Abstract
Using a life course approach, we examined how sexuality is related to cognitive function for partnered older adults. We utilized longitudinal data from two rounds of the National Social Life, Health, and Aging Project (NSHAP) to analyze 1,683 respondents. Cognitive function was measured using a continuous Montreal Cognitive Assessment (MoCA) score. We considered both sexual frequency and sexual quality (i.e., physical pleasure, emotional satisfaction). We estimated cross-lagged models to consider the potential reciprocal relationship between sexuality and cognitive function. Results indicated that sexuality was not related to later cognitive function in the total sample, but the pattern varied by age and gender. For adults aged 62–74, better sexual quality (i.e., feelings of physical pleasure and emotional satisfaction) was related to better cognitive functioning, while for those aged 75–90, more frequent sex was related to better cognitive functioning. Feelings of physical pleasure were related to better cognitive functioning for men but not women. There was no evidence of cognitive functioning being related to later sexuality. The findings highlight the importance of age and gender in modifying the link between sexuality and cognition in later life.
Keywords: cognition, dementia, sexual frequency, sexual quality, NSHAP
Introduction
A large segment of older adults in the U.S. are living with poor cognitive functioning. In 2020, over 12 million Americans aged 65 and older had mild cognitive impairment (MCI) and among whom, roughly 6.1 million progressed into Alzheimer’s dementia (Rajan et al., 2021). Dementia, with Alzheimer’s as the most common cause, is a progressive disease where cognitive and emotional capabilities decline to the point where daily functioning is impacted (Alzheimer’s Association, 2021). It is preceded by MCI, a period characterized by “subtle cognitive changes that do not interfere with everyday activities” (Alzheimer’s Association, 2021, p. 330). The prevalence of poor cognitive health has encouraged research into modifiable risk factors that may prevent, delay, and slow the progression of cognitive impairment. Involvement in social activities is one way suggested to improve cognition and reduce the risk of dementia (Prince et al., 2014). Yet, less is clear about how sexual activities, an intimate type of social activity, and their quality may shape cognitive health in later life.
The current study examined how sex, defined as any “mutually voluntary activity with another person that involves sexual contact, whether or not intercourse or orgasm occurs” (Lindau et al., 2007, p. 763), is related to cognitive function among community-residing, partnered older adults. In this study, we recruited older adults who were 62 years old and older, following sampling strategies in the dataset (O’Muircheartaigh et al., 2014; O’Muircheartaigh et al., 2021). Guided by the gendered life course perspective, we used nationally representative, longitudinal data from the National Social Life, Health, and Aging Project (NSHAP) to address two main research questions: (1) are sexual frequency and sexual quality related to later cognitive function? and (2) does this relationship vary by age or gender?
The growing projections and substantial costs of dementia highlight the importance of understanding behaviors related to cognitive health. Alzheimer’s disease cases are expected to more than double in the next forty years in the United States if current rates continue (Rajan et al., 2021), and the various health care costs for dementia among adults 65 and older total to $355 billion (Alzheimer’s Association, 2021). Our findings help to contextualize a multifaceted understanding of healthy aging and speak to clinical practices and policy decisions regarding cognitive health, and in particular how it may be related to sexual life, an often overlooked area for older adults.
Sexuality among Older Adults
Many adults continue to be sexually active in later life (Lindau et al., 2007), although frequency of sex does decrease with age (Karraker et al., 2011). Among older adults, sexual inactivity is often influenced by partners availability due to some adults outliving their partner (DeLamater & Moorman, 2007). However, sexual satisfaction continues, and can even grow in later years and some older adults report increased sexual quality, likely because they have honed sexual preferences and skills within their relationship (Forbes et al., 2017; Lindau et al., 2007). A qualitative comparison of couples at midlife versus later life revealed that the older couples had deeper emotional and physical connections despite a decrease in their sexual frequency (Lodge & Umberson, 2012). Data from a representative U.S. sample of older adults further indicated that most older adults, including those with and without cognitive impairment, report their sexual lives to be physically pleasurable and emotionally satisfying (Lindau et al., 2018).
Sexuality, both sexual frequency and quality, may shape health in later life. Sexual activity has been shown to be beneficial for a variety of mental and physical health outcomes, including cardiovascular health, psychological distress, happiness, and subjective well-being (Lee et al., 2016; Liu et al., 2016; Zhang & Liu, 2020). Sexual quality is also suggested to be important for older adults’ physical and mental health. For example, a study using a nationally representative sample of German adults aged 40–95 found that sexual satisfaction was related to physical health, including better self-rated health and less physical illness (Buczak-Stec et al., 2021). Furthermore, longitudinal research using a representative U.S. sample of adults aged 57 and older at baseline found that greater sexual quality was beneficial for mental health, specifically better self-rated mental health, greater happiness, and less psychological distress (Zhang & Liu, 2020).
Sexuality and Cognition: Limited Empirical Evidence
A few studies have suggested that sexuality is related to cognition among older adults, but most of these studies were based either on non-representative samples or samples outside the U.S. (Hartmans et al., 2014). For instance, a cross-sectional analysis of 73 adults aged 50–83 in the United Kingdom found that older men and women who had sex weekly performed better on cognitive functioning tests than their counterparts who were sexually inactive (Wright et al., 2019). Another U.S. study of 155 adults aged 55 and older found that those with higher sexual satisfaction at the baseline survey were less likely than those with lower sexual satisfaction to develop MCI or dementia over the 10-year follow-up (Smith et al., 2021). One nationally representative study in England examined over 6,000 adults 50 and older and found that both more frequent sex and greater emotional closeness were associated with better memory performance at baseline and two years later (Allen, 2018).
The only studies that we are aware of that used nationally representative U.S. samples to study sexuality and cognition were by Lindau et al. (2018) and Waite et al. (2022). Lindau and colleagues’ (2018) analysis was cross-sectional, with an emphasis on variations in prevalence of sexuality across cognitive status groups. While informative, this study did not consider the longitudinal process of sexuality shaping cognition. Waite and colleagues (2022) did test two waves of NSHAP data and found similar likelihoods of being sexually active across cognitive states. Yet, they only focused on one partnered sexuality measure (i.e., being sexually active) and did not consider how different stages of later life may contribute to differences in the relationships between sexuality and cognition. Most importantly, the key focus of Waite et al.’s (2022) study was how cognition may affect later sexuality, while the present study used a different framework to perceive the relationship between sexuality and cognition. Specifically, we worked from a life course perspective to assess how sexuality may shape later-life cognitive function over time while taking into account potential reverse causality and paying special attention to age and gender variations.
A Gendered Life Course Perspective on Sexuality Links to Cognition
The changes people experience as they age have an effect on their life, including their sexual life and their health (Liu et al., 2016). The life course perspective emphasizes that human development occurs across life trajectories and is influenced by sociohistorical and spatial contexts, transitions, and the linked lives with whom individuals are interrelated (Elder, 1998). As people move through the life course, they experience changes in both sexuality and cognition. Older adults may experience decreases in or changes to their sexual behaviors (Karraker et al., 2011), yet sexual ability continues into old age and many older adults still report satisfaction with their sexual lives (Lodge & Umberson, 2012). While research indicates that the risk of dementia increases with age (Alzheimer’s Association, 2021), sexual activity continues for some older adults with poor cognitive functioning. Lindau et al. (2018) found that partnered sexual activity continued in over 60% of adults with dementia aged 62–69, over 50% of adults with dementia aged 70–79, and over 40% of adults with dementia aged 80–91.
