Significance
Pseudomonas aeruginosa causes biofilm-based infections and is known for its production of colorful phenazine derivatives. Among these, the methylated phenazines are the most toxic and can cause condition-dependent damage to their producer. Here, we show that the methylated phenazine pyocyanin also has a beneficial effect in that it supports metabolic activity at depth in biofilms, where oxygen limitation would otherwise stall metabolism. We describe a link between P. aeruginosa global regulators, which control methylated phenazine production in a manner that limits the toxicity of these compounds while simultaneously enabling their contribution to metabolism. These results expand our understanding of the strategies that enable P. aeruginosa survival in multicellular structures, which is key to its success during chronic host colonization.
Keywords: biofilm physiology, pyocyanin, carbon catabolite repression, metabolic heterogeneity, self-resistance
Abstract
Within biofilms, gradients of electron acceptors such as oxygen stimulate the formation of physiological subpopulations. This heterogeneity can enable cross-feeding and promote drug resilience, features of the multicellular lifestyle that make biofilm-based infections difficult to treat. The pathogenic bacterium Pseudomonas aeruginosa produces pigments called phenazines that can support metabolic activity in hypoxic/anoxic biofilm subzones, but these compounds also include methylated derivatives that are toxic to their producer under some conditions. In this study, we uncover roles for the global regulators RpoS and Hfq/Crc in controlling the beneficial and detrimental effects of methylated phenazines in biofilms. Our results indicate that RpoS controls phenazine methylation by modulating activity of the carbon catabolite repression pathway, in which the Hfq/Crc complex inhibits translation of the phenazine methyltransferase PhzM. We find that RpoS indirectly inhibits expression of CrcZ, a small RNA that binds to and sequesters Hfq/Crc, specifically in the oxic subzone of P. aeruginosa biofilms. Deletion of rpoS or crc therefore leads to overproduction of methylated phenazines, which we show leads to increased metabolic activity—an apparent beneficial effect—in hypoxic/anoxic subpopulations within biofilms. However, we also find that under specific conditions, biofilms lacking RpoS and/or Crc show increased sensitivity to phenazines indicating that the increased metabolic activity in these mutants comes at a cost. Together, these results suggest that complex regulation of PhzM allows P. aeruginosa to simultaneously exploit the benefits and limit the toxic effects of methylated phenazines.
Cellular growth at high density promotes the formation of resource gradients, and multicellular structures therefore contain microenvironments with distinct conditions. To maintain metabolic activity, cells within multicellular structures undergo physiological differentiation, which enhances robustness of the overall population (1–5). Coexistence in a multicellular structure also allows differentiated subpopulations to interact in beneficial ways, for example, by exchanging metabolites that are optimally produced in one region and consumed in another. The physiological heterogeneity of multicellular structures is problematic in infections or cancers, where the effectiveness of a drug often depends on the metabolic status of target cells (6). Approaches that provide insight into physiological differentiation while retaining information about structure can help us to understand the unique biology of multicellularity and can also inform treatment methods.
Microbial biofilms are multicellular structures that exhibit metabolic differentiation and developmental responses to resource limitation, with parallels in metazoans and plants (2, 7). Studies in diverse bacteria have indicated that sigma factors, which are regulatory proteins that mediate transcriptional responses to environmental and physiological cues, are key players in biofilm biology (8–13). The sigma factor is a component of the RNA polymerase that confers specificity for target sequences in promoters, and bacterial genomes typically encode more than one sigma factor with different specificities (8, 14). Thus, condition-dependent shifts in the sigma factor pool allow bacteria to elicit global changes in their transcriptional profiles, which adapt their physiology to their current environment. One of the best-studied sigma factors is RpoS, which is conserved in proteobacteria and typically activates gene expression in the stationary phase of planktonic growth or during resource limitation (15–18).
The bacterium Pseudomonas aeruginosa is a prevalent cause of biofilm-based infections and a model for the study of biofilm development and metabolism. It can adapt to environmental changes over the long term, with its high mutation rate (19–22), and in the short term, with an arsenal of up to 26 sigma factors (depending on strain identity) and hundreds of transcription factors (23–25). These regulators form a complex network of gene expression control that we are only beginning to understand holistically (24, 25). Our knowledge about the roles of sigma factor-controlled networks in regulating gene expression and affecting P. aeruginosa physiology is particularly limited for cells surviving in more complex environments like biofilms.
RpoS is required for wild-type P. aeruginosa biofilm development in a variety of assays and across strain backgrounds. It is expressed in biofilms, particularly at the biofilm–air interface, in Escherichia coli and P. aeruginosa and its regulon includes genes involved in crucial redox metabolism in the oxic biofilm subzone (10, 26–28). Furthermore, rpoS mutants have been reported to overproduce pyocyanin (1-hydroxy-5-methylphenazine) (29), one of several phenazine compounds that allow P. aeruginosa to carry out extracellular electron transfer when respiratory electron acceptors are limiting (such as within biofilms) (30–32). While studies in biofilms have indicated that this electron-shuttling property of phenazines supports metabolic activity, recent work has also shown that phenazines can be toxic to P. aeruginosa under specific conditions or when their production and localization are not properly controlled (33–35).
In this study, we examined the role of RpoS in P. aeruginosa PA14 biofilm physiology and were intrigued to find that this sigma factor has an inhibitory effect on metabolic activity at depth in biofilms. At first glance, an inhibition of metabolic activity seems detrimental, and we sought to understand how and why a major regulator would mediate this effect. Here, we describe the identification of a regulatory pathway and of phenazine products that act downstream of RpoS to modulate metabolic activity. Our findings implicate this pathway in methylated phenazine resistance and suggest that modulation of phenazine methylation is critical for balancing the toxic and beneficial effects of these reactive compounds in P. aeruginosa biofilms.
Results
RpoS Attenuates Biofilm Metabolic Activity.
Prior work has indicated that RpoS is active in biofilms and that it confers fitness during conditions of either carbon source or oxygen limitation in P. aeruginosa (36, 37). We therefore sought to test whether RpoS contributes to metabolic activity in macrocolony biofilms, which contain opposing oxygen and carbon-source gradients (38, 39). Our group has previously used stimulated Raman scattering (SRS) microscopy to map metabolic activity across biofilm depth and found that it is affected by growth conditions and genotype (31, 39). SRS microscopy is an optical imaging technique that detects the vibration of specific chemical bonds (40). For this method, we transfer mature biofilms onto medium containing deuterated water for 5 h before harvesting and preparing thin sections and then use SRS to detect the D-C bonds that arise from incorporation of deuterium into biomass (an indicator of metabolic activity) (Fig. 1A). We used this approach and observed two changes in ΔrpoS biofilms when compared to WT: i) a shift in the localization of maximum metabolic activity, from a depth of ~50 to 30 µm, and ii) an overall increase in metabolic activity over a broad region starting at a depth of about 50 µm and continuing toward the biofilm base (Fig. 1B).
Fig. 1.
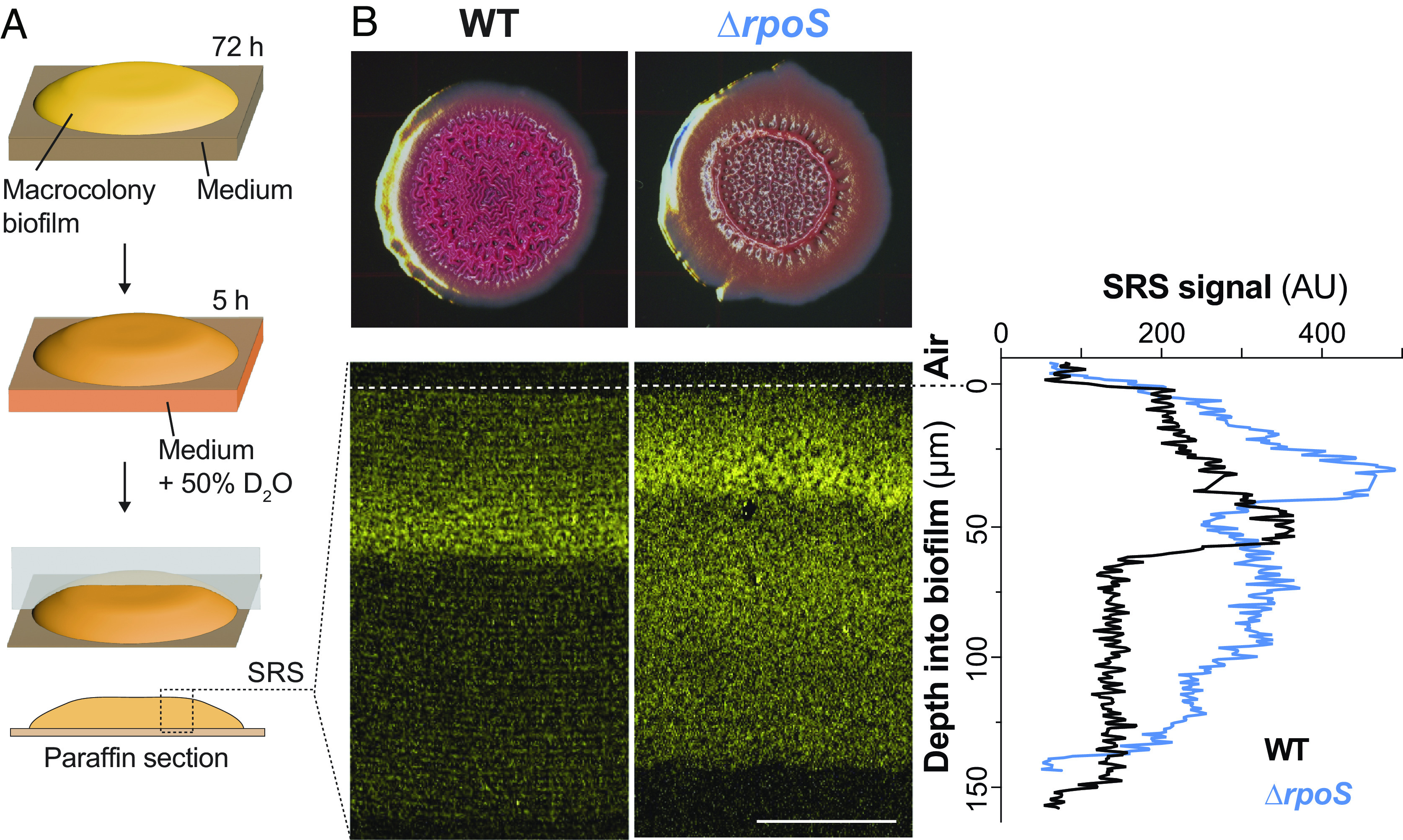
ΔrpoS biofilms show increased metabolic activity at depth when compared to those formed by WT PA14. (A) Scheme depicting the preparation of macrocolony biofilm thin sections for imaging by stimulated Raman scattering (SRS) microscopy. (B) Top: PA14 and ΔrpoS biofilms grown for 72 h on 1% tryptone, 1% agar medium containing the dyes Congo red and Coomassie blue. Bottom: SRS microscopy images of PA14 and ΔrpoS biofilm thin sections. The air interface of each biofilm is indicated by a dotted line. The SRS signal is indicative of metabolic activity and is false-colored yellow. Right: quantification of average SRS signal across depth. Experiments were performed in biological triplicate and representative images are shown (Scale bar, 50 µm).
