Abstract
Prior studies, which have relied upon the use of pseudovirions generated in heterologous cell types, have led to sometimes conflicting conclusions regarding the role of the minor capsid protein of papillomaviruses, L2, in the viral life cycle. In this study we carry out analyses with true virus particles assembled in the natural host cell to assess L2's role in the viral infectious life cycle. For these studies we used the organotypic (raft) culture system to recapitulate the full viral life cycle of the high-risk human papillomavirus HPV31, which was either wild type or mutant for L2. After transfection, the L2 mutant HPV31 genome was able to establish itself as a nuclear plasmid in proliferating populations of poorly differentiated (basal-like) human keratinocytes and to amplify its genome to high copy number, support late viral gene expression, and cause formation of virus particles in human keratinocytes that had been induced to undergo terminal differentiation. These results indicate that aspects of both the nonproductive and productive phases of the viral life cycle occur normally in the absence of functional L2. However, upon the analysis of the virus particles generated, we found an approximate 10-fold reduction in the amount of viral DNA encapsidated into L2-deficient virions. Furthermore, there was an over-100-fold reduction in the infectivity of L2-deficient virus. Because the latter deficiency cannot be accounted for solely by the 10-fold decrease in encapsidation, we conclude that L2 contributes to at least two steps in the production of infectious virus.
Papillomaviruses are small icosahedral viruses with double-stranded, circular DNA genomes that infect the stratified squamous epithelial tissues of vertebrates. The infection commonly results in a papilloma, or wart, formation. A small subset of the human-specific genotypes termed the high-risk human papillomaviruses (HPVs), including HPV31, have the capacity to cause cancer, most notably cervical cancer (35). Understanding the papillomaviral life cycle may allow us to establish the means to inhibit high-risk papillomaviral infections and thereby prevent HPV-associated cancers. In this study, we investigated the role the minor capsid protein, L2, plays in the papillomaviral life cycle.
The life cycle of papillomaviruses is intricately tied to the differentiation of their host tissue, stratified squamous epithelium (23). In the poorly differentiated basal cells, which comprise the proliferating compartment of the epithelium and where papillomaviral infection is thought to arise, the viral genome takes up residence as a stable, nuclear plasmid that is maintained at low copy number. Only a subset of viral genes, the early genes, is selectively expressed in the basal compartment, and therefore no new virus is made there. Consequently, we refer to the infective state in the basal compartment as the nonproductive phase of the life cycle. As basal cells proliferate and undergo cell division, daughter cells that lose contact with the underlying basement membrane begin migrating superficially into the upper layers of the stratified squamous epithelia and initiate a terminal differentiation process. It is in these suprabasal cells that the productive phase of the viral life cycle in which progeny virus are made arises. A number of unique events occur in these suprabasal cells, including the virus reprogramming these normally postmitotic cells to support DNA synthesis, the consequent amplification of the viral genome, the synthesis of the viral structural genes, L1 and L2, which encode the viral major and minor capsid proteins, respectively, the assembly of viral capsids, and the encapsidation of the viral genomes to generate progeny virus. The ability to study both phases of the HPV life cycle in vitro only became possible with the application to the study of papillomaviruses of the organotypic or raft culture system, which allows one to induce the normal differentiation and stratification of human keratinocytes (18). Using this culture system one can induce the complete life cycle, including the production of progeny virus, by using cervical epithelial or human foreskin-derived cell lines harboring HPV genomes extrachromosomally (19, 22). The subsequently gained knowledge for establishing the nonproductive state of the viral life cycle (nuclear plasmid state) in keratinocytes with transfected recombinant HPV genomes (11) led to the recent successes in analyzing the functions of several viral genes in the viral life cycle (8, 9, 12, 27).
To study papillomavirus particle assembly, structure, and early steps in infection, such as binding and uptake of virus particles by cells, researchers traditionally have relied upon alternative means than raft cultures for generating virus particles. This is largely because raft cultures yield limited amounts of virus and because this culture system is somewhat cumbersome to establish and use successfully. L1 is sufficient to form virus-like particles when expressed in a variety of cell types, and when L2 is coexpressed it is also incorporated into the particles (33, 34). A variety of labs have taken advantage of this and developed a broad range of systems that produce artificial virions, also called pseudovirions. In these systems L1 and L2 are overexpressed in the presence of a reporter plasmid that can be encapsidated in cells as different as Saccharomyces cerevisiae, insect cells, and mammalian cells, and pseudovirions are allowed to spontaneously assemble inside or outside of the cells (4, 15, 24-26, 28, 29, 32, 33). Some of these pseudovirions have DNA on the outside of the particle (28).
These pseudovirions have proven quite useful in structural studies as well as studies on early steps of the infectious process, such as cell binding and uptake. It has been quite well established that pseudovirions composed of only L1 cannot transduce their reporter plasmid into cells as well as their L1/L2 counterparts (25, 29). Studies that examined this process more closely have found that L2 seems to be involved in the translocation of the virus to the nucleus after cell entry (30). Studies supporting a role for L2 in cell entry have also been reported (14, 30). These studies, however, do not examine the role of L2 in the context of a complete viral life cycle. Using pseudovirions, researchers have attempted to establish whether L2 is involved in the process of virion assembly. Different investigators have obtained conflicting results. Some found that in their system L2 was required for the encapsidation of viral or reporter DNA (4, 20, 25, 26, 32, 33). In other systems L2 was not required (28, 29). These differing results are likely due to the differences in the systems, specifically the type of cell used in the production of the pseudovirions and whether assembly takes place inside or outside of cells.
In this study we addressed the role of L2 in the life cycle by naturally recapitulating the life cycle by using the organotypic (raft) culture system. Not only were we able to thereby establish the natural conditions for virion assembly, but also the other parts of the viral life cycle could be monitored. Using this system, we compared a wild-type HPV31 virus to an HPV31 virus that cannot express L2. We monitored all parts of the viral life cycle, including establishment and maintenance of the viral genome after transfection, induction of suprabasal DNA synthesis, amplification of viral DNA, expression of capsid proteins, virion assembly, and infection. Evidence will be provided indicating that L2 contributes to multiple steps in the infectious life cycle.
MATERIALS AND METHODS
DNA reagents.
A recombinant plasmid containing the HPV31a genome inserted into the EcoRI site of pBR322 was obtained from L. Laimins (Northwestern University) and is hereafter termed pHPV31. The L2 mutant HPV31a genome, containing two nucleotide substitutions within the L2 open reading frame, was generated by a two-step, site-directed mutagenesis strategy that employed PCR with a high-fidelity and highly processive, thermostable DNA polymerase, Pfu Turbo. Following amplification, the DNA was digested with DpnI, which selectively cleaved the input, bacterially synthesized, deoxyadenasine methylase-methylated, template DNA, leaving the PCR amplimers containing the desired mutation undigested. Following transformation of and selection for the amplimer into XL10 Ultracompetent Escherichia coli cells (Stratagene, La Jolla, Calif.), the plasmid DNAs were isolated and the entire viral genome was sequenced to verify the correct incorporation of the nucleotide substitutions and the absence of spurious mutations elsewhere in the viral genome. The first point mutation changed an A to a T at nucleotide 4180 by using the two complementary primers 5′ CCATGCGTCCtAACGCTCTACAAAACG 3′ and 5′ CGTTTTGTAGAGCGTTaGGACCGCATGG 3′ (mutations are shown in lowercase).
Clones were screened for the desired mutation, and the mutant plasmid was used in the second PCR. The second PCR introduced an A-to-T transversion at nucleotide 4321, which again amplified the entire genome with the following complementary primers: 5′ GGTATGGTAGTtTGGGTGTTTTTTTTGG 3′ and 5′ CCAAAAAAAACACCCAaACTACCATACC 3′ (mutations are shown in lowercase).
For both PCRs, Pfu Turbo DNA polymerase was used with the following program: 95°C for 1 min and 18 repetitions of 95°C for 50 s, annealing temperature for 50 s and 68°C for 21 min, with a final extension of 68°C for 7 min. The annealing temperature for the first PCR was 60°C, and the annealing temperature for the second PCR was 55°C.
Cell culture.
The NIKS cell line (also referred to previously as the BC1-ep/SL cell line) is a spontaneously immortalized keratinocyte cell line characterized previously (1) and which supports the HPV life cycle (10). The CIN-612 9E cell line was established from a CIN1 lesion and harbors HPV31b genome extrachromosomally (2). NIKS cells harboring HPV31a (established as detailed below), as well as the CIN-612 9E cell line, were maintained on a feeder layer of mitomycin C-treated fibroblasts in supplemented F medium as previously described (12). The HaCaT cell line is an immortalized keratinocyte cell line established from adult human skin cells (3). This cell line was maintained without feeders in F medium containing 10% fetal bovine serum, 100 U of penicillin/ml, and 100 μg of streptomycin/ml. The cells were passaged by dispersal with 0.1% trypsin-0.5 mM EDTA at 37°C.