We anticipate that sex is related to cognition for several reasons. First, sex is a type of physical exercise (Butt, 1990), and exercise is linked to better cognitive performance by increasing the blood flow and supply in the brain, reducing inflammation in the body, and increasing proteins that induce neuron growth and survival (Kirk-Sanchez & McGough, 2014). Physical activity promotes cardiovascular health through these pathways which can protect against neurodegenerative diseases and brain aging to help maintain older adults’ cognition (Kirk-Sanchez & McGough, 2014). Second, stress is an underlying factor related to both cognition and sex. Stress prevents the new formation of neurons in the hippocampus, an area of the brain associated with memory (Mirescu & Gould, 2006). For adults in satisfying relationships, sex has been found to relieve stress (Ein-Dor & Hirschberger, 2012). Older adults who enjoy satisfying sexual activities may experience decreased stress that may in turn protect neurogenesis. Third, sex may improve cognitive function through the release of the neurotransmitter dopamine. Dopamine is produced during sexual arousal and orgasm (Blum et al., 2012), and more sexual activity is related to greater dopamine release (Melis & Argiolas, 1995). In older adults, dopamine has been found to improve episodic memory (Chowdhury et al., 2012). Taken together, as people move across the life course and into old age, sex may be one way to combat atrophy in the brain due to its ability to decrease cardiovascular risk, reduce stress, and release dopamine. Considering these relationships, we formed the following hypotheses:
Hypothesis 1: Older adults who have more frequent sex will have better subsequent cognitive function than those who have less frequent sex.
Hypothesis 2: Older adults with better sexual quality will have better subsequent cognitive function than those with worse sexual quality.
Age Differences
Life course scholars often adopt age as a key indicator for life course stages (Elder & O’Rand, 1995). With adults living longer, some researchers have distinguished between different phases of later life, for example younger-old versus older-old (Min et al., 2023; Neugarten, 1974). In this study, we compared adults who were aged 62–74 (i.e. younger-old) to those who were aged 75–90 (i.e. older-old). Sexuality’s effect on cognition may be contingent on age because (dis)advantages may have cumulative effects on health and cognition (Dannefer, 2003). There may be age differences in the relationship between sex and cognition because sexual activity does decrease with age (Lindau et al., 2007), and the anticipated linkages between sex and cognition may not be as pronounced with lower sexual activity. However, sexual quality may enhance with age (Lodge & Umberson, 2012), so the cognitive benefits of decreased stress from a satisfying relationship may be seen among older adults. While older adults with poor cognitive function can be sexually active, there is limited research on age differences in how sexuality may be linked to subsequent cognitive function. Data from two waves of the English Longitudinal Study of Ageing (ELSA) yielded no evidence for age variation in the relationship between feeling emotionally close during sex and memory performance (Allen, 2018). Interview data from couples aged 55–87 where one partner had dementia revealed that younger couples attributed the changes in their sexual lives to dementia, while older couples felt that the broader context of their aging experience affected their sexual relationship (Sandberg, 2020). This qualitative study (i.e., Sandberg, 2020) indicates that age may influence the relationship between sexuality and cognition. Given how sexuality changes in later life and that cognitive impairment is a progressive disease, we formulated a third hypothesis:
Hypothesis 3: The relationship between sexuality and cognitive function will be stronger at older ages.
Gender Differences
The gendered life course perspective recognizes that an accumulation of gendered experiences, from education to employment to retirement, can result in different health and relationship experiences for older men and women (Moen, 2001). Older cohorts were socialized to have certain beliefs about sex, e.g., that sexuality should only occur within marriage (Karraker et al., 2011). Sexual agency also changes at different life stages. In later life, sex is decoupled from pregnancy but there may be new caregiving responsibilities for one’s spouse which detracts from the desire to be sexually intimate with them (Drummond et al., 2013), and lowers sexual satisfaction, especially when caring for a partner with poor cognitive functioning (Davies et al., 2010; Nogueira et al., 2017). Moreover, although incidence rates of dementia are similar for both men and women in the U.S. (Alzheimer’s Association, 2021), representative data on older adults with dementia suggested that a greater percentage of men with dementia (59%) than women with dementia (51%) were sexually active (Lindau et al., 2018). Furthermore, more women than men are caregiving for a spouse with dementia (Erol et al., 2015), and women experience a greater caregiver burden than men (Pöysti et al., 2012; Xiong et al., 2020). Older women also report lower sexual quality than older men (Træen et al., 2019). All of these gender differences may modify the general beneficial linkages between sex and cognition we anticipated.
The current empirical evidence for gender differences in the link between sexuality and cognition suggests that this relationship appears to be more pronounced for men than women. For example, a cross-sectional (Wright & Jenks, 2016) and a longitudinal (Smith et al., 2020) analysis of ELSA both found that sexual activity was related to memory recall for older men but not older women. In a U.S. study, older men with dementia reported significantly lower sexual satisfaction compared to the normal cognitive group; there were no differences for women (Lindau et al., 2018). Yet, Allen (2018) found no gender differences in sexual frequency and memory using two waves of ELSA data. We drew on the limited evidence of gender differences in sexuality and cognition to form a fourth hypothesis:
Hypothesis 4: The relationship between sexuality and cognitive function will be stronger for men than for women.
Reverse Causality
Although our main framework, guided by the life course perspective, suggests that sexuality may shape cognition, it is possible that cognitive function may also contribute to variations in sexuality. Only a few studies have examined this relationship. In their review article of eight studies, Hartmans et al. (2014) found that poor cognitive functioning was related to less sexual activity among older adults, although the overall results were inconclusive. Waite and colleagues’ (2022) representative data analysis found that older adults with early dementia were less likely to have masturbated, but there was no relationship between cognitive function and being sexually active. Focus group data from 23 spousal caregivers for people with mild memory or cognitive impairment indicated that most experienced decreased sexual activity (Davies et al., 2010). Evidence from qualitative studies of couples where one partner is cognitively impaired indicates how dementia influences sexual quality. For example, interview data from couples in Brazil (Nogueira et al., 2017) and Finland (Eloniemi-Sulkava et al., 2002) showed that spouses reported experiencing a negative sexual change, including unattended sexual desires and sexual dissatisfaction, which correspond to the onset or progression of dementia in their partner. Given the possibility of cognitive function affecting sexuality suggested in the literature, we also explored this potential reverse causality in our analysis.
Method
Data
We used data from the second (2010–2011) and third (2015–2016) rounds of the National Social Life, Health, and Aging Project (NSHAP) to consider longitudinal relationships between sexuality and cognition, guided by the gendered life course approach. NSHAP is a nationally representative sample of community-dwelling older adults in the United States. The first round of data (2005–2006) included 3,005 adults aged 57–85 and oversampled for African Americans and Latinos. Round 2 surveyed 3,377 respondents, including 2,261 respondents from Round 1 and 1,116 new interviews for partners and those who declined to participate in Round 1 (Waite et al., 2014). Round 3 surveyed 4,777 respondents, including 2,368 new respondents and 2,409 respondents from Round 2 (Waite et al., 2017). Because consistent cognitive measures are only available in Rounds 2 and 3 in NSHAP, we restricted the analysis to the 2,409 respondents who completed both Rounds 2 and 3 in order to analyze change over time. To focus on partnered sexuality, we further restricted our analytic sample to the 1,683 respondents who were 62 and older with complete data on cognitive function and who were married, cohabiting, or had a romantic, intimate, or sexual partner at Round 2. The final analytic sample size varied slightly across models as we excluded missing values for the focal sexual variable in the specific model. NSHAP only surveyed respondents with the cognitive ability to complete the questions, so those with severe dementia were likely excluded from the sample (Kotwal et al., 2015), suggesting that our sample excluded the most vulnerable group. Thus, our conclusions may be conservative because the patterns might have been stronger if we included the most vulnerable group.
Measures
Cognitive Function
NSHAP employed a survey adaptation of the Montreal Cognitive Assessment (MoCA-SA) to measure cognitive function during Rounds 2 and 3 (Kotwal et al., 2015). Pilot testing of the MoCA-SA showed validity and internal reliability (Kotwal et al., 2015; Shega et al., 2014). The assessment covered six cognitive domains (attention, executive function, language, memory, orientation, and visuospatial skills) through a series of 18 questions. Respondents scored between 0 and 20 points. We applied a recommended equation (see Shega et al., 2014) to convert the MoCA-SA score to a MoCA score that ranged from 0–30 points. Lower MoCA scores indicate worse cognitive function.