The Methylated Phenazine Pyocyanin Supports Metabolic Activity across Depth in PA14 Biofilms.
We have previously reported that phenazine production (Fig. 2A) enhances metabolic activity at depth in PA14 biofilms (31), and we therefore hypothesized that the effects of RpoS on biofilm metabolic activity could be mediated by effects on phenazine production. To test this, we used SRS microscopy to image biofilm thin sections from a ΔrpoSΔphz mutant, which is unable to produce phenazines. Removing the capacity to produce phenazines abolished the enhanced metabolic activity of the ΔrpoS parent strain, indicating that this phenotype is indeed phenazine-dependent (Fig. 2B). We note that the absence of phenazines did not affect the depth at which the maximum metabolic activity was detected in ΔrpoS biofilms. The localization of maximum metabolic activity may be affected by differences in oxygen consumption in ΔrpoS biofilms as we observed steeper oxygen gradients in this mutant background when compared to respective parent-strain backgrounds (Fig. 2C).
Fig. 2.
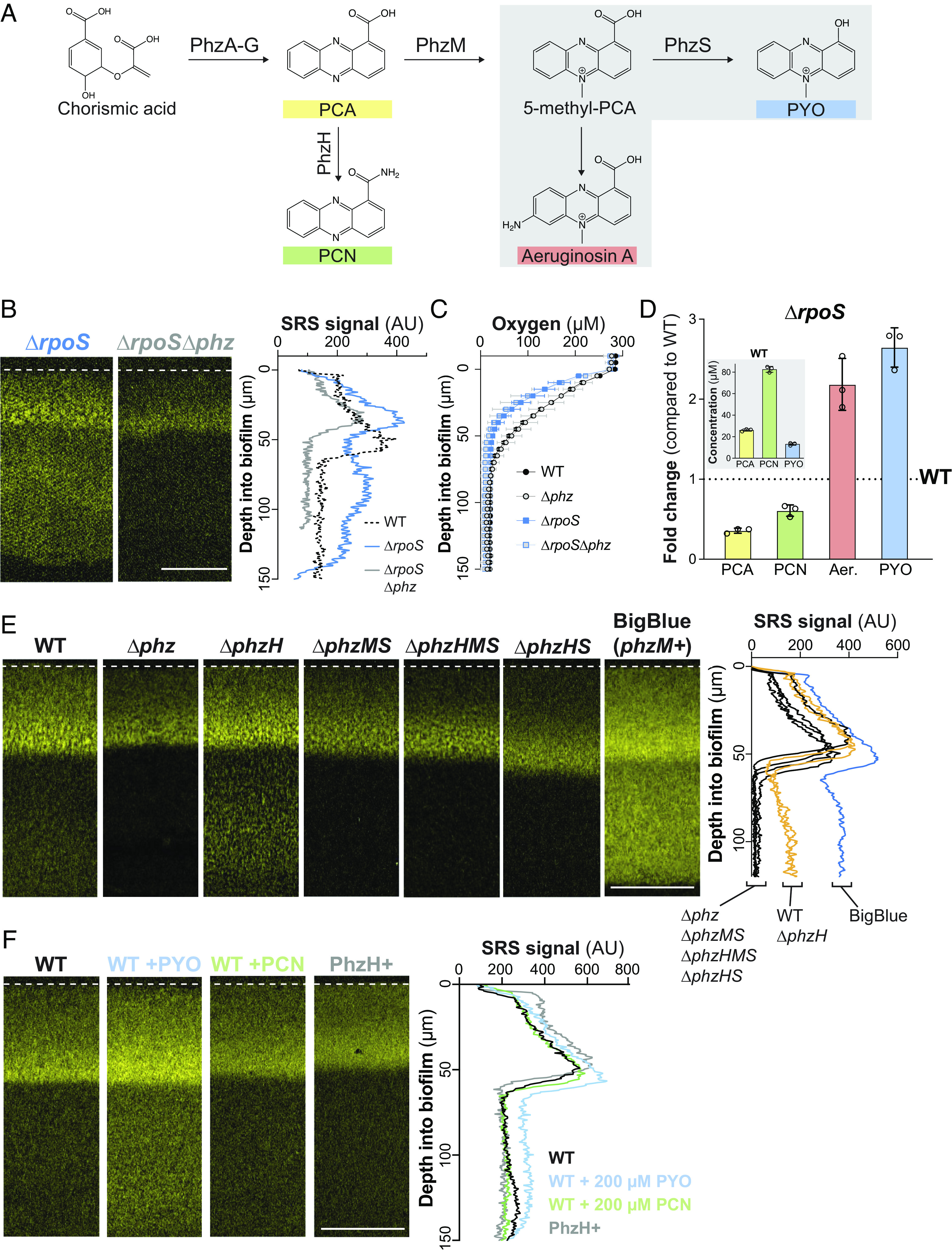
Biofilm metabolic activity at depth is supported specifically by the production of methylated phenazine derivatives. (A) The phenazine biosynthetic pathway. A series of enzymes convert two molecules of chorismate to phenazine-1-carboxylic acid (PCA). PCA can be aminated by PhzH to produce phenazine-1-carboxamide (PCN) or methylated by PhzM to produce 5-methyl-PCA. Further modification of 5-methyl-PCA abiotically by free amine groups or enzymatically by PhzS produces aeruginosins (one example structure is shown) or pyocyanin (PYO), respectively. (B) SRS microscopy images of thin sections prepared from ΔrpoS and ΔrpoSΔphz macrocolony biofilms. (C) Change in oxygen concentration along the depth of biofilms. Data shown are the mean of biological triplicates and error bars represent SD. (D) Change in phenazine production by ΔrpoS biofilms relative to biofilms formed by WT PA14. Inset: absolute concentrations of phenazines from WT colonies. Biofilms were grown on 1% tryptone, 1% agar for 72 h. Aer., aeruginosins. Individual points represent biological replicates and error bars represent SD. (E) SRS microscopy images of thin sections prepared from PA14 WT and phenazine biosynthetic mutant biofilms. (F) SRS microscopy images of WT and PhzH+ (containing an extra copy of phzH under the control of a constitutive promoter) biofilm thin sections. Where indicated, WT biofilms were exposed to 0.2 M of the specified phenazines for 5 h. For (B, E, and F), SRS signal is false-colored yellow; quantification of the average SRS signal across depth is shown in the right-hand panels, and experiments were performed in biological triplicate and representative images are shown (Scale bars, 50 µm).
Due to the importance of phenazines in the high metabolic activity of ΔrpoS biofilms, we investigated how RpoS affects the production of specific phenazine derivatives. P. aeruginosa phenazines are produced in biofilms and in the stationary phase during growth in shaken liquid cultures (41). The first derivative produced in the P. aeruginosa phenazine synthesis pathway is phenazine-1-carboxylic acid (PCA), which can be converted to i) phenazine-1-carboxamide (PCN) via the PhzH transamidase, or ii) 5-methyl-PCA via the PhzM methyltransferase. 5-methyl-PCA, an unstable intermediate, is converted to other methylated phenazines including pyocyanin and aeruginosin A (42–44) (Fig. 2A). It has been reported that RpoS inhibits production of PhzM and that RpoS-deficient mutants show increased pyocyanin and decreased PCA production (29, 45). HPLC and spectrophotometer analysis of extracts from biofilms and the underlying growth medium confirmed that flux through the PhzM step of the phenazine biosynthetic pathway was increased in ΔrpoS biofilms (Fig. 2D). Furthermore, PCA levels in PA14 and ΔrpoS biofilms lacking the phenazine modification enzymes PhzH, PhzM, and PhzS (ΔphzHMS) were similar, providing further evidence that the changes in phenazine differentiation observed in ΔrpoS biofilms is due to changes in regulation of one or more enzyme(s) that modify PCA (SI Appendix, Fig. S1A).