Stable transfection of NIKS cells and establishment of clonal cell lines.
Five micrograms of recombinant, wild-type, or L2 mutant HPV31a plasmid was digested with EcoRI to release from the pBR322 bacterial vector, the EcoRI was then heat inactivated, the digest was diluted to 2.5 μg per ml in 1× ligase buffer, 10,000 U of T4 ligase (New England Biolabs, Beverly, Mass.) was added, and the solution was incubated at 16°C overnight. The ligation was diluted to 10 ml with buffer PB (QIAGEN, Valencia, Calif.) and then aspirated through a mini-prep column (QIAGEN). The DNA on the column was eluted with 50 μl of warm double-distilled H2O (65°C). Five microliters of the eluate was run on an agarose gel and stained with ethidium bromide to verify concentration and to ensure that ligations were primarily intramolecular. The remainder of the eluate was cotransfected with 1.2 μg of pEGFP-N1 (Clontech, Palo Alto, Calif.), which contains the neomycin resistance gene, into 3 × 105 NIKS cells as detailed previously (12).
Preparation and analysis of total genomic DNA.
NIKS cells grown to subconfluence on feeders had their feeders removed by EDTA treatment prior to being trypsinized and pelleted. The pellet was resuspended in 3 ml of lysis buffer (0.15 M NaCl, 0.10 M EDTA, 0.02 M Tris [pH 8], 1% sodium dodecyl sulfate [SDS]), supplemented with 100 μg of proteinase K/ml, and incubated at 37°C for 3 h. The lysate was extracted with an equal volume of phenol followed by phenol-chloroform-isoamyl alcohol (25:24:1) and finally chloroform-isoamyl alcohol (24:1). High-molecular-weight DNA was precipitated by the addition of two volumes of 100% ethanol (EtOH) to the aqueous phase, retrieved with a pipette, dried, and resuspended in 300 μl of Tris-EDTA (TE; pH 8) overnight. The low-molecular-weight DNA was precipitated by leaving the remaining ethanol lysate mixture at −20°C overnight, pelleting at 7,000 × g for 30 min at 4°C. The pellet was resuspended in 300 μl of TE (pH 8) and added to the high-molecular-weight DNA. Following incubation with 120 μg of RNase A at room temperature for 1 h, the DNA was extracted with an equal volume of phenol-chloroform-isoamyl alcohol (25:24:1) followed by chloroform-isoamyl alcohol (24:1), dialyzed against TE (pH 8) overnight, sheared by passing it through a 22-gauge needle 40 times, ethanol precipitated at −20°C overnight, pelleted at 14,000 rpm, 4°C for 30 min in an Eppendorf model 5415C microcentrifuge, resuspended in 50 μl of double-distilled H2O, and quantified by spectrophotometry at a 260-nm wavelength.
To examine for the presence of extrachromosomal, open circular and supercoiled forms of HPV31 DNA, 20 μg of total genomic DNA was digested with BamHI, which restricts neither HPV31a nor HPV31b. To determine copy number and to assess for the presence or absence of integrated forms of the viral genomes, 10 μg of total genomic DNA was digested with either EcoRI (for HPV31a clonal NIKS cell lines) or HindIII (for the CIN-612 9E cell line) to linearize the HPV31a or HPV31b genomes, respectively. Copy number controls were made with serial dilutions of known amounts of linearized pHPV31 genomes. The DNA samples were analyzed by Southern blot hybridization by using an [α-32P]dCTP-labeled HPV31 DNA probe made with a random primer labeling kit (Amersham, Piscataway, N.J.). Following high-stringency washes, the Southern blot was analyzed using a phosphorimager.
Raft culture keratinocytes were grown in organotypic (raft) cultures to induce differentiation as described previously (12). To prepare raft cultures for immunohistochemistry, rafts were incubated with medium containing 10 μM bromodeoxyuridine (BrdU; Sigma) for 8 h on day 11 postlifting, just prior to harvest. The entire raft culture, including the dermal equivalent, was embedded in 2% molten agar-1% formalin, fixed in 4% formalin overnight, processed, embedded in paraffin, and cut into 5-μm-thick cross-sections. Alternatively, to prepare rafts for harvesting virus, the stratified keratinocyte tissue was separated from the dermal equivalent by using a tweezer, and the tissue was frozen at −80°C until used for recovering virus (see below).
Immunohistochemistry.
Cells that had incorporated BrdU were detected in the paraffin-embedded sections as described previously (12). Percentage of BrdU incorporation was quantified by counting BrdU-positive cells in the supraparabasal layer of raft cultures and dividing by the total number of BrdU-positive cells. A minimum of 10 fields of view (400× magnification) were counted per raft culture, and five clones of wild-type and five clones of L2 mutants from six independent rafting experiments were counted. For CIN-612 9E and NIKS, four different rafts were counted. Relative percentage of BrdU incorporation was determined by setting the average percentage of BrdU incorporation of the wild-type clones from each rafting experiment equal to 1.0. The percentage of BrdU incorporation of each raft was then divided by the average percentage of rdU incorporation of the wild-type clones to obtain a relative percentage of BrdU incorporation. These numbers were compared using the Wilcoxon rank sum test on the Mstat program.
To detect the L1 and L2 proteins, sections of rafts were deparaffinized in xylene and rehydrated through a series of alcohols, and endogenous peroxidase activity was quenched by incubation in 3% H2O2 in methanol for 15 min, washed in phosphate-buffered saline (PBS), unmasked by boiling in 10 mM sodium citrate, pH 6, for 20 min, cooled to room temperature, washed in PBS, and blocked in 1.5% horse serum (Vector) in PBS for 30 min at room temperature. The primary antibody was applied in 1.5% horse serum-PBS and incubated at room temperature for 3 h. The sections were washed in PBS, and the primary antibody was detected with the Vectastain ABC kit (Vector) as described by the manufacturer, using the supplied secondary antibody and developed using the DAB kit (Vector, Burlingame, Calif.) as described by the manufacturer. Sections were counterstained with hematoxylin (Vector) as described by the manufacturer. For L1 staining, H16.D9 (generous gift of Neil Christensen [5]) was used at a 1:1,000 dilution. For L2 staining, rabbit antiserum to full-length six-His-tagged HPV31 L2 (generous gift of Richard Roden), and herein called αHPV31-L2, was used at a 1:2,000 dilution.
FISH.
To detect cells that harbored amplified viral DNA, sections of raft cultures were probed with a digoxigenin (DIG)-labeled DNA probe specific for HPV31 DNA. The probe was generated using the BioPrime DNA labeling system (Invitrogen, Carlsbad, Calif.) as described by the manufacturer, except that a unique mixture of deoxynucleoside triphosphates (2 mM dATP, 2 mM dCTP, 2 mM dGTP, 1.3 mM dTTP, and 0.7 mM DIG-11-dUTP) was used in place of the dexoxynucleoside triphosphates provided. Linearized pHPV31 was used as a template. The probe was then purified using a spin column (Bio-Rad, Hercules, Calif.), precipitated using 0.5 volumes of Cot-1 DNA (Invitrogen), 0.02 volumes of herring sperm DNA (Gibco, Carlsbad, Calif.), 0.33 volumes of 7.5 M sodium acetate, and two volumes of 100% EtOH. The pellet was resuspended in 200 μl of Hybrisol VII CEP hybridization buffer (Vysis, Downers Grove, Ill.) for every 50 μl of original probe synthesis reaction mixture. Just before application, this probe mixture was heated to 75°C for 5 min and then kept warm at 37°C until application. Histology slides containing the raft sections were incubated at 65°C overnight, deparaffinized with xylenes three times at 15 min each, dehydrated with 100% EtOH two times for 10 min each, air dried, boiled in 10 mM sodium citrate buffer (pH 6) for 30 min, washed with 2× SSC (0.3 M NaCl plus 0.03 M sodium citrate), further unmasked by a 10-s incubation with Digest-All-3 (Zymed, San Francisco, Calif.) at 37°C, washed again in 2× SSC, dehydrated through a series of alcohols, air dried, treated with a denaturing buffer (70% formamide, 2× SSC) for 5 min, dehydrated through a series of cold (4°C) alcohols (70 to 100%), and allowed to air dry. For the hybridization step, 20 μl of probe solution was added to each slide, the solution was sealed in with a coverslip and nail polish, and the slides were incubated in a moist chamber at 37°C for 1 week. Following removal of the coverslips, the slides washed for 2 min in 0.5× SSC heated to 76°C and washed with 2× SSC and 0.1% NP-40 (room temperature). Twenty microliters of fluorescein isothiocyanate-conjugated anti-DIG antibody (Roche) diluted 1:400 in CAS block (Zymed) was applied to each slide, covered with a coverslip to evenly disperse the antibody, and incubated at 37°C for 25 min. The slides were washed with 2× SSC and 0.1% NP-40 at room temperature. Then, VectaMount medium containing 4′,6′-diamidino-2-phenylindole (DAPI; Vector) was applied, covered with a coverslip, and sealed with nail polish (Wet ‘n’ Wild, City of Industry, Calif.). Fluorescent in situ hybridization (FISH) was detected using a Zeiss Axiovert fluorescence microscope, and images were obtained using a Zeiss digital camera system.