Sexuality
We used three measures of partnered sexuality to consider both sexual frequency and sexual quality, following previous research (e.g., Liu et al., 2016). In this study, sexuality referred to “mutually voluntary activity with another person that involves sexual contact, whether or not intercourse or orgasm occurs” (Lindau et al., 2007, p. 763). Sexual frequency measured how often the respondent engaged in sex during the past 12 months, with responses of never (reference), once a month, 2–3 times a month, and once a week or more. Previous work suggested that having sex once a week or more is considered frequent sex at this age range (Liu et al., 2016). For sexual quality, we considered the physical and emotional dimensions separately as previous studies highlight the importance of distinguishing these two dimensions of sexual quality even though the two measures are correlated (Laumann et al., 2006; Lawrance & Byers, 1992; Liu et al., 2016). We measured how much physical pleasure and how much emotional satisfaction respondents got from their sexual relationship. Both sexual quality measures were coded as none/slightly/moderately pleasurable or satisfying (reference; collapsed due to skewness) and very/extremely pleasurable or satisfying.
Covariates
We controlled for covariates that have been associated with sexuality and cognitive function (Alzheimer’s Association, 2021; Lindau et al., 2007). All covariates came from Round 2 of the data. Gender was coded as men (0) and women (1). Age was separated into two groups: the younger-old (62–74) and the older-old (75–90). Race-ethnicity was coded as non-Hispanic white (reference), non-Hispanic Black, Hispanic, and others. Education included less than a high school degree (reference), high school degree, some college, and college graduate and higher. Family income was a relative measure, with respondents reporting that in comparison to other families, they were below average (reference), average, and above average. Marital status was coded as being married or cohabiting (0) or not (1). We also included self-rated health and depression measures to consider respondents’ overall health status (Idler & Benyami, 1997). Self-rated health was coded continuously, ranging from poor (1) to excellent (5) (Idler & Benyami, 1997). Depression was measured using the Center for Epidemiological Studies Depression Scale (CES-D; Cronbach’s alpha=0.79; Radloff, 1977). The CES-D asked 11 questions about respondents’ experiences feeling lonely, happy, sad, etc. in the previous week. Responses ranged from 0 (rarely or none of the time) to 3 (most of the time), with higher values indicating greater depression. The final CES-D scale totaled the scores from the 11 questions.
Analytic Approach
We started with descriptive analysis of all analytic variables for the total sample as well as by gender and age groups. We conducted t-tests for continuous variables and proportion tests for categorical variables comparing the two age groups (based on Round 2 age) and men and women. To understand the potential reciprocal relationships between sexuality and cognition, we applied cross-lagged models (illustrated in Figure 1) which have been widely used in the analysis of longitudinal data (Finkel, 2004). Specifically, we used Round 2 sexuality to predict Round 3 cognitive function and used Round 2 cognitive function to predict Round 3 sexuality. In each prediction equation, we controlled for Round 2 cognitive function, Round 2 sexuality, and all other Round 2 covariates. Due to concerns of multicollinearity across sexuality variables, we ran three separate cross-lagged models to assess sexual frequency (Model A), physical (Model B) and emotional (Model C) dimensions of sexual quality, respectively, in relation to cognitive function. The specific prediction equation varied by the measurement of the endogenous variables. Specifically, we applied Ordinary Least Squares regressions to predict cognitive function, ordinal logistic regressions to predict sexual frequency, and binary logistic regressions to predict physical pleasure and emotional satisfaction. We first ran the cross-lagged models among the total sample and then separately by gender and age groups in order to examine potential group differences. All models were tested using Stata 15 (StataCorp, 2017).
Figure 1.
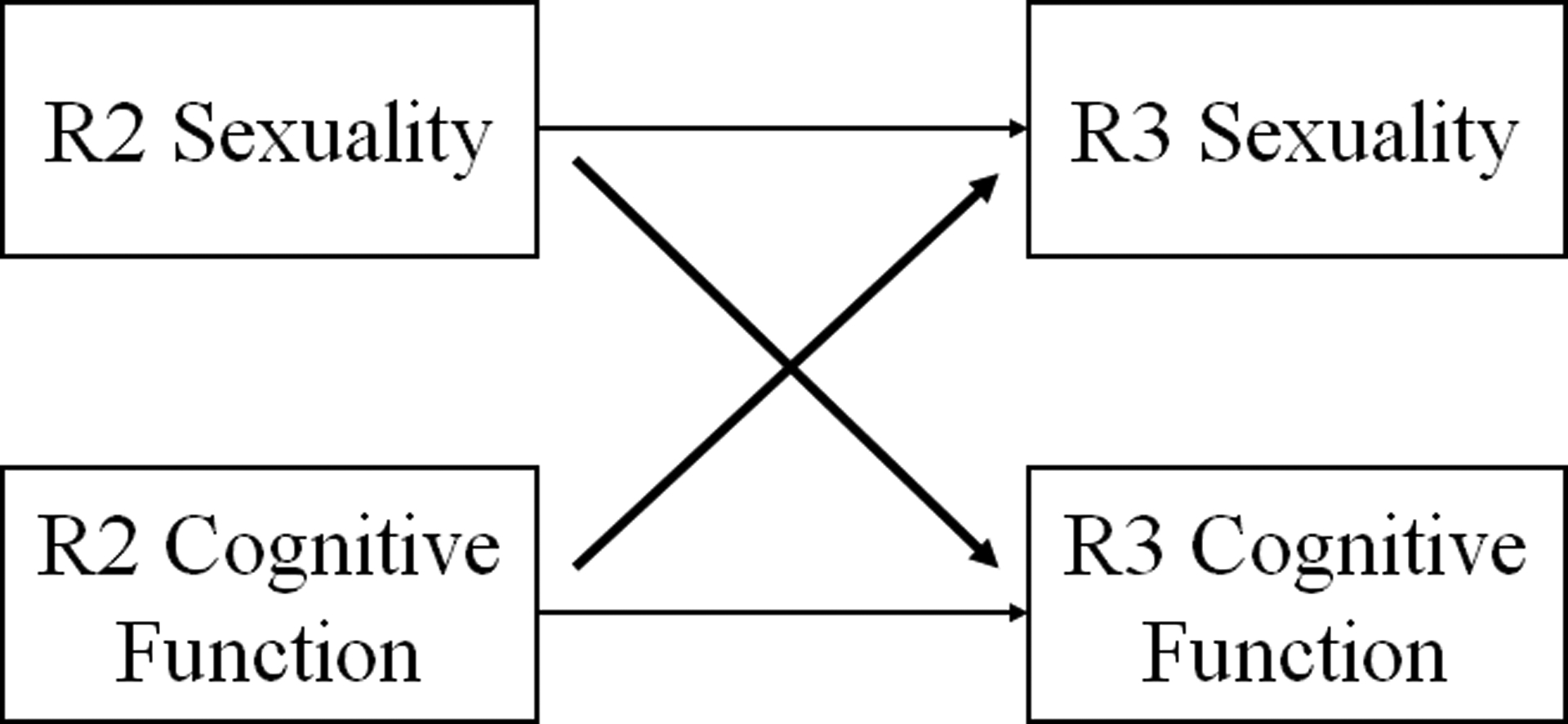
Cross-Lagged Model for Sexuality and Cognition.
All analyses were weighted. Missing values for categorical exogenous covariates were flagged as separate missing categories. Cases with missing values (about 0.04%) on the continuous exogenous covariates were replaced with the mean. We further applied the approach developed by Heckman (1979) to adjust the sample selection biases due to lost-to-follow-up in the longitudinal survey design. This approach consists of modeling the probability that a respondent would be lost to follow-up between waves, using logistic regression models, conditional on a set of predictors measured at the baseline survey. Then, for individuals who remained in the final sample, cognition or sexuality outcomes were modeled as a function of a set of independent variables, including the estimated probabilities of lost-to-follow-up. Following this Heckman-type correction, estimates should be interpreted as being adjusted for factors that may affect that risk, as well as for the tendency to be lost-to-follow-up.