To evaluate whether specific phenazine derivatives contribute to metabolic activity in biofilms and whether this underpins the ΔrpoS phenotype, we incubated mutant biofilms lacking genes for phenazine-modification enzymes on deuterated water, prepared thin sections, and imaged via SRS microscopy. We observed a clear correlation between the production of pyocyanin and the amount of metabolic activity across depth in biofilms, most notably in the hypoxic/anoxic zone. Consistent with the notion that one or more methylated phenazines promote metabolic activity, a strain (“BigBlue”) that contains a second copy of phzM on the chromosome (46) and therefore overproduces methylated phenazines (SI Appendix, Fig. S1B), showed the strongest SRS signal (Fig. 2E). Among the mutants we tested was ΔphzHS, which would be expected to produce increased levels of 5-methyl-PCA, aeruginosin A, and other abiotic 5-methyl-PCA derivatives (relative to WT) without producing pyocyanin. Interestingly, ΔphzHS biofilms exhibited an SRS profile that resembled that of ΔphzHMS biofilms, showing that specific disruption of pyocyanin synthesis abolishes metabolic activity in the biofilm anoxic zone. By adding endogenous pyocyanin to mature biofilms, we also found that this methylated phenazine is sufficient to promote metabolic activity at depth (Fig. 2F). In addition, since PCN is the most abundant phenazine in macrocolony biofilms (Fig. 2D) (41), we tested the effect of this compound on metabolic activity. In contrast to our observations for pyocyanin, our results for engineered strains with altered PCN production and for biofilms exposed to PCN indicated that this phenazine does not contribute to metabolic activity at depth in biofilms (Fig. 2 E and F).
RpoS Regulates Phenazine Derivatization Via the P. aeruginosa Carbon Catabolite Repression System.
We sought to further understand the regulatory pathway that allows RpoS to control phenazine methylation. The phzM transcript is one of >200 mRNA targets of the Hfq/Crc (“catabolite repression control”) protein complex (Fig. 3A) (47–50). The Hfq/Crc complex mediates the terminal regulatory step of the carbon catabolite repression (CCR) pathway. Canonically, the CCR pathway acts to inhibit translation of mRNAs that support the use of nonpreferred carbon sources when preferred substrates are available (51, 52), though recent studies have indicated that it has broad effects on P. aeruginosa physiology beyond primary metabolic functions (53–55). Consistent with the notion that RpoS acts via this pathway to control phenazine methylation, a recent study (45) found that the translation of phzM is inhibited by RpoS. To further probe this model, we created Δcrc and ΔrpoSΔcrc mutants and measured phenazine production by biofilms. We found that like ΔrpoS biofilms, Δcrc and ΔrpoSΔcrc biofilms show enhanced production of methylated phenazines (Fig. 3B). Δcrc biofilms differ from ΔrpoS biofilms in that they do not show a substantial decrease in PCA and PCN production compared to WT. This indicates that although both Hfq/Crc and RpoS have global regulatory effects (18, 50), the impact of Crc on nonmethylated phenazines is negligible or small compared to that of RpoS. RpoS affects the expression of over 700 genes (18), one or more of which likely has downstream effects on the production of the nonmethylated phenazines.
Fig. 3.
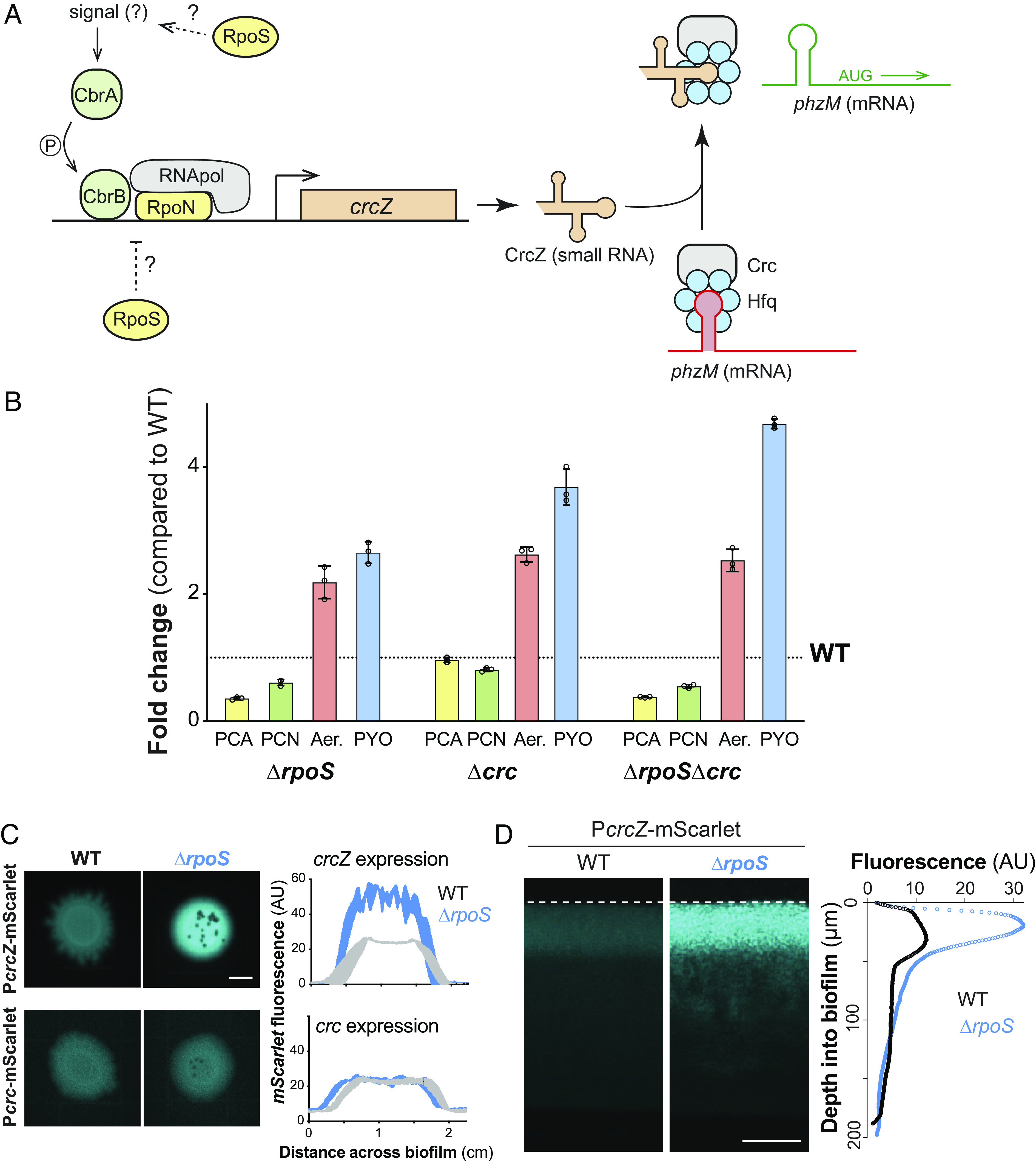
RpoS inhibits phenazine methylation by modulating the carbon catabolite repression pathway at the biofilm-air interface. (A) Schematic of the P. aeruginosa carbon catabolite repression pathway, which controls translation of phzM in a carbon source-dependent manner. RpoN is the canonical sigma factor that is associated with this pathway and it acts in conjunction with the transcription factor CbrB. When CrcZ levels are low, the Crc/Hfq complex binds to phzM transcript and inhibits translation. When CrcZ levels are high, CrcZ binds and sequesters Crc/Hfq, allowing translation of the phzM transcript. (B) Changes in production of phenazine derivatives by ΔrpoS, Δcrc, and ΔrpoSΔcrc biofilms relative to biofilms formed by WT PA14. Individual points represent biological triplicates and error bars represent SD. (C) Representative whole-biofilm fluorescence images of PA14 WT and ΔrpoS strains expressing mScarlet under control of PcrcZ or Pcrc (Scale bar, 5 mm). mScarlet fluorescence is false-colored cyan. The average levels of fluorescence produced by each strain along the diameter of three independent, biological-replicate biofilms with the Pcrc-mScarlet or PcrcZ-mScarlet reporters are shown in the right-hand plots. Shading indicates the SD. (D) Fluorescence microscopy of thin sections prepared from WT PA14 and ΔrpoS biofilms expressing PcrcZ-mScarlet (Scale bar, 50 µm). mScarlet fluorescence is false-colored cyan. Average fluorescence across depth of a representative biofilm section is shown in the right-hand plot.
RpoS Inhibits crcZ Expression.
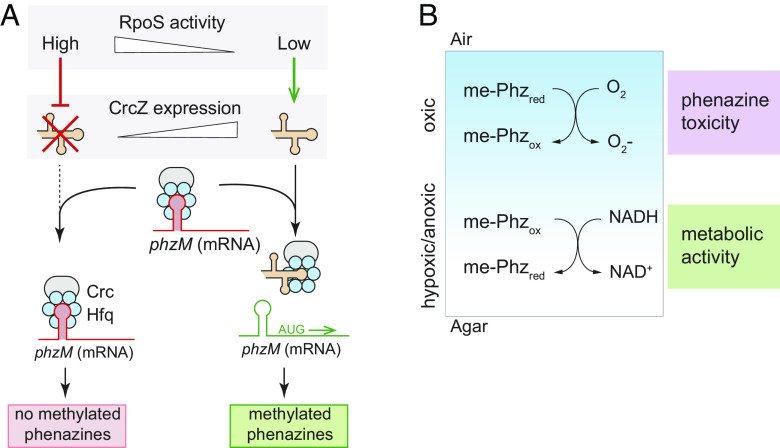
Binding of the Hfq/Crc complex to target transcripts can be prevented by the small RNA CrcZ (Fig. 3A), which itself can bind and thereby sequester Hfq/Crc under appropriate conditions. This regulatory mechanism allows for condition-dependent translation of Hfq/Crc targets (52). RpoS could therefore affect Hfq/Crc activity by modulating transcription of either crcZ or crc. To test this, we created constructs in which the upstream promoter regions of crcZ or crc are cloned in front of mScarlet (PcrcZ and Pcrc, respectively) and then placed these in a neutral site on the chromosome in WT and ΔrpoS backgrounds. We found that in both biofilms and liquid (planktonic) cultures, removal of RpoS did not affect activity of Pcrc, but, in contrast, led to a strong enhancement of PcrcZ activity (Fig. 3C and SI Appendix, Fig. S2A). This indicates that RpoS inhibits crcZ expression, possibly by competing with RpoN for RNA polymerase (56) (Fig. 3A) or another more indirect mechanism.