The percentage of FISH-positive cells was quantified by counting FISH-positive cells and dividing by the total number of cells (DAPI-positive cells). A minimum of 10 fields of view (400× magnification) were counted per raft culture, and three clones of the wild type and three clones of L2 mutants from four independent rafting experiments were counted. For CIN-612 9E, four different rafts were counted. Positive cells were not observed in the NIKS rafts and were therefore not counted. A relative percentage of FISH-positive cells was determined by setting the average percentage of FISH-positive cells of the wild-type clones from each rafting experiment equal to 1.0. The percentage of FISH-positive cells of each raft was then divided by the average percentage of FISH-positive cells of the wild-type clones to obtain a relative percentage of FISH-positive cells. These numbers were compared using the Wilcoxon rank sum test on the Mstat program.
Isolation of virions.
Virions were isolated from 10 rafts of each cell line by a slightly modified version of a protocol developed previously (22). Following the high-speed centrifugation at 130,000 × g, the supernatant was carefully poured off and the pellet was drip dried. The pellet was resuspended in 500 μl of buffer (50 mM NaCl, 50 mM sodium phosphate; pH 7.4) and transferred to a 2-ml Dounce homogenizer and homogenized, by hand, 12 to 15 times to break apart any clumps. The resulting homogenate was centrifuged at 8,000 × g in a microcentrifuge at 4°C for 10 min. The supernatant was reserved, and the pellet was reextracted as described above. The pooled supernatant was termed the crude preparation of virus.
Note: the above virus isolation protocol may produce aerosols of infectious HPV and therefore was carried out under modified biosafety level 2 conditions that included the following critical requirements. Personnel wore disposable lab coats, two layers of gloves, a HEPA-filtered mask, and eye protection. All handling of virus samples was carried out in a biosafety cabinet. All waste was autoclaved before being disposed, and any reusable equipment that had come into contact with the virus preparation was soaked in a virucidal agent (such as CiDecon or Vesphene) for 10 min before being washed. Any equipment in the hood that had not come into contact with the preparation was treated with 70% EtOH before being removed from the hood.
Transmission electron microscopy (TEM).
Virus particles were visualized under an electron microscope by using a negative stain. Briefly, 5 μl of sample was mixed with an equal volume of Nano-W (methylamine tungstate; Nanoprobes, Inc.). Pioloform (Ted Pella, Inc.)-coated grids were exposed to the sample negative stain mixture for approximately 1 min to allow adsorption to the grid surface. Excess moisture was removed by wicking with filter paper until dry. The grids were immediately viewed and documented on a Philips CM120 (FEI Corp., Eindhoven, The Netherlands) at 80 kV.
Production of GST-L1 and GST-L2 standards.
To produce purified, recombinant glutathione S-transferase (GST)-L1 and GST-L2 for quantification purposes, the HPV31 L1 and L2 genes were cloned into the multiple cloning site of the pGEX-3X vector (Amersham Biosciences). Using PCR, the L1 open reading frame was amplified with pHPV31 as a template and the following primers, which surround the L1 open reading frame and include additional BamHI and EcoRI/XmaI linkers, respectively: 5′ cgaggatccAGATGTCTCTGTGGCGGCCTAGCGAG 3′ and 5′ gctgaattcccgggTATTACATACACATCCATTACTTTTTAG 3′ (linkers are shown in lowercase). The PCR was done using Pfu Turbo, and the reaction was cycled as follows: 95°C for 1 min; five cycles of 95°C for 30 s, 50°C for 30 s, and 72°C for 2 min; 30 cycles of 95°C for 30 s, 65°C for 30 s, and 72°C for 2 min; and 72°C for 7 min. To amplify L2, pHPV31 was used as the template and the following primers, which surround the L2 open reading frame and contain BamHI and EcoRI/XmaI linkers, respectively, were used: 5′ cgaggatccCCATGCGGTCCAAACGCTCTACAAAACG 3′ and 5′ gctgaattcccgggGGTAAGTAGACAGTAGCCTCGCTAG 3′ (linkers are shown in lowercase).
The PCR was carried out with Pfu Turbo, and the reaction was cycled as follows: 95°C for 1 min; five cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 2 min; 30 cycles of 95°C for 30 s, 64°C for 30 s, and 72°C for 2 min; and 72°C for 7 min. Both PCR products were cut with BamHI and EcoRI and ligated into pGEX-3X, also cut with BamHI and EcoRI. Both plasmids were transformed into BL-21 E. coli cells (Stratagene), and clones were identified and confirmed by restriction enzyme analysis. Expression of GST-L1 and GST-L2 fusion proteins was induced as follows. Overnight cultures were diluted 1:200 and allowed to grow to an optical density of 0.6 to 0.8 at 37°C with shaking. Isopropyl-β-d-thiogalactopyranoside was added to 0.3 mM, and the cultures were shifted to 20°C and incubated with shaking for 1.5 h. The bacteria were collected by centrifugation, resuspended in NETN lysis buffer (20 mM Tris-HCl [pH 8.0], 100 mM NaCl, 1 mM EDTA, 0.5% NP-40, 0.1 mM phenylmethylsulfonyl fluoride [PMSF], 0.02 mg of aprotinin/ml), and sonicated six times for 10 s at 4°C, and the lysate was clarified by centrifugation at 14,500 × g for 10 min. The supernatant was incubated with glutathione-Sepharose beads (Pharmacia), with mixing at 4°C for 1 h. The beads were collected by centrifugation (500 × g, 4°C, 5 min), washed twice with NETN buffer, and washed twice with wash buffer (50 mM Tris-HCl [pH 8.0], 50 mM NaCl, 1 mM EDTA, 0.01% NP-40, 0.1 mM PMSF), and the fusion proteins were eluted by rotating beads two times with an equal volume of elution buffer (wash buffer plus 10 mM reduced glutathione), for 30 min each time. The purified GST-fusion proteins were quantified by running dilutions on an SDS-10% polyacrylamide gel along with dilutions of a known bovine serum albumin standard. The gel was stained with Coomassie blue, and the intensities of staining of the relevant protein bands were compared to obtain approximate GST-fusion protein concentrations.
Western blotting of L1 and L2.
Various volumes of the crude preps were analyzed by SDS-10% polyacrylamide gel electrophoresis along with size markers and either dilutions of GST-L1 or GST-L2. The protein was transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore). For both L1 and L2 detection, the membrane was blocked overnight at 4°C in 5% milk-PBST (PBS with 0.1% Tween 20). The membrane was incubated with primary antibody at room temperature for 3 h. The secondary antibody was applied for 30 min at room temperature. For L1 detection, H16.D9 (see “Immunohistochemistry”) was diluted in 5% milk-PBST at a 1:10,000 dilution and detected with a peroxidase-conjugated anti-mouse secondary antibody (Jackson ImmunoResearch) at a 1:10,000 dilution in 5% milk-PBST. For L2 detection, αHPV31-L2 (see “Immunohistochemistry” above) was diluted 1:2,000 in 5% milk-PBST. The secondary antibody was a peroxidase-conjugated anti-mouse antibody (Sigma) diluted 1:10,000 in 5% milk-PBST. The peroxidase was detected using the ECL Plus reagent (Amersham Biosciences) according to the manufacturer's instructions. The blot was initially exposed to film and then scanned in a phosphorimager set to the fluorescence setting (100 μm, 900 V for the blue wavelength). The bands were quantified using ImageQuant and compared to the GST standards to determine protein amounts, and those amounts were divided by the volumes to obtain approximate L1 and L2 concentrations.
DNase treatment.