Results
Descriptive Results
Table 1 shows descriptive statistics of all analytic variables for the total sample as well as by gender and age groups. Sexual frequency was lower among the older-old group (i.e. aged 75–90) than the younger-old group (i.e., aged 62–74), although there were less consistent and significant age differences in physical pleasure and emotional satisfaction with sex. Compared to women, men were more likely to have more frequent sex and felt more physical pleasure and emotional satisfaction with sex. In terms of cognition, cognitive functioning was significantly worse among the older-old group than the younger-old group, and among men than women. The findings also suggest that the older-old group reported poorer health than the younger-old group, and women were more likely than men to be non-Hispanic White, to be less educated, to report average income, and to have higher depression scores.
Table 1.
Weighted Descriptive Statistics, NSHAP Rounds 2 and 3
| Total (N=1683) |
Age 62–74 (N=1237) |
Age 75–90 (N=446) |
Men (N=870) |
Women (N=813) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | R3 | R2 | R3 | R2 | R3 | R2 | R3 | R2 | R3 | |
| Sexuality | ||||||||||
| Sexual Frequency | ||||||||||
| Never (ref) | 33.16 | 41.58 | 28.32* | 40.22* | 49.59* | 46.19* | 27.19* | 40.22 | 39.53* | 43.03 |
| Once a month | 21.37 | 16.26 | 21.68 | 17.75* | 20.30 | 11.19* | 24.13* | 17.11 | 18.41* | 15.35 |
| 2–3 times a month | 19.85 | 15.95 | 22.40* | 17.81* | 11.18* | 9.65* | 21.28 | 17.70* | 18.32 | 14.09* |
| Once a week or more | 16.78 | 12.07 | 19.10* | 12.34 | 8.90* | 11.16 | 17.73 | 12.24 | 15.77 | 11.89 |
| Missing | 8.84 | 14.14 | 8.50 | 11.88* | 10.03 | 21.81* | 9.67 | 12.73 | 7.78 | 15.64 |
| Sexual Quality | ||||||||||
| Physical Pleasure | ||||||||||
| None/slight/moderate (ref) | 25.60 | 29.03 | 25.61 | 30.38* | 25.58 | 24.46* | 17.43* | 24.60* | 34.33* | 33.77* |
| Very/extreme pleasurable | 70.95 | 60.22 | 71.22 | 61.33 | 70.04 | 56.46 | 79.58* | 67.11* | 61.72* | 52.86* |
| Missing | 3.45 | 10.75 | 3.18 | 8.30* | 4.39 | 19.08* | 2.99 | 8.29* | 3.95 | 13.37* |
| Emotional Satisfaction | ||||||||||
| None/slight/moderate (ref) | 24.08 | 24.12 | 23.99 | 25.55* | 24.40 | 19.25* | 16.21* | 19.49* | 32.49* | 29.07* |
| Very/extreme satisfied | 73.96 | 66.81 | 74.05 | 67.44 | 73.66 | 64.66 | 82.16* | 73.53* | 65.21* | 59.62* |
| Missing | 1.95 | 9.07 | 1.96 | 7.00* | 1.94 | 16.09* | 1.63 | 6.98* | 2.30 | 11.30* |
| Cognitive Function | 23.82 (3.69) | 23.12 (4.21) | 24.21* (3.53) | 23.72* (3.91) | 22.49* (3.88) | 21.09* (4.57) | 23.43* (3.60) | 22.91* (4.18) | 24.24* (3.73) | 23.35* (4.24) |
| Covariates at Round 2 | ||||||||||
| Gender | ||||||||||
| Men | 51.66 | 50.04* | 57.17* | |||||||
| Women | 48.34 | 49.96* | 42.83* | |||||||
| Age | ||||||||||
| 62–74 | 77.28 | 74.86* | 79.87* | |||||||
| 75–90 | 22.72 | 25.14* | 20.13* | |||||||
| Race-ethnicity | ||||||||||
| Non-Hispanic white (ref) | 83.48 | 82.71 | 86.09 | 81.75* | 85.32* | |||||
| Non-Hispanic black | 7.21 | 7.43 | 6.46 | 8.11 | 6.25 | |||||
| Hispanic | 6.47 | 7.05 | 4.50 | 7.54 | 5.33 | |||||
| Others | 2.84 | 2.81 | 2.96 | 2.60 | 3.10 | |||||
| Education | ||||||||||
| Less than high school (ref) | 11.98 | 11.32 | 14.22 | 13.26 | 10.62 | |||||
| High school | 23.93 | 23.18 | 26.48 | 21.89* | 26.10* | |||||
| Some College | 31.79 | 32.68 | 28.77 | 27.07* | 36.84* | |||||
| College graduate | 32.30 | 32.82 | 30.54 | 37.78* | 26.66* | |||||
| Relative family income | ||||||||||
| Below average (ref) | 22.10 | 21.76 | 23.25 | 20.80 | 23.49 | |||||
| Average | 40.92 | 40.40 | 42.69 | 37.87* | 44.18* | |||||
| Above average | 24.70 | 25.42 | 22.24 | 27.62* | 21.58* | |||||
| Missing | 12.28 | 12.42 | 11.81 | 13.71 | 10.75 | |||||
| Marital Status | ||||||||||
| Married/cohabiting | 94.81 | 94.86 | 94.63 | 93.73* | 95.97* | |||||
| Not married/cohabiting | 5.19 | 5.14 | 5.37 | 6.27* | 4.03* | |||||
| Self-rated health | 3.46 (1.01) | 3.49* (1.01) | 3.35* (1.02) | 3.44 (1.00) | 3.47 (1.02) | |||||
| Depression | 4.10 (4.40) | 4.03 (4.43) | 4.32 (4.30) | 3.65* (4.18) | 4.57* (4.59) | |||||
| Lost to follow-up R2 to R3 | 0.22 (0.11) | 0.18* (0.07) | 0.38* (0.10) | 0.27* (0.12) | 0.18* (0.09) | |||||
Note. NSHAP = National Social Life, Health, and Aging Study. All values are percents, except for cognitive function, self-rated health, depression, and lost to follow-up which reports mean and standard deviation. R2 = Round 2; R3 = Round 3.
Significant differences at p < 0.05 level comparing the two age groups (based on R2 age) or comparing men and women.
Results from Cross-lagged Models
Tables 2 and 3 show the results from the cross-lagged models for the total sample as well as by gender and age groups. We first discuss the results for sexuality (Round 2) predicting later cognitive function (Round 3) in Table 2, followed by the results for cognitive function (Round 2) predicting later sexuality (Round 3) in Table 3. Results in Table 2 show that among the total sample, none of the sexuality measures were related to later cognitive functioning.
Table 2.