The resource gradients that form across depth in macrocolony biofilms lead to physiological differentiation and subpopulations with distinct patterns of gene expression (2, 4, 5, 26, 57). RpoS-dependent effects on gene expression could be localized to a specific biofilm subzone, perhaps where this activity would be most physiologically relevant. Indeed, results from previous studies suggest that RpoS is most expressed and active at the P. aeruginosa biofilm–air interface (26, 27, 58, 59). Moreover, pyocyanin synthesis requires oxygen, and prior work by our group has indicated that production of 5-Me-PCA may also be limited to oxygen-containing biofilm subzones (60). When we prepared thin sections of WT and ΔrpoS biofilms expressing PcrcZ-mScarlet and imaged by fluorescence microscopy, we found that the effect of RpoS on crcZ expression was indeed localized to the biofilm-air interface (Fig. 3D), similar to what has been observed for direct RpoS targets such as the promoter for coxB (26, 38). The observation that RpoS inhibits crcZ expression specifically at the biofilm-air interface, which is the biofilm region furthest from the growth medium, is consistent with a role for RpoS and the CCR system in inhibiting methylated phenazine production. Because phenazines are excreted small molecules with the potential to diffuse through the biofilm, changes in phenazine derivatization due to changes in RpoS activity at the biofilm surface can negatively impact metabolic activity at depth in biofilms (Fig. 1B).
Evidence suggests that crcZ transcription in P. aeruginosa is driven directly by the regulator CbrB and the sigma factor RpoN (Fig. 3A and SI Appendix, Fig. S2 A and B) (24, 52, 61). CbrB is part of the CbrAB two-component system, which responds to an as-yet-unidentified cue. Our observation that RpoS inhibits crcZ transcription raised the question of whether RpoS might be acting upstream of CbrAB. To test this, we deleted cbrB in the ΔrpoS PcrcZ-mScarlet reporter strain and found that this abolished PcrcZ activity (SI Appendix, Fig. S2B). These results indicate that CbrB is required for crcZ repression in the ΔrpoS background and are consistent with a model in which RpoS acts upstream of CbrAB, the first apparent step of the P. aeruginosa CCR pathway, to inhibit phzM translation and therefore methylated phenazine production. In other words, RpoS might inhibit CbrB-induced crcZ expression, leading to a larger pool of unsequestered Crc available to bind phzM transcript, and therefore lower levels of phzM translation in the WT when compared to ΔrpoS.
CCR-pathway Mutant Phentoypes Are Consistent with Roles of the Pathway in Regulating PhzM Translation and Carbon Source Utilization.
Our observations linking RpoS to the CCR pathway and the inhibition of metabolic activity in biofilms suggest that RpoN, which is required for crcZ expression, could promote metabolic activity in biofilms. We addressed this by imaging thin sections of WT and ΔrpoN biofilms via SRS microscopy and found that ΔrpoN biofilms showed lower levels of metabolic activity across depth than those formed by the WT (SI Appendix, Fig. S3). This observation is consistent with RpoN’s role in the CCR pathway and the abrogation of methylated phenazine production in this mutant (47, 62). A second prediction that arises from the fact that RpoS inhibits crcZ expression (Fig. 3D) is that RpoS might hinder P. aeruginosa’s ability to transition to growth on nonpreferred carbon sources, as crcZ relieves Crc/Hfq repression of target mRNAs involved in the use of these substrates (63). To test this idea, we used a defined liquid growth medium with a highly preferred carbon source (i.e., succinate, on which P. aeruginosa shows low levels of crcZ expression) or with a nonpreferred carbon source (i.e., mannitol, which induces high levels of crcZ expression) (64). We found that the ΔrpoS mutant indeed showed a large increase in crcZ expression (SI Appendix, Fig. S4), and enhanced growth during the lag and exponential phases (SI Appendix, Fig. S5), during growth on mannitol when compared to WT. During growth on succinate, ΔrpoS did not show a large increase in crcZ expression or enhanced growth during the lag or exponential phases of growth. The ΔrpoS mutant did, however, show an increased growth yield relative to WT during growth on succinate, which may arise from independent effects of this mutation.
Due to its role in stabilizing Hfq’s interaction with target transcripts such as that of phzM, mutants lacking Crc overproduce PhzM and methylated phenazines (47, 65) (Fig. 3B). Having observed that ΔrpoS and BigBlue—two mutants with elevated methylated phenazine production—show increased metabolic activity at depth in biofilms, we wondered whether this correlation would extend to the Δcrc mutant. We used SRS microscopy to image Δcrc and ΔphzΔcrc biofilm thin sections and found that the Δcrc mutant also showed increased metabolic activity across depth in a phenazine-dependent manner (Fig. 4A).
Fig. 4.
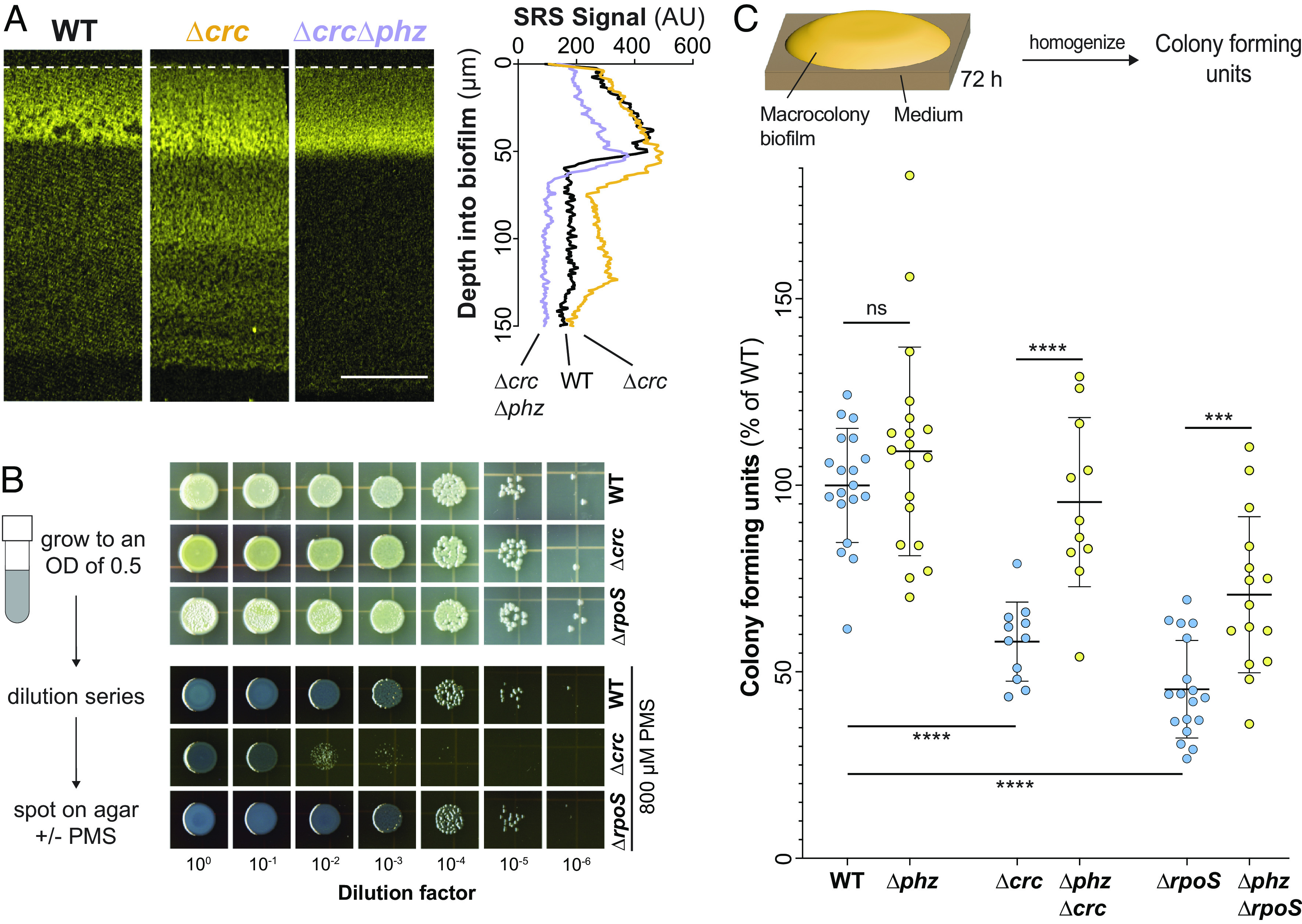
Like rpoS, crc dampens phenazine-supported metabolic activity but contributes to phenazine resistance. (A) SRS images of thin sections prepared from WT, Δcrc, and ΔcrcΔphz biofilms. SRS signal is false-colored yellow and the average signal across depth is shown in the right-hand plot (Scale bar, 50 µm). (B) Left: Scheme depicting experimental design. Right: Growth of WT, ΔrpoS, and Δcrc on 1% tryptone, 1% agar medium without or with 800 µM PMS. For (A) and (B), experiments were performed with biological triplicates and representative data are shown. (C) Top: Scheme depicting experimental design. Bottom: Quantification of colony-forming units from 3-d-old biofilms, displayed as a percentage of the WT value. Each point represents a biological replicate. P values were calculated using unpaired, two-tailed t tests (***P < 0.001 and ****P < 0.0001).