Crude virus preps were incubated in DNase buffer (50 mM NaCl, 50 mM Na-phosphate [pH 7.4], 10 mM MgCl2, 0.1 mM PMSF) with 3 ng of spike DNA (pUC19) and 10 U of DNase (Roche). The digest was incubated at 37°C for 1 h. The DNase was inactivated with the addition of EDTA to 50 mM. After mixing and incubating for 5 min at room temperature, dithiothreitol was added to 10 mM to help dissociate the virus. Immediately, an equal volume of phenol was added, the mixture was extracted for the aqueous phase, and the extraction was repeated with equal volumes of phenol-chloroform-isoamyl alcohol (25:24:1) and then with chloroform-isoamyl alcohol (24:1). The aqueous phase was then dialyzed 1:100 against TE (pH 8) overnight with one change of buffer. The DNA was precipitated with a 1/10 volume of NaOAc, 10 μg of tRNA/ml, and 2.5 volumes of 100% EtOH at −20°C overnight. After centrifugation, the pellet was resuspended in 30 μl of TE (pH 8), and the entire amount was loaded onto a 0.8% agarose gel along with size markers and copy number standards (pHPV31, diluted appropriately). The gel was then Southern blotted as described above. The DNA bands were quantified using a phosphorimager and ImageQuant software. The amount of DNase-resistant DNA was determined by comparing to the copy number standards. The amount of DNA was divided by the amount of L1 that had been in the initial DNase digest. This number (copies of HPV31 per nanogram of L1) was set relative to the wild type by dividing each number by the average of the wild type for each experiment.
Infections and detection of infections.
On the day prior to infection, 3 × 105 HaCaT cells were seeded into a 12-well dish. The next day the cells were washed with PBS, and virus (equal amounts of L1) was added to 300 μl of serum-free medium. Tenfold dilutions (with serum free medium) were made, and the cells were then incubated with 250 μl of these virus mixtures at 4°C with gentle rocking for 1 h. After 1 h the virus was aspirated and the cells were washed twice with PBS. New medium was added, and the cells were incubated at 37°C for 2 days.
Total RNAs were extracted from cells using TRIzol reagent (Invitrogen Life Technologies), and their concentrations were determined by quantifying absorbance at 260 nm. RNA concentrations and quality were verified by electrophoresis through agarose gels containing ethidium bromide. RNAs (3.0 to 5.0 μg) were reverse transcribed using random hexamer primers in a final volume of 20 μl. End-point and quantitative PCR (qPCR) were performed using GeneAmp RNA PCR reagents and AmpliTaq Gold DNA polymerase (Applied Biosystems) under conditions previously reported (21, 22). For qPCR 5 μl of each cDNA reaction mixture was analyzed in triplicate. HPV31b primers E7.4A and E4B (21, 22) were used at 200 nM each and the Taqman probe spanning the E1^E4 splice site (6-carboxyfluorescein-CAG TGA CGA AAT ATC CTT TGC TGG GAT TGT T-tetramethyl carboxyrhodamine) was used at final concentration of 100 nM with 2.5 mM MgCl2. Oligonucleotides were synthesized by Sigma Genosys. An ABI 5700 qPCR machine was used for the PCR amplifications and data analysis. The PCR thermocycling profile was as follows: 2 min at 50°C, 12 min at 95°C, 40 to 50 cycles at 95°C for 15 s, and 65°C for 30 s. Copy number controls from 105 to 101 were made from serial 10-fold dilutions of a cDNA template of cloned E1^E4 or of PCR amplicons. Acceptable slope values were between −3.2 and −3.5, and correlation values were between −0.9800 and −0.9999. Results are shown as the average of two to three values within zero to four threshold cycle (Ct) standard deviations (N. Patterson, J. Smith, and M. Ozbun, submitted for publication).
RESULTS
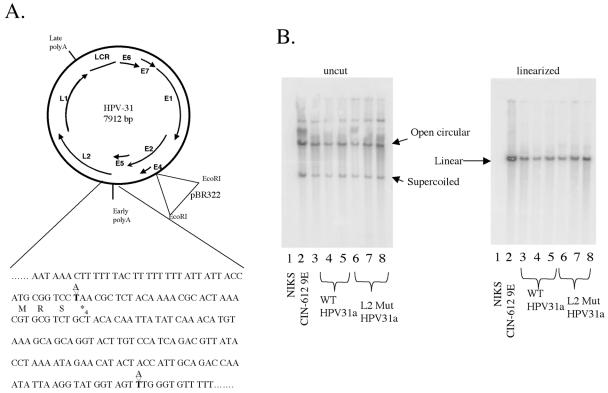
To determine the function of L2 in the HPV life cycle, we compared the properties of the wild-type HPV31a genome to that of an L2 mutant HPV31a genome in an immortalized human foreskin keratinocyte cell line (NIKS) that is known to support the full HPV life cycle (10). To eliminate L2 expression, we introduced two point mutations in the L2 translational open reading frame by using site-directed mutagenesis (Fig. 1A). The first mutation changed the fourth codon in the coding region from a lysine to a stop codon. The second mutation substituted an internal ATG codon at position 51 to a TTG to eliminate any possibility of alternative translational initiation within the L2 open reading frame. The entire genome was sequenced to ensure that no additional mutations were introduced during the amplification reactions.
FIG. 1.
Establishment of human keratinocyte cell populations harboring wild-type or L2 mutant HPV31. (A) Mutation of the L2 open reading frame. AAA of codon 4 was mutated to TAA to introduce a stop codon. ATG of codon 51 was mutated to TTG to eliminate the possibility of reinitiation of translation. (B) Southern analysis of total genomic DNA from clonal NIKS populations transfected with HPV31 DNA. Equal amounts of total genomic DNA were loaded for each cell line. The three lanes labeled WT HPV31a are from three different wild-type clonal populations, and the lanes labeled L2 Mut HPV31a are from three different L2 mutant clonal populations. Left panel: DNA was loaded uncut. Right panel: DNA was cut with a restriction enzyme that linearized the HPV31 genome. Position of open circular, supercoiled, and linear HPV31 DNA bands are indicated based upon the migration pattern of the positive control DNA (CIN-612 9E) loaded in the second lane on each gel and based upon molecular weight standards (not shown). The negative control was total genomic DNA isolated from untransfected NIKS cells (lane 1 in each gel).
L2 is not required for the establishment and maintenance of the genome after transfection.
Circularized wild-type and L2 mutant HPV31a genomes were transfected in the presence of a plasmid encoding the neomycin resistance gene into NIKS cells, and colony clones were isolated. Following transfection, cells were selected with G418 for 4 days, drug-resistant cells were allowed to grow out for approximately 10 days, colonies of transfected cells were cloned, and the resulting clonal cell lines were established and expanded. We used the CIN-612 9E cell line as a positive control, as this cell line harbors the HPV-31b genome extrachromosomally and has been shown to yield infectious virions in raft cultures (22).
Keratinocytes grown subconfluently in F medium on fibroblast feeders retain a poorly differentiated state reminiscent of the basal compartment of stratified squamous epithelia, the compartment in which papillomaviruses establish a nonproductive state characterized by the establishment and maintenance of the viral genome as a low-copy, extrachromosomal plasmid. To determine whether L2 is required for this nonproductive infectious state, we monitored the presence and state of the viral genomic DNA in the clonal lines of transfectant cells grown on feeders in F medium by analyzing total genomic DNA using Southern analysis. When we analyzed total genomic DNA digested with a restriction enzyme that does not cleave the HPV31 genomes, both the wild-type and the L2 mutant HPV31a genomic DNA in the transfected NIKS cell clonal populations banded in a pattern characteristic of an extrachromosomal plasmid. The presence of open circular as well as supercoiled HPV31 DNA was identical to that seen in the positive control CIN-612 9E (Fig. 1B, left panel). When the total genomic DNA was cut with a restriction enzyme that linearizes the HPV genome (Fig. 1B, right panel), the viral DNA banded uniquely at approximately 8,000 bp, the expected size for unit-length viral genomes, indicating an absence of any detectable rearrangements or integration of the viral DNA within these transfected cell populations. The copy number generally was found to be equivalent (between 25 and 50 copies per cells) among the clonal cell lines, regardless of whether the genomes were mutant or wild type for L2. The copy number in the CIN-612 9E cell line was slightly higher, at about 50 copies per cell. Because no difference was seen in the presence and state of the viral DNA in the cell populations transfected with wild-type versus L2 mutant HPV31a genomes, we conclude that L2 is not required for the establishment and maintenance of the viral genome, a hallmark of the nonproductive infectious state, upon transfection.
L2 is not required for the induction of suprabasal DNA synthesis.