Estimated Regression Coefficients of Sexuality Predicting Cognitive Function from Cross-Lagged Models, NSHAP Rounds 2 and 3.
| Cognitive Function (OLS Regression) |
|||||
|---|---|---|---|---|---|
| Total | Age 62–74 | Age 75–90 | Men | Women | |
| A. Sex Frequency (ref: none) | |||||
| Once a month | −0.11 | −0.29 | 0.27 | 0.38 | −0.46 |
| (−0.59–0.36) | (−0.83–0.26) | (−0.69–1.22) | (−0.36–1.12) | (−1.25–0.33) | |
| 2–3 times a month | −0.11 | −0.24 | −0.06 | 0.27 | −0.40 |
| (−0.58–0.36) | (−0.71–0.24) | (−1.66–1.54) | (−0.54–1.08) | (−1.09–0.29) | |
| Once a week or more | 0.21 | −0.11 | 1.50* | 0.72 | −0.12 |
| (−0.24–0.67) | (−0.60–0.39) | (0.27–2.73) | (−0.02–1.46) | (−0.90–0.65) | |
| (N=1331) | (N=999) | (N=332) | (N=708) | (N=623) | |
| B. Physical pleasure (ref: not very pleasurable) | |||||
| Very/extremely pleasurable | 0.43 | 0.51* | 0.11 | 0.74* | 0.22 |
| (−0.02–0.88) | (0.01–1.02) | (−0.89–1.11) | (0.06–1.42) | (−0.30–0.73) | |
| (N=1430) | (N=1084) | (N=346) | (N=771) | (N=659) | |
| C. Emotional satisfaction (ref: not very satisfying) | |||||
| Very/extremely satisfied | 0.43 | 0.58* | −0.15 | 0.54 | 0.43 |
| (−0.04–0.90) | (0.07–1.10) | (−1.20–0.90) | (−0.25–1.33) | (−0.15–1.00) | |
| (N=1486) | (N=1118) | (N=368) | (N=795) | (N=691) | |
Note. NSHAP = National Social Life, Health, and Aging Study. 95% confidence intervals in parentheses. All models controlled for age (excluded in the age stratified models), gender (excluded in the gender stratified models), race, education, relative income, marital status, self-rated health and depression, all measured at Round 2. Round 2 cognitive function was also controlled in the prediction of Round 3 cognitive function.
p<0.001,
p<0.01,
p<0.05
Table 3.
Estimated Odds Ratios of Cognitive Function Predicting Sexuality from Cross-Lagged Models, NSHAP Rounds 2 and 3.
| A. Sexual Frequency | B. Physical Pleasure | C. Emotional Satisfaction | |
|---|---|---|---|
| (Ordinal Logit) | (Binary Logit) | (Binary Logit) | |
| Total sample | |||
| Cognitive Function | 1.02 | 0.99 | 1.00 |
| (0.97–1.07) | (0.95–1.04) | (0.95–1.05) | |
| (N=1331) | (N=1430) | (N=1486) | |
| Age 62–74 | |||
| Cognitive Function | 1.01 | 1.00 | 1.00 |
| (0.96–1.07) | (0.95–1.07) | (0.94–1.06) | |
| (N=999) | (N=1084) | (N=1118) | |
| Age 75–90 | |||
| Cognitive Function | 1.06 | 0.95 | 0.99 |
| (0.97–1.16) | (0.87–1.04) | (0.90– 1.10) | |
| (N=332) | (N=346) | (N=368) | |
| Men | |||
| Cognitive Function | 1.00 | 0.99 | 1.00 |
| (0.95–1.06) | (0.93–1.06) | (0.94–1.07) | |
| (N=708) | (N=771) | (N=795) | |
| Women | |||
| Cognitive Function | 1.04 | 0.99 | 1.00 |
| (0.98–1.12) | (0.93–1.06) | (0.93–1.08) | |
| (N=623) | (N=659) | (N=691) |
Note. NSHAP = National Social Life, Health, and Aging Study. 95% confidence intervals in parentheses. All models controlled for age (excluded in the age stratified models), gender (excluded in the gender stratified models), race, education, relative income, marital status, self-rated health and depression, all measured at Round 2. Round 2 sexuality was also controlled in the prediction of Round 3 sexuality.
p<0.001,
p<0.01,
p<0.05,
p<0.10
The findings further suggest that the relationship between sexuality and cognitive function varied by age groups. Specifically, results in Model A of Table 2 show that among sexually active older-old adults, having sex once a week or more was related to better cognitive functioning five years later compared to their counterparts who had no sex in the last year (b = 1.50, p = .017), while frequency of sex was not related to cognitive functioning among the younger-old group. In contrast, sexual quality was related to cognitive functioning among the younger-old group but not among the older-old group. Specifically, younger-old respondents who felt that sex was very or extremely pleasurable (b = 0.51, p = 0.045, Model B of Table 2) and who felt that sex was very or extremely satisfying (b = 0.58, p = 0.026, Model C of Table 2) had better cognitive functioning five years later than their counterparts who did not feel so, while sexual quality was not related to later cognitive function among the older-old group.
Additionally, results in the gender stratified models in Table 2 indicate that the relationship between sexuality and cognitive function varied by gender. Sexual quality was related to cognition for men but not women. Specifically, men who felt that sex was very or extremely pleasurable (b = 0.74, p = 0.034, Model B of Table 2) had better cognitive functioning five years later than their counterparts who did not feel so. There were no significant gender differences in the relationship between sexual frequency and cognitive function.
Table 3 shows the results for the reverse causal pathway in which cognitive function at Round 2 predicts later sexuality at Round 3, suggesting no significant effects of cognition on later sexuality.
Discussion
Older adults’ sexual lives are often overlooked, and they may be further ignored when they experience poor cognitive health. There is little research on sexuality as it relates to cognition in this population, although sexual activity has been positively related to other physical and mental health outcomes (Lee et al., 2016; Liu et al., 2016; Zhang & Liu, 2020). This study is one of the first population-based studies on how partnered sexuality is related to cognitive function over time among older adults in the United States while also examining reverse causality in this relationship. The results provide novel evidence to partially support our hypotheses, with the key relationship between sexuality and cognition varying by age group and gender.
First, we did not find any evidence that having more frequent sex was related to cognitive functioning among the younger-old group (i.e., aged 62–74 in our sample). This is in line with Waite and colleagues’ (2022) work which also found no evidence of a link between sex and cognitive impairment in a longitudinal sample of older individuals and intimate dyads. Whereas Waite et al.’s (2022) work measured sexual activity using a single measure of having had sex in the past year, our work explored sexual frequency. Even this more nuanced measure of sexuality yielded no results for the younger-old group, lending additional support to Waite and colleagues’ (2022) findings. While having sex provides physical exercise which promotes circulatory health and is beneficial for the aging brain (Kirk-Sanchez & McGough, 2014), our findings did not support a link between sexual frequency and cognitive functioning among younger-old adults.
In contrast, among the older-old group (i.e., aged 75–90 in our sample), we found that having sex more frequently was related to better cognitive functioning five years later. Specifically, among older adults aged 75–90, those who engaged in sexual activity at least once a week had a cognitive score that was 1.5 units higher five years later compared to their counterparts who reported no sexual activity in the past year. This effect size was comparable to education, another factor known to positively impact cognitive function in this age group (Alzheimer’s Association, 2021). For example, compared to having less than a high school degree, the estimated effect size of having some college education was 1.72 and having a college degree was 1.88 in the Table 2 model (not shown but available upon request). That sexual frequency matters in older-old ages may be because having any sex in these later years, regardless of sexual quality, is beneficial for health outcomes. Sexual activity can foster cognitive health because it is a form of physical activity (Kirk-Sanchez & McGough, 2014). This may be especially true at older-old ages as physical inactivity increases with age (Sallis, 2000) and older-old adults have high rates of sedentary behavior (Matthews et al., 2008). Thus, any circulatory benefits from having sex may be important at very old age.
Moreover, we did find some support for sexual quality impacting cognition, especially among the younger-old group, as younger-old adults who felt their sexual relationships were very pleasurable and satisfying had better cognitive functioning five years later than their counterparts who did not feel so. This finding is consistent with a recent study suggesting that emotional closeness with a sexual partner was related to older adults’ better scores on memory tests (Allen, 2018). This result may be due to pleasure hormones associated with sexually satisfying relationships. Dopamine is a neurotransmitter released during sex (Blum et al., 2012; Melis & Argiolas 1995). Sexual satisfaction is associated with orgasm (Laumann et al., 2000), and an orgasm releases a rush of dopamine (Blum et al., 2012). Thus, people with more sexually satisfying relationships may experience higher levels of dopamine, which has been linked to improved memory in older adults (Chowdhury et al., 2012).
