults were less likely to receive laser treatment and surgery for glaucoma. These findings have important implications for disease monitoring and treatment in frail older adults with glaucoma, as inadequate monitoring and prevention of vision loss in this population may have severe consequences for morbidity and mortality.
Supplementary Material
Financial support:
National Eye Institute (K23 EY032634-01) and National Institute on Aging (K24AG073527), National Institutes of Health, Bethesda, Maryland; The sponsors or funding organizations had no role in the design or conduct of this research.
Abbreviations and Acronyms:
- IRR
incident rate ratio
- ED
emergency department
- VF
visual field
- RNFL OCT
retinal nerve fiber layer optical coherence tomography
- SLT
selective laser trabeculoplasties
- SD
standard deviation
- CI
confidence interval
- ICD-9/ICD-10
International Classification of Diseases, Ninth Revision or Tenth Revision, respectively
- CFI
claims-based frailty index
- CPT
Current Procedural Terminology
- SES
socioeconomic status
Footnotes
Conflict of interest: The following authors have no conflicts of interest: OAH, JK, AAP. NZ is a consultant for Character Biosciences Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Xue QL. The Frailty Syndrome: Definition and Natural History. Clin Geriatr Med. 2011;27(1). doi: 10.1016/j.cger.2010.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394(10206). doi: 10.1016/S0140-6736(19)31786-6 [DOI] [PubMed] [Google Scholar]
- 3.Ensrud KE, Kats AM, Schousboe JT, et al. Frailty Phenotype and Healthcare Costs and Utilization in Older Men. J Am Geriatr Soc. 2020;68(9). doi: 10.1111/jgs.16522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vermeiren S, Vella-Azzopardi R, Beckwée D, et al. Frailty and the Prediction of Negative Health Outcomes: A Meta-Analysis. J Am Med Dir Assoc. 2016;17(12):1163.e1–1163.e17. doi: 10.1016/J.JAMDA.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 5.Buckinx F, Rolland Y, Reginster JY, Ricour C, Petermans J, Bruyère O. Burden of frailty in the elderly population: Perspectives for a public health challenge. Arch Public Heal. 2015;73(1). doi: 10.1186/s13690-015-0068-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzales-Turín JM, Rodríguez-Laso Á, Carnicero JA, García-García FJ, Rodríguez-Mañas L. Relationship between self-reported visual impairment and worsening frailty transition states in older people: a longitudinal study. Aging Clin Exp Res. 2021;33(9):2491–2498. doi: 10.1007/S40520-020-01768-W/TABLES/3 [DOI] [PubMed] [Google Scholar]
- 7.Liljas AEM, Carvalho LA, Papachristou E, et al. Self-reported vision impairment and incident prefrailty and frailty in English community-dwelling older adults: Findings from a 4-year follow-up study. J Epidemiol Community Health. 2017;71(11):1053–1058. doi: 10.1136/jech-2017-209207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swenor BK, Lee MJ, Tian J, Varadaraj V, Bandeen-Roche K. Visual Impairment and Frailty: Examining an Understudied Relationship. J Gerontol A Biol Sci Med Sci. 2020;75(3):596–602. doi: 10.1093/GERONA/GLZ182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varadaraj V, Lee MJ, Tian J, Ramulu PY, Bandeen-Roche K, Swenor BK. Near Vision Impairment and Frailty: Evidence of an Association. Am J Ophthalmol. 2019;208:234–241. doi: 10.1016/j.ajo.2019.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaumberg DA, Christen WG, Buring JE, Glynn RJ, Rifai N, Ridker PM. High-sensitivity C-reactive protein, other markers of inflammation, and the incidence of macular degeneration in women. Arch Ophthalmol. 2007;125(3). doi: 10.1001/archopht.125.3.300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanapuru B, Ershler WB. Inflammation, Coagulation, and the Pathway to Frailty. Am J Med. 2009;122(7). doi: 10.1016/j.amjmed.2009.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shang X, Zhu Z, Wang W, Ha J, He M. The Association between Vision Impairment and Incidence of Dementia and Cognitive Impairment: A Systematic Review and Meta-analysis. Ophthalmology. 2021;128(8):1135–1149. doi: 10.1016/J.OPHTHA.2020.12.029 [DOI] [PubMed] [Google Scholar]
- 13.Cao GY, Chen ZS, Yao SS, et al. The association between vision impairment and cognitive outcomes in older adults: a systematic review and meta-analysis. Aging Ment Health. Published online 2022. doi: 10.1080/13607863.2022.2077303 [DOI] [PubMed] [Google Scholar]
- 14.Bramley T, Peeples P, Walt JG, Juhasz M, Hansen JE. Impact of vision loss on costs and outcomes in medicare beneficiaries with glaucoma. Arch Ophthalmol. 2008;126(6). doi: 10.1001/archopht.126.6.849 [DOI] [PubMed] [Google Scholar]
- 15.Gutienez P, Roy Wilson M, Johnson C, et al. Influence of glaucomatous visual field loss on health-related quality of life. Arch Ophthalmol. 1997;115(6). doi: 10.1001/archopht.1997.01100150779014 [DOI] [PubMed] [Google Scholar]