The productive stage of the viral life cycle, in which progeny virus is made, arises within the terminally differentiating compartment of stratified squamous epithelia. This stage of the viral life cycle can be recapitulated in tissue culture using an organotypic or raft culturing technique (10, 18). We therefore analyzed the role of L2 in the productive stage of the viral life cycle by placing the clonal NIKS populations harboring wild-type or L2 mutant HPV31a genomes in raft cultures. Briefly, cells grown on feeders were trypsinized and plated onto a dermal equivalent composed of fibroblasts embedded in collagen. The keratinocytes were allowed to grow to confluence prior to being lifted above the air-liquid interface. Eleven days postlifting, when maximum differentiation is seen, raft cultures were harvested, fixed, and embedded in paraffin for histological analysis.
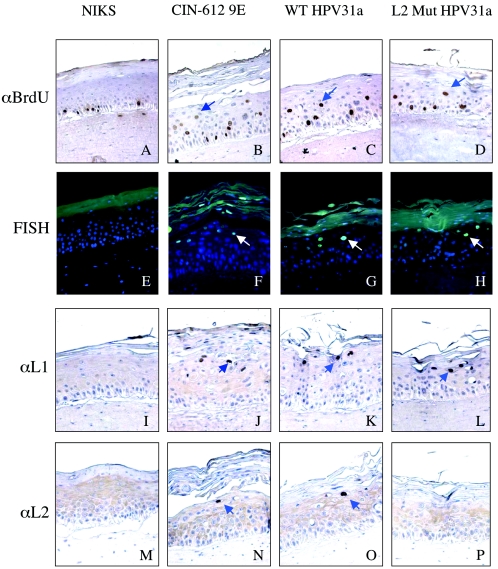
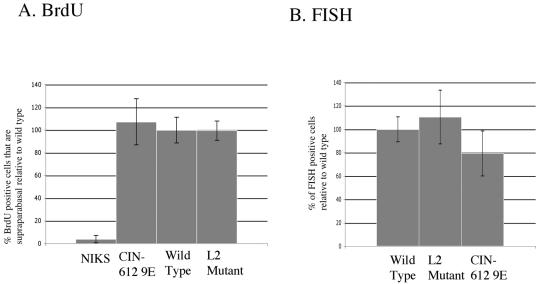
One of the best-known hallmarks of HPV infection is the induction of DNA synthesis in cells found above the basal layer that have normally exited the cell cycle. This reprogramming of suprabasal cells to support DNA synthesis is thought to be required for amplification of the viral DNA that is necessary for production of progeny virus. To monitor for this suprabasal DNA synthesis, we incubated our raft cultures in the presence of BrdU, a nucleotide analogue that is incorporated into any nascent DNA, for 8 h prior to their being harvested. We then performed BrdU-specific immunohistochemistry on the resulting histological cross-sections of the various raft cultures (Fig. 2A to D). In HPV-negative NIKS cells (Fig. 2A), BrdU incorporation, indicative of cells supporting DNA synthesis, was restricted to the basal compartment. This is equivalent to what is seen in normal stratified squamous epithelia in vivo and reflects the fact that, normally, only basal cells are able to enter S phase to initiate new rounds of DNA synthesis. In contrast, in the HPV-positive CIN-612 9E cells (Fig. 2B), BrdU-positive cells were observed both in the basal and suprabasal compartments, consistent with prior studies demonstrating an ability of HPVs to reprogram suprabasal cells to support DNA synthesis. The same pattern of BrdU-positive cells in both the basal and suprabasal compartment was observed in the NIKS cells harboring the wild-type (Fig. 2C) or L2 mutant (Fig. 2D) HPV31a genome. We quantified the abundance of BrdU-positive cells in the suprabasal compartment in raft cultures generated with at least three independent clonal populations harboring wild-type or L2 mutant genomes and compared that to the values seen in HPV-negative cells or the HPV-positive, CIN-612 9E cells (Fig. 3A). We saw no difference in the level of suprabasal DNA synthesis in the L2 mutant versus wild-type HPV31 cell populations (P = 0.7 by Wilcoxon rank sum test). From these experiments we conclude that L2 does not play a role in the induction of suprabasal DNA synthesis.
FIG. 2.
Immunohistochemical analyses of rafts. (A to D) Immunohistochemistry for BrdU incorporation. (E to H) FISH with fluorescein isothiocyanate-conjugated antibody specific for DIG-labeled HPV31 DNA probe. (I to L) Immunohistochemistry for L1 expression with H16.D9 antibody. (M to P) Immunohistochemistry for L2 expression with αHPV31-L2 antibody. For all panels displaying immunohistochemical staining (A to D and I to P), brown cells are positive and are indicated with blue arrows. Counterstain was done with hematoxylin. For all panels displaying immunofluorescent staining (E to H), cells positive for FISH signal have green nuclei (white arrows), whereas FISH-negative cells have blue nuclei (a consequence of the DAPI counterstain). Sections present in the first column (A, E, I, and M) are taken from the negative control NIKS rafts, those in the second column (B, F, J, and N) are from the positive control CIN-612 9E rafts, those in the third column (C, G, K, and O) are from wild-type (WT) HPV31a-harboring rafts, and those in the fourth column (D, H, L, and P) are from L2 mutant HPV31a-harboring rafts.
FIG. 3.
L2 is not needed to induce suprabasal DNA synthesis or for amplification of viral DNA. (A) Quantification of suprabasal BrdU incorporation. Bars show percent BrdU-positive nuclei that are supraparabasal, relative to the average value obtained for rafts harboring wild-type HPV31. (B) Quantification of the amplification of viral DNA. Bars represent percent nuclei (detected by DAPI) that are positive for amplified viral DNA relative to the average value obtained for rafts harboring wild-type HPV31. These data were quantified as described in Materials and Methods.
L2 is not required for the amplification of viral DNA.
To observe whether viral genomes are amplified when L2 is mutated, we hybridized sections of our raft cultures with a labeled probe specific for HPV31 DNA. We then detected which nuclei harbored amplified viral DNA by using FISH. We were able to find nuclei that had amplified viral DNA in the more superficial layers of the suprabasal compartment in sections of rafts generated with CIN-612 9E (Fig. 2F), as well as the NIKS cells harboring wild-type (Fig. 2G) or L2 mutant (Fig. 2H) HPV31 genome, but not in the sections from rafts made with HPV-negative NIKS (Fig. 2E). We quantified the abundance of cells with amplified viral DNA in at least three independent clonal populations of cells harboring either wild-type or L2 mutant HPV31 DNA (Fig. 3B). We saw no statistically significant difference (P = 0.47 by Wilcoxon rank sum test) in the frequency of FISH-positive cells between populations harboring wild-type versus L2 mutant genomes. Nor did we see any overt differences in the intensity of fluorescence in those cells that were positive for FISH between populations harboring wild-type versus L2 mutant genomes (Fig. 2). Thus, within the limitations of the experimental methodology employed, we found no evidence to support a role of L2 in viral DNA amplification.
L2 is not required for the expression of L1.
We next evaluated the expression of the two capsid proteins in the raft cultures. First we monitored L2 expression by immunohistochemistry. Strong nuclear staining for L2 was observed in cells in the more superficial layers of the suprabasal compartment in raft cultures generated with the positive control, CIN-612 9E (Fig. 2N), as well as NIKS harboring wild-type HPV31a genomes (Fig. 2O), confirming that these raft cultures support late gene expression. Diffuse, light staining in the cytoplasm seen throughout the strata in these sections represents background staining, as evidenced by a similar diffuse, light cytoplasmic staining in the HPV-negative NIKS cells (Fig. 2M). Importantly, no cells with strong nuclear staining were seen in the rafts of NIKS cells harboring L2 mutant genomes (Fig. 2P), as was the case in the HPV-negative NIKS (Fig. 2M), confirming that the L2-mutation had inhibited L2 expression. The absence of L2, however, had no effect on L1 expression. Strong L1-positive nuclear staining was seen in the more superficial layers of the suprabasal compartment in rafts generated with CIN-612 9E (Fig. 2J) and NIKS harboring wild-type HPV31a genomes (Fig. 2K), as well as those harboring L2 mutant genomes (Fig. 2L). We therefore conclude that expression of L2 is not required for the expression of L1.
L2 is not required for the formation of virus particles.
The next stage of the viral life cycle we monitored was the formation of virus particles. To evaluate virus particle production, raft cultures were homogenized and the homogenate was subjected to low-speed centrifugation to pellet unbroken cells and large cellular debris. The supernatant then was subjected to high-speed centrifugation to pellet the virus (22). This pellet was resuspended and hereafter is called the crude preparation of virus.