CCR-pathway Mutants Are Sensitive to Phenazines.
The phenotypes we observed for the ΔrpoS (Fig. 1B) and Δcrc mutants indicate that RpoS and Hfq/Crc inhibit physiological processes–leading to decreased metabolic activity and growth–raising the question of whether RpoS and Hfq/Crc activity have any benefits for PA14 under the conditions used here. In other words, do RpoS and/or Hfq/Crc have positive impacts that outweigh their inhibition of metabolic activity and growth? Because previous work has shown that pyocyanin can be toxic to P. aeruginosa under specific conditions (33), we sought to test whether RpoS and/or Hfq/Crc may protect P. aeruginosa from potential negative effects of phenazines.
We took three approaches to testing whether RpoS and/or Hfq/Crc mitigate the toxicity of phenazines. First, we plated the WT and the Δcrc and ΔrpoS mutants on medium containing the synthetic compound phenazine methosulfate (PMS). PMS is structurally similar to the methylated phenazines produced by P. aeruginosa and we have shown that other mechanisms of P. aeruginosa self-protection are active against this compound (34, 35). We found that Δcrc, but not ΔrpoS, showed a greater inhibition of growth by PMS than the WT (Fig. 4B). The Δcrc phenotype is in line with previous work in P. aeruginosa strain PAO1, which indicated that the Δcrc mutation affects redox homeostasis, tolerance of oxidizing agents, and expression of genes involved in the oxidative stress response (66). In the absence of Crc, target mRNAs whose translation would be repressed by Hfq/Crc in the wild-type background are translated. Evidence suggests that these include proteins that carry out steps in the Entner-Doudoroff and pentose phosphate pathways and that their increased levels in Δcrc lead to effects on redox balancing. In the ΔrpoS background, RpoS-dependent effects on crcZ levels are absent but Crc is still present, and it is possible that other regulators modulate crcZ levels to allow Crc to contribute to oxidative stress resistance in the presence of PMS.
In our second approach, we grew WT and mutant biofilms under standard conditions before harvesting, homogenizing, and plating for colony-forming units (CFUs). We found that ΔrpoS and Δcrc biofilms contain ~50% fewer CFUs than their WT counterparts and that this effect is partially reversed in the phenazine-null background (Fig. 4C). In our third and final approach, we sought to identify which biofilm region may benefit most from RpoS and/or Crc activity. We incubated mature biofilms on medium containing propidium iodide (PI), a dye that shows increased staining in biofilm regions with low viability. We observed increased staining at the biofilm-air interface in ΔrpoS biofilms when compared to those formed by the WT (SI Appendix, Fig. S6). This result is in line with our observation that RpoS inhibits crcZ expression specifically in this zone. Taken together, our findings highlight the long-range effects of localized sigma factor activity and metabolite production on the metabolism and survival of physiologically differentiated subpopulations in biofilms.
Discussion
Besides P. aeruginosa (67), several other pseudomonad species including P. chlororaphis and P. synxantha make the phenazine PCA (38, 68, 69). However, among these species, P. aeruginosa is unusual in its ability to produce methylated phenazines (35). Out of the variety of P. aeruginosa phenazines, those that are methylated have the most dramatic effects on biofilm matrix production and morphogenesis (34), antibiotic tolerance (31, 70), and—as shown in this study—metabolic activity. In this context, it is interesting that, as indicated by our findings, phenazine methylation is linked to global regulatory pathways that respond to carbon source identity and abundance. Phenazine production itself is a carbon- and nitrogen-consuming process (71), but in addition, methylated phenazines—which have relatively high redox potentials and reactivities—in particular have the effect of oxidizing the cytoplasm and activating cellular defense mechanisms including the expression of efflux pumps (70, 72–74). Because nutritional conditions affect the cellular carbon/nitrogen balance and could serve as proxy signals for environments in which these mechanisms contribute to fitness, it could be beneficial for P. aeruginosa to control phenazine methylation so that it occurs when specific types of carbon sources are available (75).
Fig. 5 A and B depict models of the integrated effects of RpoS and the CCR pathway on metabolic activity and survival in PA14 biofilms. The two-component system CbrAB, which functions as the first step in the pseudomonad CCR pathway, is sensitive to carbon source identity but its precise activating signal has not been described. Several studies have suggested that the CbrA-activating signal is intracellular and representative of the carbon/nitrogen balance (52, 64, 76, 77). Once activated, CbrA transfers a phosphoryl group to CbrB, which binds directly to the crcZ promoter and recruits RpoN-containing RNA polymerase to stimulate transcription (52). The sRNA CrcZ binds and sequesters Crc/Hfq, allowing translation of phzM mRNA and thereby promoting phenazine methylation (47). In WT PA14 biofilms (Fig. 1) metabolic activity is supported by oxygen available at the biofilm-air interface and by methylated phenazine available at depth in the hypoxic/anoxic region. In ΔrpoS and Δcrc biofilms (Figs. 1B and 4A) metabolic activity is enhanced throughout the biofilm due to increased production of methylated phenazine. In the Δcrc and ΔrpoS backgrounds, this enhanced metabolic activity apparently comes at a cost as evidenced by increased sensitivity to phenazines (Fig. 4B and C). Experiments in liquid cultures have indicated that the methylated phenazine pyocyanin is toxic to producing cells that are starved for carbon (33). Indeed, the fact that we observe maximal metabolic activity at a depth of ~30 to 50 µm, rather than at the biofilm–air interface, hints at the presence of an electron donor/carbon source gradient emanating from the biofilm base. Crc has also been shown to prevent the accumulation of reactive oxygen species and the activation of oxidative stress pathways (66). This suggests a tradeoff may exist in biofilms between the self-preservation of oxic cells and the potential metabolic activity of cells in hypoxic/anoxic regions of the biofilm, balanced by the dampening of phenazine methylation by RpoS acting via the CCR system.
Fig. 5.
RpoS affects pyocyanin production via the CCR pathway and is simultaneously beneficial for survival and detrimental for metabolic activity in distinct biofilm subzones. (A) Scheme depicting the regulatory relationship between RpoS activity and phzM translation. High RpoS activity indirectly inhibits production of the sRNA CrcZ, liberating the Crc/Hfq complex, which in turn represses phzM translation. When RpoS activity is low, crcZ is transcribed and the CrcZ sRNA binds to and sequesters the Crc/Hfq complex; this allows phzM translation to proceed. (B) Model depicting the differential effects of methylated phenazines in the oxic and hypoxic/anoxic subzones of a biofilm. We propose that methylated phenazines are toxic in the oxic subzone, due to low nutrient availability and the reactivity of phenazines with oxygen, and that cells in this region benefit from RpoS’ activities, including its role in dampening phenazine methylation. Cells in the hypoxic/anoxic subzone, in contrast, benefit from phenazine methylation because methylated phenazine (specifically PYO) serves as an alternate acceptor of cellular reducing equivalents and support metabolic activity. NADH is shown as a representative carrier of cellular reducing equivalents. The blue shading represents the O2 gradient.
We conclude that RpoS promotes expression of genes that enhance survival during carbon source limitation, but also inhibits expression of crcZ, at the biofilm–air interface. The mechanism of this inhibition is unclear but could arise from the effects of the RpoS regulon on P. aeruginosa metabolism; this regulon includes genes involved in primary metabolic pathways and RpoS activity could stimulate a shift in the P. aeruginosa metabolome that is sensed by CbrAB (18, 24). An alternative explanation could be that RpoS inhibits crcZ expression by competing with RpoN for binding to the RNA polymerase. However, previous analysis of sigma factor deletion strains does not show correlative positive associations between expression of RpoS-dependent genes in rpoN-deletion strains, nor vice versa (24), suggesting that this is not the mechanism of RpoS-dependent crcZ repression. Regardless, we have observed that phenazine methylation is limited by an RpoS-dependent inhibition of crcZ expression at the surface of P. aeruginosa biofilms. Because methylated phenazine contributes to metabolic activity in the hypoxic/anoxic biofilm subzone, RpoS’ effects “trickle down” to this region. RpoS- and Crc-dependent inhibition of methylated phenazine production in biofilms may therefore represent a physiological tradeoff that limits toxicity at the biofilm-air interface at the expense of metabolic activity at depth.
Materials and Methods
Strains and Growth Conditions.
All bacterial strains used in this study are listed in SI Appendix, Table S1. For routine liquid culture growth, single colonies of P. aeruginosa UCBPP-PA14 were used to inoculate lysogeny broth (LB) or 1% tryptone, as specified in the text, and shaken at 37 °C, 200 rpm. Precultures for biofilm experiments were grown in liquid LB for 12–16 h, then diluted 1:100, or 1:50 for ΔrpoN backgrounds, in 2 mL LB and grown for 2.5 h at 37 °C to an OD(500 nm) of 0.4–0.6. These liquid cultures were spotted onto 1% tryptone 1% agar plates and incubated at 25 °C. Biofilm morphology was determined as described previously (78) on morphology assay plates made of 1% tryptone and 1% agar and containing 20 µg/mL Congo red and 30 µg/mL Coomassie blue (79). Images of colonies were taken using a Keyence VHX-1000 digital microscope. All planktonic cultures and biofilms were incubated in the dark.