- 16.Ramulu PY, Mihailovic A, West SK, Friedman DS, Gitlin LN. What Is a Falls Risk Factor? Factors Associated with Falls per Time or per Step in Individuals with Glaucoma. J Am Geriatr Soc. 2019;67(1). doi: 10.1111/jgs.15609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring Frailty in Medicare Data: Development and Validation of a Claims-Based Frailty Index. Journals Gerontol - Ser A Biol Sci Med Sci. 2018;73(7). doi: 10.1093/gerona/glx229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim DH, Glynn RJ, Avorn J, et al. Validation of a Claims-Based Frailty Index Against Physical Performance and Adverse Health Outcomes in the Health and Retirement Study. J Gerontol A Biol Sci Med Sci. 2019;74(8):1271–1276. doi: 10.1093/GERONA/GLY197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halawa OA, Kolli A, Oh G, et al. Racial and Socioeconomic Differences in Eye Care Utilization among Medicare Beneficiaries with Glaucoma. Ophthalmology. 2022;129(4). doi: 10.1016/j.ophtha.2021.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samson LW, Finegold K, Ahmed A, Jensen M, Filice CE, Joynt KE. Examining Measures of Income and Poverty in Medicare Administrative Data. Med Care. 2017;55(12):e158e163. doi: 10.1097/MLR.0000000000000606 [DOI] [PubMed] [Google Scholar]
- 21.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–759. doi: 10.1016/J.JCLINEPI.2010.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandra Miao Shi, Steinberg N, Oh G, et al. (In Press). Change in a Claims-Based Frailty Index, Mortality, and Healthcare Costs in Medicare Beneficiaries. Journals Gerontol - Ser A Biol Sci Med Sci Gerontol Med Sci. Published online 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chesney TR, Haas B, Coburn N, et al. Association of frailty with long-term homecare utilization in older adults following cancer surgery: Retrospective population-based cohort study. Eur J Surg Oncol. 2021;47(4). doi: 10.1016/j.ejso.2020.09.009 [DOI] [PubMed] [Google Scholar]
- 24.Ter Chao C, Wang J, Chien KL, et al. Both pre-frailty and frailty increase healthcare utilization and adverse health outcomes in patients with type 2 diabetes mellitus 11 Medical and Health Sciences 1103 Clinical Sciences. Cardiovasc Diabetol. 2018;17(1). doi: 10.1186/s12933-018-0772-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNallan SM, Singh M, Chamberlain AM, et al. Frailty and healthcare utilization among patients with heart failure in the community. JACC Hear Fail. 2013;1(2). doi: 10.1016/j.jchf.2013.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wahl TS, Graham LA, Hawn MT, et al. Association of the modified frailty index with 30day surgical readmission. JAMA Surg. 2017;152(8). doi: 10.1001/jamasurg.2017.1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothenberg KA, Stern JR, George EL, et al. Association of frailty and postoperative complications with unplanned readmissions after elective outpatient surgery. JAMA Netw Open. 2019;2(5). doi: 10.1001/jamanetworkopen.2019.4330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chikwe J, Adams DH. Frailty: The Missing Element in Predicting Operative Mortality. Semin Thorac Cardiovasc Surg. 2010;22(2). doi: 10.1053/j.semtcvs.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 29.Shinall MC, Arya S, Youk A, et al. Association of Preoperative Patient Frailty and Operative Stress With Postoperative Mortality. JAMA Surg. 2020;155(1):e194620-e194620. doi: 10.1001/JAMASURG.2019.4620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamoreux EL, Chong E, Wang JJ, et al. Visual impairment, causes of vision loss, and falls: The singapore malay eye study. Investig Ophthalmol Vis Sci. 2008;49(2). doi: 10.1167/iovs.07-1036 [DOI] [PubMed] [Google Scholar]
- 31.Patino CM, McKean-Cowdin R, Azen SP, Allison JC, Choudhury F, Varma R. Central and Peripheral Visual Impairment and the Risk of Falls and Falls with Injury. Ophthalmology. 2010;117(2). doi: 10.1016/j.ophtha.2009.06.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halawa O, Mitchell W, Zebardast N. Fall-Related Eye Injury Among Older Adults in the United States. Am J Ophthalmol. 2021;229. doi: 10.1016/j.ajo.2021.03.063 [DOI] [PubMed] [Google Scholar]
- 33.Lee CS, Gibbons LE, Lee AY, et al. Association Between Cataract Extraction and Development of Dementia. JAMA Intern Med. 2022;182(2):134–141. doi: 10.1001/JAMAINTERNMED.2021.6990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armstrong GW, Miller JB. Telemedicine for the Diagnosis and Management of Age-Related Macular Degeneration: A Review. J Clin Med. 2022;11(3). doi: 10.3390/JCM11030835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brodin AC, Tamhankar MA, Whitehead G, Mackay D, Kim BJ, O’brien JM. Approach of an Academic Ophthalmology Department to Recovery During the Coronavirus Pandemic. Published online 2022. doi: 10.2147/OPTH.S342300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanayei N, Albrecht MM, Martin DC, et al. Outcomes of a Hybrid Ophthalmology Telemedicine Model for Outpatient Eye Care During COVID-19. JAMA Netw open.2022;5(8):E2226292. doi: 10.1001/JAMANETWORKOPEN.2022.26292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clark DO, Maddox GL. Racial and social correlates of age-related changes in functioning. Journals Gerontol. 1992;47(5). doi: 10.1093/geronj/47.5.S222 [DOI] [PubMed] [Google Scholar]
- 38.Usher T, Buta B, Thorpe RJ, et al. Dissecting the racial/ethnic disparity in frailty in a nationally representative cohort study with respect to health, income, and measurement. Journals Gerontol - Ser A Biol Sci Med Sci. 2021;76(1). doi: 10.1093/GERONA/GLAA061 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.