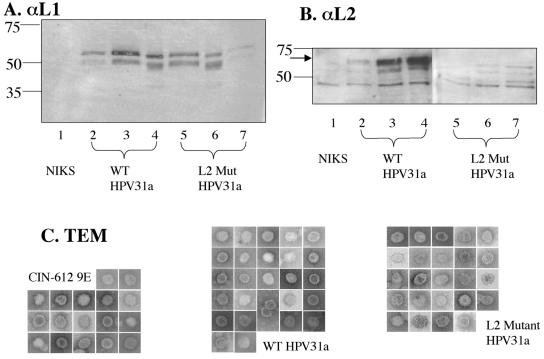
We first analyzed these crude preps by Western blotting and TEM (Fig. 4). We were able to detect L1 in the crude preps from rafts generated with NIKS harboring wild-type or L2 mutant HPV31a, or HPV31b-positive CIN-612 9E, but not in the crude preps from rafts generated with the HPV-negative NIKS cells (Fig. 4A). We consistently found two bands for L1, one migrating around the predicted size (54 kDa) and one migrating slightly faster. Furthermore, we consistently observed that the faster-migrating band was proportionately more abundant in the crude preps from rafts harboring the L2 mutant HPV31a genome. In contrast, the slower-migrating band dominated in the crude preps from rafts harboring the wild-type HPV31a or HPV31b. To quantify the amount of L1 in our crude preps, we compared the signal from both bands in each lane to known amounts of bacterially synthesized GST-HPV31 L1 protein. We found no difference in the yield of L1 from the L2 mutant HPV31-harboring rafts compared to the wild-type HPV31-harboring rafts. If all the L1 contained in these high-speed fractions was present in virus particles, the average yield of virus particles would be 2 × 107 particles per raft culture. This yield is similar to that obtained standardly in the Ozbun laboratory (Patterson et al., submitted).
FIG. 4.
Analysis of crude preparations of virus. (A and B) Immunoblot (Western) analyses of equal volumes of crude virus preparations from rafts of three different clonal NIKS populations each harboring wild-type (WT; lanes 2 to 4) or L2 mutant (L2 Mut; lanes 5 to 7) HPV31a genomes, along with a sample prepared in parallel from rafts of the negative control (untransfected) NIKS cell line (lane 1). Numbers at left indicate molecular mass in kilodaltons. In panel A, L1 was detected using the monoclonal antibody H16.D9. Note the lower levels of L1 detected in lanes 2 and 7, which reflect these particular samples yielding less virus. In panel B, L2 was detected using a polyclonal antibody to HPV31 L2. The arrow indicates the L2-specific band. Note the presence of multiple nonspecific bands that migrated faster than the L2-specific band and are present in the negative control sample isolated from rafts of untransfected NIKS (lane 1). (C) TEMs of particles of about 55 nm in diameter. The side of each square is 100 nm long. Images are shown at 110,00× magnification. No particles of 55 nm in diameter were seen in the crude preps of NIKS cells.
When we immunoblotted for the L2 protein, we were able to detect L2 protein in the crude preps from rafts harboring wild-type HPV31a or HPV31b, as indicated by the band migrating above the position of faster-migrating background bands, that were also seen in the HPV-negative NIKS crude prep with this polyclonal antibody (Fig. 4B). Importantly, the L2-specific band was not evident in the crude preps from rafts harboring the L2 mutant HPV31a genome (Fig. 4B).
We performed negative-staining TEM to detect and characterize virus particles. Icosahedral virus particles of approximately 50 nm in diameter were evident in crude preps from rafts harboring wild-type or L2 mutant HPV31a genomes (Fig. 4C), but not in the crude preps from NIKS cells (data not shown). Therefore, L2 is not required for the formation of virus particles. While one cannot accurately quantify the yield of virus particles using the EM methodologies employed here, no gross differences were observed in the abundance of particles visualized among multiple samples analyzed from rafts harboring wild-type or L2 mutant HPV31a genomes.
L2 is quantitatively required for the production of DNase-resistant viral genomes.
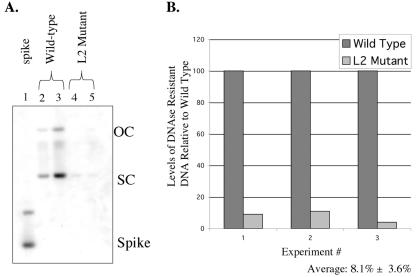
We next measured the amount of viral genomes that had been encapsidated in virus particles. In the absence of DNase treatment, there was a large amount of both HPV31 DNA as well as nonspecific cellular DNA in the crude preps, based upon ethidium bromide staining of DNAs resolved on agarose gels (data not shown). DNase treatment was therefore carried out to degrade the DNA in our preps that was not encapsidated. We monitored the completeness of DNase digestion by assessing the completion of digestion of “spike” bacterially synthesized DNA that was added to the crude preps prior to the addition of DNase. After DNase digestion, and inactivation of the DNase, we extracted the remaining DNA and performed Southern analysis (Fig. 5A). Completion of digestion was evident by the lack of detectable spike DNA. We found that the L2 mutant crude viral preps consistently harbored less DNase-resistant DNA than the wild-type viral crude preps. To quantify the amount of DNase-resistant DNA, we measured the hybridization signal with a phosphorimager and normalized the signal to the amount of L1 in each crude prep (as quantified by Western blotting using known amounts of GST-L1 protein). The yield of DNase-resistant DNA was measured in crude preps from three different clonal populations of NIKS harboring wild-type or L2 mutant HPV31a genomes. We found, on average, that there was only 8.1% of the level of DNase-resistant HPV31 DNA in the L2 mutant preps compared to that in the wild-type crude preps (Fig. 5B). Were all the L1 in the crude preps present in virus particles, these values would translate into, on average, 1 out of 20 wild-type HPV31 particles harboring encapsidated, DNase-resistant DNA, compared to approximately 1 out of 200 L2 mutant particles harboring encapsidated, DNase-resistant DNA.
FIG. 5.
Virus particles lacking L2 contain less DNase-resistant DNA. (A) Representative Southern blot of DNase-resistant DNA obtained from virus preps harvested from rafts of NIKS harboring wild-type (lanes 2 and 3) or L2 mutant (lanes 4 and 5) genomes. Lane 1, bacterially synthesized plasmid DNA that was spiked into the preparations of virus prior to DNase treatment to evaluate the completeness of DNase digestion. Note the absence of spike DNA in lanes 2 to 5. (B) Bar graph providing the quantification of the relative levels of DNase-resistant viral DNA in virus preps from rafts harboring wild-type or L2 mutant HPV31a genomes. Results are provided for three independent experiments. In each experiment, DNA was quantified from phosphorimager analyses of Southern analyses (an example of which is shown in panel A), evaluating levels of DNase-resistant DNA in virus preps from rafts of one or more independent clonal NIKS populations harboring either wild-type (dark bars) or L2 mutant (light bars) HPV31a genomes. For each experiment, the average level of DNase-resistant DNA in virus preps for rafts harboring wild-type HPV31a genomes was set to 100. Based upon the values obtained in these three independent analyses, the average relative amount of DNase-resistant DNA in the L2 mutant virus preps was 8.1% of that observed in the wild-type virus preps.
L2 is required for the production of infectious virus particles.
The final stage of the viral life cycle that we monitored was infectivity. For this purpose we used the HaCaT immortalized keratinocyte cell line (3), as prior studies demonstrated this cell epithelial cell line to be highly susceptible to HPV31b infection as judged by the level of early viral transcripts that accumulate in these cells at early times postexposure to HPV31b virus particles (21, 22). Following the prior established protocol, we incubated HaCaT cells with our crude virus preps and 10-fold serial dilutions of the virus preps at 4°C for 1 h to allow for binding, but not entry, of virus particles. Then, any unbound virus was washed away and the cells were incubated at 37°C for 2 days. Total RNA was then harvested, and we used reverse transcription-PCR (RT-PCR) to specifically amplify two early spliced viral mRNA transcripts, as well as a control cellular mRNA (β-actin).