The emphasis on the importance of sexual quality for the younger-old adults’ cognition specifically may be because at this earlier stage of later life, which may include transitions into retirement or grandparenthood, individuals are concerned about the quality of the sex. With potentially more free time to focus on their relationship, younger-old adults may have higher expectations for the quality of their sex lives. Qualitative data which compared young-old and older-old couples in Sweden found that Alzheimer’s was viewed more negatively by the younger couples than their older counterparts due to the changes it had on their sexual lives, such that it interrupted their intimate relationship with their partner (Sandberg, 2020). However, as seen in our sample, promoting sexual quality among younger-old couples may be a way to combat the interruptions which people anticipate to come with aging, and these feelings of sexual quality may manifest in their later cognitive health. Future work should explore potential neurological pathways through which positive feelings and hormones generated from sexual satisfaction may shape cognitive health, especially among younger-old adults.
Additionally, we found some evidence of gender differences in sexual quality being related to later cognition, as men who experienced high physical pleasure from their sexual relationships had better cognitive functioning five years later than men who did not. This finding was seen only in men, which is consistent with previous findings using representative data from English older adults that found sexual activity was linked to men’s memory recall but not women’s (Smith et al., 2020; Wright & Jenks, 2016). Given that men’s gender socialization places more emphasis on seeking sexual pleasure while women’s encourages more sexual passivity (Basson, 2000), physical pleasure may mean more to men. Research also finds that older men report more interest in sex than older women (Waite et al., 2009), so men may be more invested in their sexual pleasure than women due to gender socialization differences accumulating across the life course. Thus, physical pleasure may be important for older men, and this manifests in their cognitive well-being more so than for older women. Furthermore, it may also be that men equate physical pleasure with experiencing an orgasm, and male orgasms are beneficial for their cardiovascular health (Krüger et al., 1998), which can further protect against brain aging (Kirk-Sanchez & McGough, 2014).
Finally, our tests of the relationship between cognitive function and the sexuality measures yielded no significant results, indicating no support for reverse causality. This lack of evidence is consistent with previous literature which finds little evidence that cognition has an effect on the sexual activity of the partner with dementia (Eloniemi-Sulkava et al., 2002; Hartmans et al., 2014; Waite et al., 2022). As Waite and colleagues (2022) suggested, it may be that sexual activity can withstand the challenges of aging and the sexual relationship continues despite poor cognitive functioning. This is supported by Lindau and colleagues’ (2018) analysis of a nationally representative sample of older adults which found that they remained sexually active in various states of cognitive decline. Some older adults’ sex lives continue despite poor cognitive health. The ability to weather cognitive changes may be especially true in later life when couples have likely spent years together adapting to different challenges and have grown to know each other’s sexual preferences (Lodge & Umberson, 2012) which they can take into consideration when faced with cognitive functioning problems.
Limitations
There were several limitations to this study. First, cognitive measures were only included in two rounds of NSHAP. Although we tested for reverse causality in our models, we are cautious about making any causal claims based on our cross-lagged models that used only two waves of data (Finkel, 2004). Additionally, while there were five years between the rounds of data collection, cognitive decline becomes more pronounced over an extended period of time (Alzheimer’s Association, 2021). NSHAP will soon be releasing a fourth round of data that contains these cognitive measures. This will provide panel data over ten years and allow researchers to consider the relationship between sexuality and cognition over longer trajectories and provide better testing of causality.
Second, following previous studies using this dataset (e.g., Liu et al., 2016, McFarland et al., 2011; Siegel et al., 2021), we separately tested frequency of sex, physical pleasure, and emotional satisfaction. Yet, sexuality measures can be correlated with one another, and our models did not control for this. Further, our results may be limited by using control measures, such as self-reported health, to account for individual heterogeneity related to other factors which may influence cognition, such as diet or physical activity. Although self-reported health is a global health measure (Idler & Benyami, 1997), it is possible that other lifestyle factors important to cognitive functioning could influence the results.
Third, response bias may be present in the sexuality measures, as these are sensitive topics and there can be gender bias in self-reporting sexuality measures (Hyde et al., 2010). Fourth, NSHAP does not contain data on whether the sex is consensual. While there is research that addresses the ability to consent within the context of dementia, especially on populations living in care facilities (Hillman, 2017), we were not able to include that measure in our models. Fourth, there was also limited data on sexual orientation, as this question was not asked until Round 3. In Round 3, only twenty-seven respondents identified as gay, lesbian, or bisexual in our study sample. Models that excluded these respondents (not shown but available upon request) indicated that our key results did not change.
Finally, the definition of sex that NSHAP provided to respondents was broad and could include acts such as hugging and kissing. While many older adults may consider these intimate acts as sexual activity (Waite et al., 2009), there are nuances in what is considered in the sexuality of older adults and what activities may be beneficial for their cognition. Similarly, the sexual quality measures in NSHAP are left open to each respondent’s interpretation of sexual satisfaction and physical pleasure. Respondents’ sexual relationships across the life course could have been objectively different, with varying levels of happiness or conflict, and they may have responded based on the present state of their sexual relationship or based on any improvement or worsening of their sex life. While these measures of sexual quality are widely used (Laumann et al., 2000; Lee et al., 2016; Lindau et al., 2007), it is important to remember the subjective nature of these variables.
Recent studies have revealed worrisome cognitive trends across cohorts in American older adults (Zheng, 2021), highlighting the emerging importance of identifying preventive factors that will curb the worsening cognitive trends. Sexuality is one underutilized but important aspect of later life that has broad health implications. Our findings moved beyond current work (Lindau et al., 2018; Waite et al., 2022) by considering age and gender differences in multiple sexuality measures over time to suggest that for partnered adults, there are age and gender differences in the role that sexual frequency and sexual quality play in protecting cognitive function. Having more frequent sex was related to better subsequent cognitive function for the older-old group while having better sexual quality was related to better subsequent cognitive function for the younger-old group. Moreover, sexual quality was related to better subsequent cognitive function for men but not women. Sex is important for older adults’ cognitive health. Health practitioners should develop and carefully evaluate intervention strategies that target sexual frequency and sexual quality for specific groups to encourage or preserve the cognitive health of older adults rather than avoid discussion of their sexuality or assume that they are sexually inactive. Given the limited research on cognition, aging, and sex, this study calls for more research in this direction and future research should explore the underlying processes of sexuality shaping cognitive health in late life, with specific attention to age and gender variations.
Acknowledgments
An earlier version of this paper was presented at the 2022 Population Association of America (PAA) annual meeting.
Funding
This work was supported by the National Institute on Aging under Grant R01 AG061118.
Footnotes
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement.
Data is available to download from ICPSR. Data from Round 2 of the National Social Life, Health, and Aging Project (NSHAP) can be accessed via https://doi.org/10.3886/ICPSR34921.v4. Data from NSHAP Round 3 can be accessed via https://doi.org/10.3886/ICPSR36873.v7.