To compare the growth dynamics and activities of reporters for individual strains, experiments were carried out in 96-well plate, black-sided, clear-bottomed plates (Greiner Bio-One). Strains were pregrown in glass culture tubes in liquid LB for 12–16 h, and these precultures were used to inoculate 200 µL of media at a dilution of 1:100 in 96-well plates. Plates were set to incubate at 25 °C with continuous shaking on the medium setting in a Biotek Synergy H1 plate reader. The defined medium used to test individual carbon sources was MOPS minimal medium (50 mM 4 morpholinepropanesulfonic acid (pH 7.2), 43 mM NaCl, 93 mM NH4Cl, 2.2 mM KH2PO4, 1 µg/mL FeSO4·7H2O, and 1 mM MgSO4·7H2O). Growth was assessed by taking OD readings at 500 nm and fluorescence readings at excitation and emission wavelengths of 569 nm and 599 nm, respectively, every 30 min for up to 40 h.
Fluorescent Reporter Strain Construction.
All plasmids used in this study are listed in SI Appendix, Table S2. To create reporters of promoter activity for the crcZ and crc genes, the respective 161-bp and 500-bp promoter regions upstream of the start codon were amplified by PCR using primers (SI Appendix, Table S3) that added KpnI and XhoI (Pcrc) or SpeI and XhoI (PcrcZ) digest sites to the 5′ and 3′ ends of the sequence. Purified PCR products were digested and ligated into the pLD3208 vector at the multiple cloning site, upstream of the mScarlet sequence. The resulting plasmids were transformed into E. coli strain UQ950 and verified by sequencing. They were then moved into P. aeruginosa wild-type or mutant strains, as indicated in SI Appendix, Table S1, using biparental conjugation with E. coli strain S17-1. Selection for recombinants was carried out as previously described (38).
Creation of Deletion Mutant Strains.
All plasmids used in this study are listed in SI Appendix, Table S2. Deletion constructs were made and deletion strains were produced via homologous recombination as previously described (38). Briefly, ~1-kb genomic flanks on either side of the gene to be deleted were amplified via PCR (primers listed in SI Appendix, Table S3) and ligated into digested pMQ30 plasmid (80) using Saccharomyces cerevisiae InvSc1 gap-closing repair. Successful ligations were selected with SD-Ura plates, grown, and the plasmid purified by miniprep. The plasmid was transformed into an E. coli mating strain, BW29427, and mated into the recipient P. aeruginosa strain. Plasmid integration into P. aeruginosa resulted in merodiploids, which are resolved by outgrowth in LB. Strains that successfully lost the integrated plasmid via homologous recombination were selected by growth on sucrose. The final clones were confirmed by PCR with primers flanking the excision site.
Creation of PCN-overproducing Strain.
phzH was amplified using primers LD4528 and LD4529, and the rrnB terminator was amplified from pAKN69 (81) using primers LD4530 and LD4077 such that the two fragments had ~20 bp overlapping sequence similarity. An overlap PCR was performed using primers LD528 and LD4077 and the two fragments (~75 ng each) as template. The resulting fragment (PhzH-coding region with a terminator) was restriction-digest cloned into pAKN69 using SphI and NheI restriction enzymes. Because the coding region of PhzH contains a SphI cut site, this step was completed by first digesting the insert with EcoRI and gel purified the two fragments. The fragment with the beginning of the gene was cut with SphI and the end fragment cut with NheI. Then a three-way ligation was performed to assemble the complete plasmid. The resulting plasmid was transformed into E. coli BW29427, verified by sequencing, and introduced into PA14 using biparental conjugation with E. coli BW29427. Single recombinants were selected on LB agar plates containing 100 μg/mL gentamicin.
Biofilm Thin Sectioning.
Macrocolony preparation and sectioning was performed as previously described with minor modifications (82). Bacterial subcultures were spotted onto 45 mL/15 mL two-layer 1% tryptone 1% agar in 100 mm × 15 mm square plates and grown for 72 h. The biofilms were overlaid with 25 mL of 1% molten agar, solidified, excised into histocassettes, and fixed in 4% paraformaldehyde in PBS for 24 h. After fixing, the samples were washed in a series of ethanol/PBS solutions in which the ethanol concentration was gradually increased to 100%. The samples were then washed with histoclear and subsequently infiltrated with molten paraffin wax. The samples were then placed in paraffin wax in wax molds and solidified overnight. The wax blocks containing the biofilm samples were shaped and the middle of the biofilms sectioned into 10-µm sections. The sections were floated on water and placed onto precharged glass slides. The slides were dried for 48 h, then heat fixed on a 45 °C hot plate for 45 min. After the slides had cooled, they were rehydrated by washing with histoclear followed by a series of ethanol/PBS solutions in which the ethanol concentration was gradually decreased to 0%. Once rehydrated, mounting media and a coverslip were placed on the slide, and the samples were imaged.
Stimulated Raman Scattering Microscopy.
Five microliters of a bacterial subculture was spotted onto 45 mL/15 mL two-layer 1% tryptone 1% agar plates and grown for 72 h; then, the top layer and biofilm were transferred to 2 mL 1% tryptone 1% agar 50% D2O and incubated at 25 °C for 5 h. Where indicated, the plates also contained the specified concentration of commercially available phenazines (pyocyanin: Cayman Chemical Company 10009594, PCN: Apexmol 550-89-0). The biofilms were then thin-sectioned as described above. For SRS microscopy, an integrated laser source (picoEMERALD, Applied Physics & Electronics, Inc.) was used to produce both a Stokes beam (1,064 nm, 6 ps, intensity modulated at 8 MHz) and a tunable pump beam (720–990 nm, 5–6 ps) at an 80-MHz repetition rate. The spectral resolution of SRS is FWHM = 6–7 cm−1. These two spatially and temporally overlapped beams with optimized near-IR throughput were coupled into an inverted multiphoton laser-scanning microscope (FV1200MPE, Olympus). Both beams were focused on the biofilm samples through a 25X water objective (XLPlan N, 1.05 N.A. MP, Olympus) and collected with a high N.A. oil condenser lens (1.4 N.A., Olympus) after the sample. By removing the Stokes beam with a high O.D. bandpass filter (890/220 CARS, Chroma Technology), the pump beam is detected with a large area Si photodiode (FDS1010, Thorlabs) reverse-biased by 64 DC voltage. The output current of the photodiode was electronically filtered (KR 2724, KR electronics), terminated with 50 Ω, and demodulated with a lock-in amplifier (HF2LI, Zurich Instruments) to achieve near shot-noise-limited sensitivity. The stimulated Raman loss signal at each pixel was sent to the analog interface box (FV10-ANALOG, Olympus) of the microscope to generate the image. All images were acquired with 80 µs time constant at the lock-in amplifier and 100 µs pixel dwell time (~27 s per frame of 512 × 512 pixels). Measured after the objectives, 40 mW pump power and 120 mW Stokes beam were used to image the carbon-deuterium 2,183 cm−1 and off-resonance 2,004 cm−1 channels.
Image analysis was performed using Fiji. For better visualization, all SRS sections were brightened in Fiji to a maximum brightness value of 800. Quantifications reflect the unmodified signal.
Oxygen Profiling of Biofilms.
A 25-µm-tip oxygen microsensor (Unisense OX-25) was used to measure oxygen concentrations along the depth of biofilms grown for 72 h at 25 °C. Two-point calibration was used to calibrate the oxygen microsensor. The first calibration point was atmospheric oxygen using a calibration chamber (Unisense CAL300) containing water continuously bubbled with air. The second calibration point was a “zero” point using an anoxic solution of 0.1 M sodium ascorbate 0.1 M NaOH. Oxygen measurements were taken throughout the depth of the biofilm using a measurement time of 3 s and a wait time between measurements of 3 s. The step size between measurements was 5 µm and controlled by a micromanipulator (Unisense MM33). Profiles were recorded using a multimeter (Unisense) and the SensorTrace Profiling software (Unisense).
Phenazine Detection by HPLC and Spectrophotometry.
Ten μL of bacterial subculture was spotted onto 4 mL of 1% tryptone 1% agar in 30-mm circular petri plates and grown for 72 h at 25 °C. The biofilm and agar were placed into 5 mL of 100% methanol and nutated overnight at room temperature to extract the phenazines. To measure the amount of aeruginosins, 600 μL of the methanol extract was added to 600 μL of chloroform, briefly vortexed, and allowed to settle into fractions. After separation, 100 μL of the top aqueous fraction was put into a 96-well plate and read in a plate reader. To quantify aeruginosin levels, the samples were excited at 520 nm and the emission read at 620 nm. The results presented are all normalized relative to the signal of PA14 biofilms. To measure levels of PCA, PCN, and pyocyanin, the methanol extract was filtered through 0.22-μm cellulose acetate Spin-X centrifuge tube filters (Costar); then, 200 μL was transferred to HPLC vials for analysis on an Agilent 1100 HPLC system. A 50-µL sample was loaded on a Kinetex Biphenyl reverse phase column (Phenomenex 00F-4622-E0). HPLC-based separation was carried out using a gradient of water-0.02% formic acid (solvent A) to methanol-0.02% formic acid (solvent B) at a flow rate of 0.4 mL/min using the following protocol: linear gradient from 40% to 100% solvent B for 30 min followed by 5 min of 100% solvent B. The total method time was 35 min with a 5-min postwash of 100% solvent B. The resulting peaks at 366 nm were identified and quantified relative to samples of purified PCA, PCN, and pyocyanin run in known concentrations. The retention times for pyocyanin, PCN, and PCA are 9.5 min, 26 min, and 31.5 min, respectively.