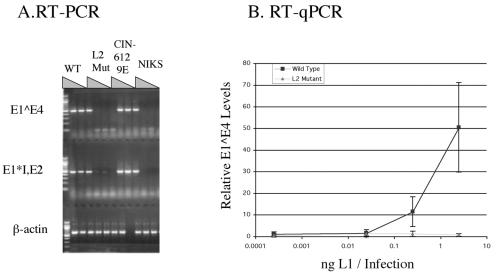
We found that while spliced E1*, E2, and E1^E4 HPV31 transcripts were easily amplified from cells infected with wild-type virus preps, the RNA harvested from cells infected with L2 mutant virus preps contained such low copies of viral transcripts that even after 40 rounds of PCR it remained undetectable or barely detectable (Fig. 6A). This experiment was semiquantitative in that we infected cells with 10-fold serial dilutions of the crude virus preps. Even at the lowest amount of inoculum (1:100 dilution), the wild-type virus infections yielded much higher relative numbers of amplimers than seen with the undiluted L2 mutant virus preps. From this we concluded that the L2 mutant virus preps must be at least 100-fold less infectious than the wild-type virus preps.
FIG. 6.
L2 is required for production of infectious particles. Shown are RT-PCR (A) and Taqman PCR (B) analyses of RNA isolated from HaCaT cells exposed to virus preps isolated from rafts of NIKS cells harboring wild-type (WT) or L2 mutant (L2 Mut) HPV31a genomes, untransfected NIKS cells harboring no viral genomes (NIKS), or the positive control cell line harboring HPV31b genomes (CIN-612 9E). (A) RT-PCR of spliced early viral transcripts, E1^E4 and E1*I, E2, and control PCR for β-actin transcripts. The three lanes of each genotype represent 10-fold serial dilutions of virus; the WT, L2 Mut, and CIN612 9E samples used had equivalent levels of L1 protein as judged by quantitative L1 Western blot analysis. For the negative control sample (NIKS), an equivalent volume of sample was that used for the other samples. A loading error accounts for the absence of the B-actin signal in one of the positive control (CIN612 9E) RT-PCR samples. (B) Quantitative RT-PCR. Tenfold dilutions of virus preps, quantified by Western blotting for L1, were used in independent infections of HaCaT cells. Equal amounts of total cellular RNA were used in RT, and triplicate cDNA samples were subjected to qPCR for E1^E4 transcripts. Relative levels of E1^E4 transcripts were determined by comparison with copy number standards. Values obtained from infections with wild-type virus are indicated by the box symbol, and for L2 mutant virus they are indicated by the triangle symbol; error bars represent standard errors of the means. Note that the level of RNA quantified in the cells infected with L2 mutant virus was not above background levels (i.e., they gave threshold values no greater than that seen with RNA from mock infections). Shown is one representative experiment. Similar results were obtained with other independent experiments using independent sources of wild-type and L2 mutant virus preps. In all cases wild-type virus gave robust RT-qPCR signals, whereas L2 mutant virus gave signals not above background levels seen with mock infections.
To quantify further the difference in efficiency of infectivity, we repeated the infections and performed real-time PCR on cDNA generated with reverse transcriptase from RNA harvested from cells infected with different dilutions of our crude virus preps (Fig. 6B). We found that the amount of viral transcripts increased with the amount of wild-type virus that was used in the infections. Viral transcripts from cells exposed to the L2 mutant viruses were undetectable above background level.
DISCUSSION
Because L2 is a late gene that is not expressed until the granular layer of cells, we expected that it would not function in any step prior to this stage in the papillomaviral life cycle. This hypothesis was supported by our studies using transfected NIKS populations. We found that there was no difference between the nonproductive stage induced by a wild-type HPV31 genome and that induced by an HPV31 genome not expressing L2. Both were established and maintained at an equal copy number, of around 30 copies per cell, after transfection of immortalized keratinocytes (Fig. 1B). Likewise, we saw no statistically significant difference between the amounts of suprabasal DNA synthesis induced by the wild-type genome and that from a genome unable to express L2 (Fig. 2 and 3). Amplification of the viral genome is observed around the time L2 is expressed. L2 has been shown to bind E2, a viral protein that is important in the replication of the viral genome (7, 13). It was therefore a distinct possibility that L2 might influence the process of viral DNA amplification. However, L2 had no measurable effect on the number of cells containing amplified viral DNA (Fig. 3) nor on the intensity of FISH signal among those cells that were positive (Fig. 2). Therefore, within the limitations of this experimental methodology, we did not observe any effect of L2 on the amplification of viral genomes in the productive stage of the viral life cycle.
Prior studies have also shown that L2 can affect the activity of E2 in transcriptional activation (13). Since L2 is expressed in the late stages, the absence of L2 might affect the expression of other late genes, including the major capsid protein, L1. However, L1 was found to be expressed in rafts harboring either the wild-type HPV31 or the L2 mutant HPV31 (Fig. 2) and gave yields of L1 in the virus preps that were similar for both the wild-type and L2 mutant HPV31a-harboring rafts (Fig. 4). Therefore, we conclude that L2 does not modulate L1 expression.
Our studies provide the first clear evidence that, in the context of the viral life cycle, L2 plays a critical role in the assembly of virions, in particular the encapsidation of the viral genome. Specifically, we found that virions derived from the raft cultures harboring the L2 mutant HPV31 contain less than 10% of the DNase-resistant genomes as wild-type virions (Fig. 5). This finding is significant, as the question of whether L2 plays a role in DNA encapsidation has been controversial based upon prior studies (see below). Importantly, those prior studies were not carried out in the context of the natural viral life cycle, as they were here. Rather, they made use of heterologous systems in which L1 and L2 were overexpressed in irrelevant cell types from heterologous promoters in the context of viral or plasmid-based expression vectors, and the viral genome or some heterologous plasmid to be packaged was provided in trans via transfection, sometimes under conditions in which the DNA being packaged was highly amplified using a simian virus 40 replication origin. These nonphysiological conditions likely contribute to the fact that in some cases L2 is observed to influence the degree of DNA encapsidation (4, 20, 25, 26, 32, 33) and in others it is not (28, 29).
How L2 functions to facilitate DNA encapsidation remains unclear. It is known to bind DNA but to do so nonspecifically. L2 can bind to E2 (13) and relocalize E2 to subnuclear domains along with L1 (7). E2 binds site specifically to the viral genome (17), which also is relocalized by L2 to the same subnuclear domains (7). Therefore, one distinct possibility is that L2 uses E2 to help recruit the viral genome to the site of virion assembly. Further study is necessary to test this hypothesis.
An alternative explanation for our finding that virions isolated from rafts harboring the L2 mutant HPV contained less DNase-resistant viral genomes is that L2-deficient virions are structurally less rigid and therefore the encapsidated DNA is more susceptible to DNase-induced degradation. Were this the case, our findings would not reflect a role of L2 in encapsidation. We did observe that the crude virus preps from rafts harboring the L2 mutant HPV genome accumulated a faster-migrating L1-specific protein band to a greater extent than the wild type (Fig. 4). This faster-migrating band, which was less abundant in virus preps from wild-type HPV-harboring rafts, may reflect a proteolytic cleavage product and, if so, could indicate that the L2-minus capsids are more permeable to proteases or that L1 exists in a different conformation in the absence of L2. Whether this inferred, increased proteolytic susceptibility of L1 is predictive of some increased susceptibility of packaged DNA to DNase degradation, however, is unknown. A role of L2 in early steps of infection was clearly demonstrated by our experiments quantitatively comparing the infectivity of the virion preparations with and without L2. We found that the infectivity was decreased by greater than 100-fold when L2 was not present, compared to that with the wild-type virions (Fig. 6). Because this reduction is greater than the 10-fold decrease in DNase-resistant DNA, we conclude that the defect in infectivity cannot be explained solely by a reduction in the abundance of DNase-resistant DNA, but rather a direct role of L2 in infectivity. This finding is consistent with recent data from other investigators in the field who have proposed a role of L2 in infection based upon analyses of virus-like particles assembled from various heterologous expression systems (29-31). Based upon these pseudovirus studies, a requirement for L2 in infectivity could reflect a role of L2 in receptor recognition (16, 30), the intracellular movement of the viral particle once it has entered (31; M. Sapp, personal communication), and/or the delivery of the viral genome to distinct domains within the nucleus (6). Further studies are needed to distinguish between these possibilities.
Acknowledgments
We especially thank Randall Massey of the UW Medical School Electron Microscopy Center who provided the TEM images and members of the McArdle Histology Facility who embedded and sectioned the raft cultures. We also thank Richard Roden for the HPV31 L2 antibody, Neil Christensen for the H16.D9 antibody, and Lou Laimins for the pHPV31 plasmid. We are indebted to Bob Garcea and Chris Buck for providing advice on protocols developed in these studies, and we thank Rolf Streeck and Martin Sapp for sharing their findings prior to publication and Teresa Compton, Paul Ahlquist, Dohun Pyeon, Ann Palmenberg, and Bill Sugden for helpful discussions.