References
- Allen MS (2018). Sexual activity and cognitive decline in older adults. Archives of Sexual Behavior, 47(6), 1711–1719. 10.1007/s10508-018-1193-8 [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association. (2021). 2021 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia, 17(3), 327–406. doi: 10.1002/alz.12328 [DOI] [PubMed] [Google Scholar]
- Basson R (2000). The female sexual response: A different model. Journal of Sex and Marital Therapy, 26(1), 51–65. doi: 10.1080/009262300278641 [DOI] [PubMed] [Google Scholar]
- Blum K, Chen ALC, Giordano J, Borsten J, Chen TJH, Hauser M, Simpatico T, Femino J, Braverman ER, & Barh D The addictive brain: All roads lead to dopamine. Journal of Psychoactive Drugs, 44(2), 134–143. doi: 10.1080/02791072.2012.685407 [DOI] [PubMed] [Google Scholar]
- Buczak-Stec E, König H-H, & Hajek A (2021). Sexual satisfaction of middle-aged and older adults: Longitudinal findings from a nationally representative sample. Age and Ageing, 50(2), 559–564. 10.1093/ageing/afaa161 [DOI] [PubMed] [Google Scholar]
- Butt DS (1990). The sexual response as exercise. Sports Medicine, 9(6), 330–343. 10.2165/00007256-199009060-00002 [DOI] [PubMed] [Google Scholar]
- Chowdhury R, Guitart-Masip M, Bunzeck N, Dolan RJ, & Düzel E (2012). Dopamine modulates episodic memory persistence in old age. Journal of Neuroscience, 32(41), 14193–14204. doi: 10.1523/JNEUROSCI.1278-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannefer D (2003). Cumulative advantage/disadvantage and the life course: Cross-fertilizing age and social science theory. The Journals of Gerontology, Series B, 58(6), S327–S337. 10.1093/geronb/58.6.S327 [DOI] [PubMed] [Google Scholar]
- Davies HD, Newkirk LA, Pitts CB, Coughlin CA, Sridhar SB, Zeiss LM, & Zeiss AM (2010). The impact of dementia and mild memory impairment (MMI) on intimacy and sexuality in spousal relationships. International Psychogeriatrics, 22(4), 618–628. doi: 10.1017/S1041610210000177 [DOI] [PubMed] [Google Scholar]
- DeLamater J, & Moorman SM (2007). Sexual behavior in later life. Journal of Aging and Health, 19(6), 921–945. doi: 10.1177/0898264307308342 [DOI] [PubMed] [Google Scholar]
- Drummond JD, Brotman S, Silverman M, Sussman T, Orzeck P, Barylak L, & Wallach I (2013). The impact of caregiving: Older women’s experiences of sexuality and intimacy. Affilia, 28(4), 415–428. 10.1177/0886109913504154 [DOI] [Google Scholar]
- Ein-Dor T, & Hirschberger G (2012). Sexual healing: Daily diary evidence that sex relieves stress for men and women in satisfying relationships. Journal of Social and Personal Relationships, 29(1), 126–139. 10.1177/0265407511431185 [DOI] [Google Scholar]
- Elder GH Jr. (1998). The life course as developmental theory. Child Development, 69(1), 1–12. 10.2307/1132065 [DOI] [PubMed] [Google Scholar]
- Elder GH Jr., & O’Rand AM (1995). Adult lives in a changing society. In Cook KS, Fine GA, & House JS (Eds.), Sociological perspectives on social psychology (pp. 452–475). Allyn and Bacon. [Google Scholar]
- Eloniemi-Sulkava U, Notkola I-L, Hamalainen K, Rahkonen T, Viramo P, Hentinen J, Kivela S-L, & Sulkava R (2002). Spouse caregivers’ perceptions of influence of dementia on marriage. International Psychogeriatrics, 14(1), 47–58. doi: 10.1017/s104161020200827x [DOI] [PubMed] [Google Scholar]
- Erol R, Brooker D, & Peel E (2015). Women and dementia: A global research review. Alzheimer’s Disease International. https://www.alzint.org/u/Women-and-Dementia.pdf [Google Scholar]
- Finkel SE (2004). Cross-lagged. In Lewis-Beck MS, Bryman A, & Futing T (Eds.), The SAGE encyclopedia of social science research methods (pp. 229–230). SAGE Publications, Inc. [Google Scholar]
- Forbes MK, Eaton NR, & Krueger RF (2021). Sexual quality of life and aging: A prospective study of a nationally representative sample. The Journal of Sex Research, 54(2), 137–148. doi: 10.1080/00224499.2016.1233315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmans C, Comijs H, & Jonker C (2014). Cognitive functioning and its influence on sexual behavior in normal aging and dementia. International Journal of Geriatric Psychiatry, 29(5), 441–446. doi: 10.1002/gps.4025 [DOI] [PubMed] [Google Scholar]
- Heckman JJ (1979). Sample selection bias as a specification error. Econometrica, 47(1), 153–61. [Google Scholar]
- Hillman J (2017). Sexual consent capacity: Ethical issues and challenges in long-term care. Clinical Gerontologist, 40(1), 43–50. 10.1080/07317115.2016.1185488 [DOI] [PubMed] [Google Scholar]
- Hyde Z, Flicker L, Hankey GJ, Almeida OP, McCaul KA, Chubb P, & Yeap BB (2010). Prevalence of sexual activity and associated factors in men aged 75 to 95 years. Annals of Internal Medicine, 153(11), 693–702. doi: 10.7326/0003-4819-153-11-201012070-00002 [DOI] [PubMed] [Google Scholar]
- Idler EL, & Benyamini Y (1997). Self-rated health and mortality: A review of twenty-seven community studies. Journal of Health and Social Behavior, 38(1), 21–37. [PubMed] [Google Scholar]
- Karraker A, DeLamater J, & Schwartz CR (2011). Sexual frequency decline from midlife to later life. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 66B(4), 502–512. 10.1093/geronb/gbr058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk-Sanchez NJ, & McGough EL (2014). Physical exercise and cognitive performance in the elderly: Current perspectives. Clinical Interventions in Aging, 9(2014), 51–62. doi: 10.2147/CIA.S39506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotwal AA, Schumm LP, Kern DW, McClintock MK, Waite LJ, Shega JW, Huisingh-Scheetz MJ, & Dale W (2015). Evaluation of a brief survey instrument for assessing subtle differences in cognitive function among older adults. Alzheimer Disease and Associated Disorders, 29(4), 317–324. doi: 10.1097/WAD.0000000000000068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozareva DA, Cryan JF, & Nolan YM (2019). Born this way: Hippocampal neurogenesis across the lifespan. Aging Cell, 18(5), e13007. 10.1111/acel.13007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger T, Exton MS, Pawlak C, von zur Mühlen A, Hartmann U, & Schedlowski M (1998). Neuroendocrine and cardiovascular response to sexual arousal and orgasm in men. Psychoneuroendocrinology, 23(4), 401–411. 10.1016/S0306-4530(98)00007-9 [DOI] [PubMed] [Google Scholar]
- Laumann EO, Gagnon JH, Michael RT, & Michaels S (2000). The social organization of sexuality: Sexual practices in the United States. University of Chicago Press. [Google Scholar]
- Laumann EO, Paik A, Glasser DB, Kang JH, Wang T, Levinson B, Moreira ED, Nicolosi A, & Gingell C (2006). A cross-national study of subjective sexual well-being among older women and men: Findings from the Global Study of Sexual Attitudes and Behaviors. Archives of Sexual Behavior, 35(2), 143–159. 10.1007/s10508-005-9005-3 [DOI] [PubMed] [Google Scholar]
- Lawrance KA, & Byers ES (1992). Development of the interpersonal exchange model of sexual satisfaction in long term relationships. Canadian Journal of Human Sexuality, 1(3), 123–128. [Google Scholar]
- Lee DM, Vanhoutte B, Nazroo J, & Pendleton N (2016). Sexual health and positive subjective well-being in partnered older men and women. Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 71(4), 698–710. doi: 10.1093/geronb/gbw018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindau ST, Dale W, Feldmeth G, Gavrilova N, Langa KM, Makelarski JA, & Wroblewski K (2018). Sexuality and cognitive status: A U.S. nationally representative study of home-dwelling older adults. Journal of the American Geriatrics Society, 66(10), 1902–1910. doi: 10.1111/jgs.15511 [DOI] [PubMed] [Google Scholar]
- Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, & Waite LJ (2007). A study of sexuality and health among older adults in the United States. New England Journal of Medicine, 357(8), 762–774. doi: 10.1056/NEJMoa067423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Waite LJ, Shen S, & Wang DH (2016). Is sex good for your health? A national study on partnered sexuality and cardiovascular risk among older men and women. Journal of Health and Social Behavior, 57(3), 276–296. 10.1177/0022146516661597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge AC, & Umberson D (2012). All shook up: Sexuality of mid- to later life married couples. Journal of Marriage and Family, 74(3), 428–443. doi: 10.1111/j.1741-3737.2012.00969.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, & Troiano RP (2008). Amount of time spent in sedentary behaviors in the United States, 2003–2004. American Journal of Epidemiology, 167(7), 875–881. 10.1093/aje/kwm390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland MJ, Uecker JE, & Regnerus MD (2011). The role of religion in shaping sexual frequency and satisfaction: Evidence from married and unmarried older adults.The Journal of Sex Research, 48(2–3), 297–308. doi: 10.1080/00224491003739993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min SH, Topaz M, Lee C, & Schnall R (2023). Understanding changes in mental health symptoms from young-old to old-old adults by sex using multiple-group latent transition analysis. GeroScience, 1–11. 10.1007/s11357-023-00729-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirescu C, & Gould E (2006). Stress and adult neurogenesis. Hippocampus, 16(3), 233–238. doi: 10.1002/hipo.20155 [DOI] [PubMed] [Google Scholar]
- Melis MR, & Argiolas A (1995). Dopamine and sexual behavior. Neuroscience & Biobehavioral Reviews, 19(1), 19–38. doi: 10.1016/0149-7634(94)00020-2 [DOI] [PubMed] [Google Scholar]
- Moen P (2001). The gendered life course. In George LK & Binstock RH (Eds.), Handbook of aging and the social sciences (pp. 179–196). Academic Press. [Google Scholar]
- Neugarten BL (1974). Age groups in American society and the rise of the young-old. The Annals of the American Academy of Political and Social Science, 415(1), 187–198. doi: 10.1177/000271627441500114 [DOI] [Google Scholar]
- Nogueira MML, Neto JPS, Sousa MFB, Santos RL, Lacerda IB, Baptista MAT, & Dourado MCN (2017). Perception of change in sexual activity in Alzheimer’s disease: Views of people with dementia and their spouse-caregivers. International Psychogeriatrics, 29(2), 185–193. doi: 10.1017/S1041610216001642 [DOI] [PubMed] [Google Scholar]
- O’Muircheartaigh C, English N, Pedlow S, & Kwok PK (2014). Sample design, sample augmentation, and estimation for Wave 2 of the NSHAP. Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 69(Suppl. 2), S15–S26. 10.1093/geronb/gbu053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Muircheartaigh C, English N, Pedlow S, & Schumm LP (2021). Sample design and estimation in the National Social Life, Health, and Aging Project: Round 3 (2015–2016). The Journals of Gerontology: Series B, 76(Suppl 3), S207–S214. doi: 10.1093/geronb/gbab182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penhollow TM, Young M, & Denny G (2009). Predictors of quality of life, sexual intercourse, and sexual satisfaction among active older adults. American Journal of Health Education, 40(11), 14–22. doi: 10.1080/19325037.2009.10599074 [DOI] [Google Scholar]
- Pöysti MM, Laakkonen ML, Strandberg T, Savikko N, Tilvis RS, Eloniemi-Sulkava U, & Pitkälä KH (2012). Gender differences in dementia spousal caregiving. International Journal of Alzheimer’s Disease, 2012, 1–5. doi: 10.1155/2012/162960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M, Albanese E, Guerchet M, & Prina M (2014). World Alzheimer report 2014. Dementia and risk reduction: An analysis of protective and modifiable risk factors Alzheimer’s Disease International. https://www.alzint.org/u/WorldAlzheimerReport2014.pdf [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- Rajan KB, Weuve J, Barnes LL, McAninch EA, Wilson RS, & Evans DA (2021). Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimer’s & Dementia, 17(12), 1966–1975. doi: 10.1002/alz.12362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallis JF (2000). Age-related decline in physical activity: A synthesis of human and animal studies. Medicine and Science in Sports and Exercise, 32(9), 1598–1600. doi: 10.1097/00005768-200009000-00012 [DOI] [PubMed] [Google Scholar]
- Sandberg LJ (2020). Too late for love? Sexuality and intimacy in heterosexual couples living with an Alzheimer’s disease diagnosis. Sexual and Relationship Therapy, 38(1), 118–139. 10.1080/14681994.2020.1750587 [DOI] [Google Scholar]
- Shega JW, Sunkara PD, Kotwal A, Kern DW, Henning SL, McClintock MK, Schumm LP, Waite LJ, & Dale W (2014). Measuring cognition: The Chicago cognitive function measure in the National Social Life, Health and Aging Project, Wave 2. Journals of Gerontology, Series B, 69(8), S166–S176. doi: 10.1093/geronb/gbu106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JK, Kung SY, Wroblewski KE, Kern DW, McClintock MK, & Pinto JM (2021). Olfaction is associated with sexual motivation and satisfaction in older men and women.The Journal of Sexual Medicine,18(2), 295–302. doi: 10.1016/j.jsxm.2020.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AG, Bardach SH, Barber JM, Williams A, Rhodus EK, Parsons KK, & Jicha GA (2021). Associations of future cognitive decline with sexual satisfaction among married older adults. Clinical Gerontologist, 44(3), 345–353. 10.1080/07317115.2021.1887420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L, Grabovac I, Yang L, Lopez-Sanchez GF, Firth J, Pizzol D, McDermott D, Veronese N, & Jackson SE (2020). Sexual activity and cognitive decline in older age: A prospective cohort study. Aging Clinical and Experimental Research, 32(1), 85–91. 10.1007/s40520-019-01334-z [DOI] [PubMed] [Google Scholar]
- StataCorp. (2017). Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC. [Google Scholar]
- Træen B, Štulhofer A, Janssen E, Carvalheira AA, Hald GM, Lange T, & Graham C (2019). Sexual activity and sexual satisfaction among older adults in four European countries. Archives of Sexual Behavior, 48(3), 815–829. 10.1007/s10508-018-1256-x [DOI] [PubMed] [Google Scholar]
- Waite LJ (2017). National Social Life, Health, and Aging Project (NSHAP): Round 3 and COVID-19 Study. ICPSR36873-v1. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor]. 10.3886/ICPSR36873.v7 [DOI] [Google Scholar]
- Waite LJ, Cagney K, Cornwell B, Dale W, Huang E, Laumann EO, McClintock M, O’Muircheartaigh CA, & Schumm LP (2014). National Social Life, Health, and Aging Project (NSHAP): Round 2 and Partner Data Collection. ICPSR34921-v1. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor]. 10.3886/ICPSR34921.v4 [DOI] [Google Scholar]
- Waite LJ, Iveniuk J, & Kotwal A (2022). Take two to tango: Cognitive impairment and sexual activity in older individuals and dyads. The Journals of Gerontology: Series B, 77(5), 992–1003. 10.1093/geronb/gbab158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite LJ, Laumann EO, Das A, & Schumm LP (2009). Sexuality: Measures of partnerships, practices, attitudes, and problems in the National Social Life, Health, and Aging Study. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 64(1), i56–i66. doi: 10.1093/geronb/gbp038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright H, & Jenks RA (2016). Sex on the brain! Associations between sexual activity and cognitive function in older age. Age and Ageing, 45(2), 313–317. doi: 10.1093/ageing/afv197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright H, Jenks RA, & Demeyere N (2019). Frequent sexual activity predicts specific cognitive abilities in older adults. The Journals of Gerontology: Series B, 74(1), 47–51. 10.1093/geronb/gbx065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong C, Biscardi M, Astell A, Nalder E, Cameron JI, Mihailidis A, & Colantonio A (2020). Sex and gender differences in caregiving burden experienced by family caregivers of persons with dementia: A systematic review. PloS One, 15(4), e0231848. 10.1371/journal.pone.0231848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H (2021). A new look at cohort trend and underlying mechanisms in cognitive functioning. The Journals of Gerontology: Series B, 76(8), 1652–1663. 10.1093/geronb/gbaa107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, & Liu H (2020). A national longitudinal study of partnered sex, relationship quality, and mental health among older adults. The Journals of Gerontology: Series B, 75(8), 1172–1782. 10.1093/geronb/gbz074 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available to download from ICPSR. Data from Round 2 of the National Social Life, Health, and Aging Project (NSHAP) can be accessed via https://doi.org/10.3886/ICPSR34921.v4. Data from NSHAP Round 3 can be accessed via https://doi.org/10.3886/ICPSR36873.v7.