PMS Sensitivity Assay.
Precultures of strains of interest were grown for 16 h at 37 °C, 200 rpm, and then subcultured at a dilution of 30 µL into 3 mL LB medium in VWR 13 x 100 mm glass culture tubes. Subcultures were grown for about 2.5 h to an OD (500 nm) of approximately 0.55, then normalized to an OD (500 nm) of 0.5 before serial dilution. Five microliters of each dilution was spotted onto 10-cm square plates containing 60 mL of 1% tryptone 1% agar medium with or without 800 µM PMS (Fisher Scientific, ICN10095510). Biofilms were incubated for 48 h at 25 °C before scanning on an Epson Expression 11000XL photo scanner. The brightness of all images was adjusted in the same way to a value of 150 using Adobe Photoshop for ease of visualization.
Colony-Forming Unit Assay.
Macrocolony biofilms were grown for 3 d on 1% tryptone 1% agar. Each biofilm was transferred to a 2-mL tube with a sealing O-ring (Benchmark Scientific D1031-T20) containing 1 mL of phosphate-buffered saline and 0.5 g of ceramic beads (Fisher Scientific 15-340-159). Samples were homogenized using an Omni Bead Ruptor 12 (Omni 19-050) on high for 90 s at 4 °C. Samples were left on ice for 10 min to allow bubbles to clear. Serial dilutions in PBS were performed until the countable dilution of 10−6. Ten microliters were spotted on 1% tryptone 1.5% agar plates, which were tilted vertically to allow the spot to run down the plate and thereby increase the growth area. After 16 h of growth at 37 °C, single colonies were counted and recorded.
Propidium Iodide Staining.
Five microliters of a bacterial subculture was spotted onto 45 mL/15 mL two-layer 1% tryptone 1% agar plates were grown for 72 h, then the top layer and biofilm was transferred to 30 mL of 1% tryptone 1% agar 50 µM propidium iodide (Sigma-Aldrich P4864-10ML) and incubated at 25 °C for 6 h. The biofilms were then thin-sectioned as described above and imaged on a ZEISS Axio Zoom.V16.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
This work was supported by NIH/NIAID grant R01AI103369 to L.E.P.D. and NIH/NIBIB grant R01EB029523 to W.M.
Author contributions
C.R.E., M.K.S., M.W., A.P.-W., and L.E.P.D. designed research; C.R.E., M.K.S., S.A.T., M.W., L.C.F., and H.D. performed research; W.M. contributed new reagents/analytic tools; C.R.E., M.K.S., S.A.T., M.W., L.C.F., H.D., A.P.-W., and L.E.P.D. analyzed data; and C.R.E., M.K.S., A.P.-W., and L.E.P.D. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Evans C. R., Kempes C. P., Price-Whelan A., Dietrich L. E. P., Metabolic heterogeneity and cross-feeding in bacterial multicellular systems. Trends Microbiol. 28, 732–743 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jo J., Price-Whelan A., Dietrich L. E. P., Gradients and consequences of heterogeneity in biofilms. Nat. Rev. Microbiol. 20, 593–607 (2022), 10.1038/s41579-022-00692-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Gestel J., Vlamakis H., Kolter R., Division of labor in biofilms: The ecology of cell differentiation. Microbiol Spectr 3, MB–0002–2014 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Williamson K. S., et al. , Heterogeneity in Pseudomonas aeruginosa biofilms includes expression of ribosome hibernation factors in the antibiotic-tolerant subpopulation and hypoxia-induced stress response in the metabolically active population. J. Bacteriol. 194, 2062–2073 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart P. S., Franklin M. J., Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 6, 199–210 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Lim Z.-F., Ma P. C., Emerging insights of tumor heterogeneity and drug resistance mechanisms in lung cancer targeted therapy. J. Hematol. Oncol. 12, 134 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou K.-T., et al. , A segmentation clock patterns cellular differentiation in a bacterial biofilm. Cell 185, 145–157.e13 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feklístov A., Sharon B. D., Darst S. A., Gross C. A., Bacterial sigma factors: A historical, structural, and genomic perspective. Annu. Rev. Microbiol. 68, 357–376 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Missiakas D., Raina S., The extracytoplasmic function sigma factors: Role and regulation. Mol. Microbiol. 28, 1059–1066 (1998). [DOI] [PubMed] [Google Scholar]
- 10.Klauck G., Serra D. O., Possling A., Hengge R., Spatial organization of different sigma factor activities and c-di-GMP signalling within the three-dimensional landscape of a bacterial biofilm. Open Biol. 8, 180066 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knobloch J. K., et al. , Biofilm formation by Staphylococcus epidermidis depends on functional RsbU, an activator of the sigB operon: Differential activation mechanisms due to ethanol and salt stress. J. Bacteriol. 183, 2624–2633 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall C. W., et al. , Pseudomonas aeruginosa biofilm antibiotic resistance gene ndvB expression requires the RpoS stationary-phase sigma factor. Appl. Environ. Microbiol. 84, e02762-17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bazire A., et al. , The sigma factor AlgU plays a key role in formation of robust biofilms by nonmucoid Pseudomonas aeruginosa. J. Bacteriol. 192, 3001–3010 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helmann J. D., Where to begin? Sigma factors and the selectivity of transcription initiation in bacteria. Mol. Microbiol. 112, 335–347 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schellhorn H. E., Function, evolution, and composition of the RpoS regulon in Escherichia coli. Front. Microbiol. 11, 560099 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mika F., Hengge R., Small RNAs in the control of RpoS, CsgD, and biofilm architecture of Escherichia coli. RNA Biol. 11, 494–507 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gottesman S., Trouble is coming: Signaling pathways that regulate general stress responses in bacteria. J. Biol. Chem. 294, 11685–11700 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuster M., Hawkins A. C., Harwood C. S., Greenberg E. P., The Pseudomonas aeruginosa RpoS regulon and its relationship to quorum sensing. Mol. Microbiol. 51, 973–985 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Rodríguez-Rojas A., Oliver A., Blázquez J., Intrinsic and environmental mutagenesis drive diversification and persistence of Pseudomonas aeruginosa in chronic lung infections. J. Infect. Dis. 205, 121–127 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Maciá M. D., et al. , Hypermutation is a key factor in development of multiple-antimicrobial resistance in Pseudomonas aeruginosa strains causing chronic lung infections. Antimicrob. Agents Chemother. 49, 3382–3386 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciofu O., Riis B., Pressler T., Poulsen H. E., Høiby N., Occurrence of hypermutable Pseudomonas aeruginosa in cystic fibrosis patients is associated with the oxidative stress caused by chronic lung inflammation. Antimicrob. Agents Chemother. 49, 2276–2282 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliver A., Cantón R., Campo P., Baquero F., Blázquez J., High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science 288, 1251–1254 (2000). [DOI] [PubMed] [Google Scholar]
- 23.Potvin E., Sanschagrin F., Levesque R. C., Sigma factors in Pseudomonas aeruginosa. FEMS Microbiol. Rev. 32, 38–55 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Schulz S., et al. , Elucidation of sigma factor-associated networks in Pseudomonas aeruginosa reveals a modular architecture with limited and function-specific crosstalk. PLoS Pathog. 11, e1004744 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang T., et al. , An atlas of the binding specificities of transcription factors in Pseudomonas aeruginosa directs prediction of novel regulators in virulence. eLife 10, e61885 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jo J., Price-Whelan A., Cornell W. C., Dietrich L. E. P., Interdependency of respiratory metabolism and phenazine-associated physiology in Pseudomonas aeruginosa PA14. J. Bacteriol. 202, e00700-19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin Y.-C., et al. , Phenazines regulate nap-dependent denitrification in Pseudomonas aeruginosa biofilms. J. Bacteriol. 200, e00031-18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito A., May T., Taniuchi A., Kawata K., Okabe S., Localized expression profiles of rpoS in Escherichia coli biofilms. Biotechnol. Bioeng. 103, 975–983 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Suh S. J., et al. , Effect of rpoS mutation on the stress response and expression of virulence factors in Pseudomonas aeruginosa. J. Bacteriol. 181, 3890–3897 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y., Kern S. E., Newman D. K., Endogenous phenazine antibiotics promote anaerobic survival of Pseudomonas aeruginosa via extracellular electron transfer. J. Bacteriol. 192, 365–369 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schiessl K. T., et al. , Phenazine production promotes antibiotic tolerance and metabolic heterogeneity in Pseudomonas aeruginosa biofilms. Nat. Commun. 10, 762 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saunders S. H., et al. , Extracellular DNA promotes efficient extracellular electron transfer by pyocyanin in Pseudomonas aeruginosa biofilms. Cell 182, 919–932.e19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meirelles L. A., Newman D. K., Both toxic and beneficial effects of pyocyanin contribute to the lifecycle of Pseudomonas aeruginosa. Mol. Microbiol. 110, 995–1010 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakhtah H., et al. , The Pseudomonas aeruginosa efflux pump MexGHI-OpmD transports a natural phenazine that controls gene expression and biofilm development. Proc. Natl. Acad. Sci. U.S.A. 113, E3538–E3547 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sporer A. J., et al. , Pseudomonas aeruginosa PumA acts on an endogenous phenazine to promote self-resistance. Microbiology 164, 790–800 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basta D. W., Bergkessel M., Newman D. K., Identification of fitness determinants during energy-limited growth arrest in Pseudomonas aeruginosa. MBio 8, e01170-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu K. D., Franklin M. J., Park C. H., McFeters G. A., Stewart P. S., Gene expression and protein levels of the stationary phase sigma factor, RpoS, in continuously-fed Pseudomonas aeruginosa biofilms. FEMS Microbiol. Lett. 199, 67–71 (2001). [DOI] [PubMed] [Google Scholar]