This study was supported by grants from the NIH (P.F.L., CA22443 and AI52049; M.A.O., CA85747). S.C.H. was supported by the NIH-funded Molecular Biosciences Predoctoral Training Grant.
REFERENCES
- 1.Allen-Hoffman, B., S. Schlosser, C. Ivarie, L. Meisner, and S. O'Connor. 2000. Normal growth and differentiation in a spontaneously immortalized near-diploid human keratinocyte cell line, NIKS. J. Investig. Dermatol. 114:444-455. [DOI] [PubMed] [Google Scholar]
- 2.Bedell, M., J. Hudson, T. Golub, M. Turyk, Hosken, M., G. Wilbanks, and L. Laimins. 1991. Amplification of human papillomavirus genomes in vitro is dependent on epithelial differentiation. J. Virol. 65:2254-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boukamp, P., R. Petrussevska, D. Breitkreutz, J. Hornung, A. Markham, and N. Fusenig. 1988. Normal keratinization in a spontaneously immortalized aneuplid human keratinocyte cell line. J. Cell Biol. 106:761-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buck, C., D. Pastrana, D. Lowy, and J. Schiller. 2004. Efficient intracellular assembly of papillomaviral vectors. J. Virol. 78:751-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen, N., J. Dillner, C. Eklund, J. Carter, G. Wipf, C. Reed, N. Cladel, and D. Galloway. 1996. Surface conformational and linear epitoes on HPV-16 and HPV-18 virus like particles as defined by monoclonal antibodies. Virology 223:174-184. [DOI] [PubMed] [Google Scholar]
- 6.Day, P., C. Baker, D. Lowy, and J. Schiller. 2004. Establishment of papillomavirus infection is enhanced by promyelocytic leukemia protein (PML) expression. Proc. Natl. Acad. Sci. USA 101:14252-14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Day, P., R. Roden, D. Lowy, and J. Schiller. 1998. The papillomavirus minor capsid protein, L2, induces localization of the major capsid protein, L1, and the viral transcription/replication protein, E2, to PML oncogenic domains. J. Virol. 72:142-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fehrmann, F., D. J. Klumpp, and L. A. Laimins. 2003. Human papillomavirus type 31 E5 protein supports cell cycle progression and activates late viral functions upon epithelial differentiation. J. Virol. 77:2819-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flores, E., B. Allen-Hoffman, D. Lee, and P. Lambert. 2000. The human papillomavirus type 16 E7 oncogene is required for the productive stage of the viral life cycle. J. Virol. 74:6622-6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flores, E., B. Allen-Hoffman, D. Lee, C. Sattler, and P. Lambert. 1999. Establishment of the human papillomavirus type 16 (HPV-16) life cycle in an immortalized human foreskin keratinocyte cell line. Virology 262:344-354. [DOI] [PubMed] [Google Scholar]
- 11.Frattini, M., H. Lim, and L. Laimins. 1996. In vitro synthesis of oncogenic human papillomaviruses requires episomal genomes for differentiation-dependent late expression. Proc. Natl. Acad. Sci. USA 93:3062-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Genther, S., S. Sterling, S. Duensing, K. Muenger, C. Sattler, and P. Lambert. 2003. Quantitative role of the human papillomavirus type 16 E5 gene during the productive stage of the viral life cycle. J. Virol. 77:2832-2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heino, P., J. Zhou, and P. Lambert. 2000. Interaction of the papillomavirus transcription/replication factor, E2 and the viral capsid protein, L2. Virology 276:304-314. [DOI] [PubMed] [Google Scholar]
- 14.Kawana, K., K. Matsumoto, H. Yosikawa, Y. Taketani, T. Kawana, K. Yosiike, and T. Kanda. 1998. A surface immunodeterminant of human papillomavirus type 16 minor capsid protein L2. Virology 245:353-359. [DOI] [PubMed] [Google Scholar]
- 15.Kawana, K., H. Yosikawa, Y. Taketani, K. Yoshiike, and T. Kanda. 1999. Common neutralization epitope in minor capsid protein L2 of human papillomavirus types 16 and 6. J. Virol. 73:6188-6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawana, Y., K. Kawana, H. Yoshikawa, Y. Taketani, K. Yoshiike, and T. Kanda. 2001. Human papillomavirus type 16 minor capsid protein L2 N-terminal region containing a common neutralizing epitope binds to the cell surface and enters the cytoplasm. J. Virol. 75:2331-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McBride, A., H. Romanczuk, and P. Howley. 1991. The papillomavirus E2 regulatory proteins. J. Biol. Chem. 266:18411-18414. [PubMed] [Google Scholar]
- 18.Meyers, C., M. Frattini, J. Hudson, and L. Laimins. 1992. Biosynthesis of human papillomavirus from a continuous cell line upon epithelial differentiation. Science 257:971-973. [DOI] [PubMed] [Google Scholar]
- 19.Meyers, C., T. Mayer, and M. Ozbun. 1997. Synthesis of infectious human papillomavirus type 18 in differentiating epithelium transfected with viral DNA. J. Virol. 71:7381-7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okun, M., P. Day, H. Greenstone, F. Booy, D. Lowy, J. Schiller, and R. Roden. 2001. L1 interaction domains of papillomavirus L2 necessary for viral genome encapsidation. J. Virol. 75:4332-4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozbun, M. 2002. Human papillomavirus type 31b infection of human keratinocytes and the onset of early transcription. J. Virol. 76:11291-11300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozbun, M. 2002. Infectious human papillomavirus type 31b: purification and infection of an immortalized human keratinocyte cell line. J. Gen. Virol. 83:2753-2763. [DOI] [PubMed] [Google Scholar]
- 23.Peh, W. L., K. Middleton, N. Christensen, P. Nicholls, K. Egawa, K. Sotlar, J. Brandsma, A. Percival, J. Lewis, W. J. Liu, and J. Doorbar. 2002. Life cycle heterogeneity in animal models of human papillomavirus-associated disease. J. Virol. 76:10401-10416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roden, R., H. Greenstone, R. Kirnbauer, F. Booy, J. Jessie, D. Lowy, and J. Schiller. 1996. In vitro generation and type-specific neutralization of a human papillomavirus type 16 virion pseudotype. J. Virol. 70:5875-5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossi, J., L. Gissmann, K. Jansen, and M. Mueller. 2000. Assembly of human papillomavirus type 16 pseudovirions in Saccharomyces cerevisiae. Hum. Gene Ther. 11:1165-1176. [DOI] [PubMed] [Google Scholar]
- 26.Stauffer, Y., K. Raj, K. Masternak, and P. Beard. 1998. Infectious human papillomavirus type 18 pseudovirions. J. Mol. Biol. 283:529-536. [DOI] [PubMed] [Google Scholar]
- 27.Thomas, J., W. Hubert, M. Ruesch, and L. Laimins. 1999. Human papillomavirus type 31 oncoproteins E6 and E7 are required for the maintenance of episomes during the viral life cycle in normal human keratinocytes. Proc. Natl. Acad. Sci. USA 96:8449-8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Touze, A., and P. Coursaget. 1998. In vitro gene transfer using human papillomavirus-like particles. Nucleic Acids Res. 26:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Unckell, F., R. Streeck, and M. Sapp. 1997. Generation and neutralization of pseudovirions of human papillmavirus type 33. J. Virol. 71:2934-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang, R., P. Day, W. Yutzy IV, K.-Y. Lin, C. Hung, and R. Roden. 2003. Cell surface-binding motifs of L2 that facilitate papillomavirus infection. J. Virol. 77:3531-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang, R., W. Yutzy IV, R. Viscidi, and R. Roden. 2003. Interaction of L2 with beta-actin directs intracellular transport of papillomavirus and infection. J. Biol. Chem. 278:12546-12553. [DOI] [PubMed] [Google Scholar]
- 32.Zhao, K.-N., X.-Y. Sun, I. Frazer, and J. Zhou. 1998. DNA packaging by L1 and L2 capsid proteins of bovine papillomavirus type 1. Virology 243:482-491. [DOI] [PubMed] [Google Scholar]
- 33.Zhou, J., D. Stenzel, X.-Y. Sun, and I. Frazer. 1993. Synthesis and assembly of infectious bovine papillomavirus particles in vitro. J. Gen. Virol. 74:763-768. [DOI] [PubMed] [Google Scholar]
- 34.Zhou, J., X. Sun, D. Stenzel, and I. Frazer. 1991. Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virions-like particles. Virology 185:251-257. [DOI] [PubMed] [Google Scholar]
- 35.Zur Hausen, H. 2001. Oncogenic DNA viruses. Oncogene 20:7820-7823. [DOI] [PubMed] [Google Scholar]