- 38.Jo J., Cortez K. L., Cornell W. C., Price-Whelan A., Dietrich L. E., An orphan cbb3-type cytochrome oxidase subunit supports Pseudomonas aeruginosa biofilm growth and virulence. eLife 6, e30205 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dayton H., et al. , Cell arrangement impacts metabolic activity and antibiotic tolerance in Pseudomonas aeruginosa biofilms. bioRxiv [Preprint] (2023). 10.1101/2023.06.20.545666 (Accessed 28 August 2023). [DOI] [PMC free article] [PubMed]
- 40.Hu F., Shi L., Min W., Biological imaging of chemical bonds by stimulated Raman scattering microscopy. Nat. Methods 16, 830–842 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Recinos D. A., et al. , Redundant phenazine operons in Pseudomonas aeruginosa exhibit environment-dependent expression and differential roles in pathogenicity. Proc. Natl. Acad. Sci. U.S.A. 109, 19420–19425 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mavrodi D. V., et al. , Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1. J. Bacteriol. 183, 6454–6465 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abu E. A., et al. , Cyclic voltammetric, fluorescence and biological analysis of purified aeruginosin A, a secreted red pigment of Pseudomonas aeruginosa PAO1. Microbiology 159, 1736–1747 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Hansford G. S., Holliman F. G., Herbert R. B., Pigments of Pseudomonas species. IV. In vitro and in vivo conversion of 5-methylphenazinium-1-carboxylate into aeruginosin A. J. Chem. Soc. Perkin 1, 103–105 (1972). [DOI] [PubMed] [Google Scholar]
- 45.Wang K., et al. , Overexpression of phzM contributes to much more production of pyocyanin converted from phenazine-1-carboxylic acid in the absence of RpoS in Pseudomonas aeruginosa. Arch. Microbiol. 202, 1507–1515 (2020), 10.1007/s00203-020-01837-8. [DOI] [PubMed] [Google Scholar]
- 46.Dietrich L. E. P., Teal T. K., Price-Whelan A., Newman D. K., Redox-active antibiotics control gene expression and community behavior in divergent bacteria. Science 321, 1203–1206 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang J., Sonnleitner E., Ren B., Xu Y., Haas D., Catabolite repression control of pyocyanin biosynthesis at an intersection of primary and secondary metabolism in Pseudomonas aeruginosa. Appl. Environ. Microbiol. 78, 5016–5020 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sonnleitner E., Bläsi U., Regulation of Hfq by the RNA CrcZ in Pseudomonas aeruginosa carbon catabolite repression. PLoS Genet. 10, e1004440 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kambara T. K., Ramsey K. M., Dove S. L., Pervasive targeting of nascent transcripts by Hfq. Cell Rep. 23, 1543–1552 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corona F., Reales-Calderón J. A., Gil C., Martínez J. L., The development of a new parameter for tracking post-transcriptional regulation allows the detailed map of the Pseudomonas aeruginosa Crc regulon. Sci. Rep. 8, 16793 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolff J. A., MacGregor C. H., Eisenberg R. C., Phibbs P. V. Jr., Isolation and characterization of catabolite repression control mutants of Pseudomonas aeruginosa PAO. J. Bacteriol. 173, 4700–4706 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sonnleitner E., Abdou L., Haas D., Small RNA as global regulator of carbon catabolite repression in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U.S.A. 106, 21866–21871 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kong W., et al. , Dual GGDEF/EAL-domain protein RmcA controls the Type III secretion system of Pseudomonas aeruginosa by interaction with CbrB. ACS Infect. Dis. 8, 2441–2450 (2022). [DOI] [PubMed] [Google Scholar]
- 54.Mould D. L., Stevanovic M., Ashare A., Schultz D., Hogan D. A., Metabolic basis for the evolution of a common pathogenic Pseudomonas aeruginosa variant. eLife 11, e76555 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pusic P., et al. , Cross-regulation by CrcZ RNA controls anoxic biofilm formation in Pseudomonas aeruginosa. Sci. Rep. 6, 39621 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farewell A., Kvint K., Nyström T., Negative regulation by RpoS: A case of sigma factor competition. Mol. Microbiol. 29, 1039–1051 (1998). [DOI] [PubMed] [Google Scholar]
- 57.Lenz A. P., Williamson K. S., Pitts B., Stewart P. S., Franklin M. J., Localized gene expression in Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 74, 4463–4471 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stewart P. S., et al. , Contribution of stress responses to antibiotic tolerance in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 59, 3838–3847 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pérez-Osorio A. C., Williamson K. S., Franklin M. J., Heterogeneous rpoS and rhlR mRNA levels and 16S rRNA/rDNA (rRNA gene) ratios within Pseudomonas aeruginosa biofilms, sampled by laser capture microdissection. J. Bacteriol. 192, 2991–3000 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bellin D. L., et al. , Electrochemical camera chip for simultaneous imaging of multiple metabolites in biofilms. Nat. Commun. 7, 10535 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abdou L., Chou H.-T., Haas D., Lu C.-D., Promoter recognition and activation by the global response regulator CbrB in Pseudomonas aeruginosa. J. Bacteriol. 193, 2784–2792 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cai Z., et al. , RpoN regulates virulence factors of Pseudomonas aeruginosa via modulating the PqsR quorum sensing regulator. Int. J. Mol. Sci. 16, 28311–28319 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bharwad K., Rajkumar S., Rewiring the functional complexity between Crc, Hfq and sRNAs to regulate carbon catabolite repression in Pseudomonas. World J. Microbiol. Biotechnol. 35, 140 (2019). [DOI] [PubMed] [Google Scholar]
- 64.Valentini M., et al. , Hierarchical management of carbon sources is regulated similarly by the CbrA/B systems in Pseudomonas aeruginosa and Pseudomonas putida. Microbiology 160, 2243–2252 (2014). [DOI] [PubMed] [Google Scholar]
- 65.Pei X. Y., et al. , Architectural principles for Hfq/Crc-mediated regulation of gene expression. eLife 8, e43158 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Corona F., Martínez J. L., Nikel P. I., The global regulator Crc orchestrates the metabolic robustness underlying oxidative stress resistance in Pseudomonas aeruginosa. Environ. Microbiol. 21, 898–912 (2019). [DOI] [PubMed] [Google Scholar]
- 67.Valot B., et al. , What it takes to be a Pseudomonas aeruginosa? The core genome of the opportunistic pathogen updated. PLoS ONE 10, e0126468 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Biessy A., Filion M., Phenazines in plant-beneficial Pseudomonas spp.: Biosynthesis, regulation, function and genomics. Environ. Microbiol. 20, 3905–3917 (2018). [DOI] [PubMed] [Google Scholar]
- 69.Garrido-Sanz D., et al. , Classification of isolates from the Pseudomonas fluorescens complex into phylogenomic groups based in group-specific markers. Front. Microbiol. 8, 413 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meirelles L. A., Perry E. K., Bergkessel M., Newman D. K., Bacterial defenses against a natural antibiotic promote collateral resilience to clinical antibiotics. PLoS Biol. 19, e3001093 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blankenfeldt W., Parsons J. F., The structural biology of phenazine biosynthesis. Curr. Opin. Struct. Biol. 29, 26–33 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dietrich L. E. P., Price-Whelan A., Petersen A., Whiteley M., Newman D. K., The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol. Microbiol. 61, 1308–1321 (2006). [DOI] [PubMed] [Google Scholar]
- 73.Dietrich Lars E., et al. , Bacterial community morphogenesis is intimately linked to the intracellular redox state. J. Bacteriol. 195, 1371–1380 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rozner M., et al. , Rewiring of gene expression in Pseudomonas aeruginosa during diauxic growth reveals an indirect regulation of the MexGHI-OpmD efflux pump by Hfq. Front. Microbiol. 13, 919539 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martínez J. L., Rojo F., Metabolic regulation of antibiotic resistance. FEMS Microbiol. Rev. 35, 768–789 (2011). [DOI] [PubMed] [Google Scholar]
- 76.Nishijyo T., Haas D., Itoh Y., The CbrA-CbrB two-component regulatory system controls the utilization of multiple carbon and nitrogen sources in Pseudomonas aeruginosa. Mol. Microbiol. 40, 917–931 (2001). [DOI] [PubMed] [Google Scholar]
- 77.Monteagudo-Cascales E., Santero E., Canosa I., The regulatory hierarchy following signal integration by the CbrAB two-component system: Diversity of responses and functions. Genes 13, 375 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Okegbe C., et al. , Electron-shuttling antibiotics structure bacterial communities by modulating cellular levels of c-di-GMP. Proc. Natl. Acad. Sci. U.S.A. 114, E5236–E5245 (2017), 10.1073/pnas.1700264114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Friedman L., Kolter R., Genes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilms. Mol. Microbiol. 51, 675–690 (2004). [DOI] [PubMed] [Google Scholar]
- 80.Shanks R. M. Q., Caiazza N. C., Hinsa S. M., Toutain C. M., O’Toole G. A., Saccharomyces cerevisiae-based molecular tool kit for manipulation of genes from Gram-negative bacteria. Appl. Environ. Microbiol. 72, 5027–5036 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lambertsen L., Sternberg C., Molin S., Mini-Tn7 transposons for site-specific tagging of bacteria with fluorescent proteins. Environ. Microbiol. 6, 726–732 (2004). [DOI] [PubMed] [Google Scholar]
- 82.Cornell W. C., et al. , Paraffin embedding and thin sectioning of microbial colony biofilms for microscopic analysis. J. Vis. Exp. 113, 57196 (2018), 10.3791/57196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.