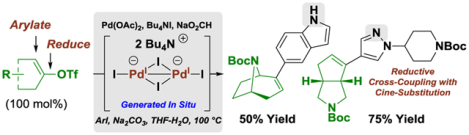
Abstract
The first deoxygenative Heck reactions are described, as illustrated by formate-mediated cine-substitutions of vinyl triflates with aryl iodides. The collective data corroborate a mechanism in which Pd(OAc)2 and Bu4NI form the dianionic iodide-bridged dimer [Pd2I6][NBu4]2, which, under reducing conditions, serves as a precursor to the palladium(I) complex [Pd2I4][NBu4]2. Dinculear oxidative addition of aryl iodide forms [Pd2I5(Ar)][NBu4]2, which dissociates to the monometallic complex [PdI2(Ar)][NBu4]. Vinyl triflate migratory insertion-sulfonate elimination delivers a palladium(IV) carbene, which upon β-hydride elimination-C-H reductive elimination gives the product of cine-substitution. These processes are the first efficient formate-mediated cross-electrophile reductive couplings beyond carbonyl addition.
Graphical Abstract
Our laboratory has developed diverse metal-catalyzed carbonyl reductive couplings mediated by the feedstock reductants (hydrogen, 2-propanol, formic acid),1,2 as well as related hydrogen auto-transfer reactions in which alcohols serve dually as reductant and carbonyl proelectrophile.2 Such methods bypass use of premetalated C-nucleophiles, which are often hazardous and expensive. In an outgrowth of this work, the first carbonyl reductive couplings of aryl halides beyond discrete arylmetal nucleophiles or metallic reductants were discovered,3a along with related formate-mediated reactions of vinyl halides or triflates.3b,c The ability to exploit sp2-halides as aryl- and vinylmetal pronucleophiles in formate-mediated carbonyl addition impelled investigations into “cross-electrophile reductive couplings” – an emerging class of C-C couplings that typically require elemental zinc or manganese as the terminal reductants.4,5,6,7 Here, we report formate-mediated reductive cross-couplings of vinyl triflates with aryl iodides, which, unlike longstanding palladium(0)-catalyzed cross-couplings of vinyl triflates,8,9 result in cine-substitution through a deoxygenative Heck-type pathway (Figure 1).10,11,12 Our collective data corroborate a mechanism in which Pd(OAc)2 and Bu4NI form the dianionic iodide-bridged dimer [Pd2I6][NBu4]2, which, under reducing conditions, serves as a reservoir for the active palladium(I) complex [Pd2I4][NBu4]2. Our data also corroborate the key role of iodide counterions vis-á-vis stabilization of palladium(I) under reductive coupling conditions.13,14,15 These transformations represent the first non-photochemical reductive C-C couplings via palladium(I)-catalysis, and highlight the distinct reactivity of dinuclear iodide-bridged palladium(I) complexes.16,17,18
Figure 1.
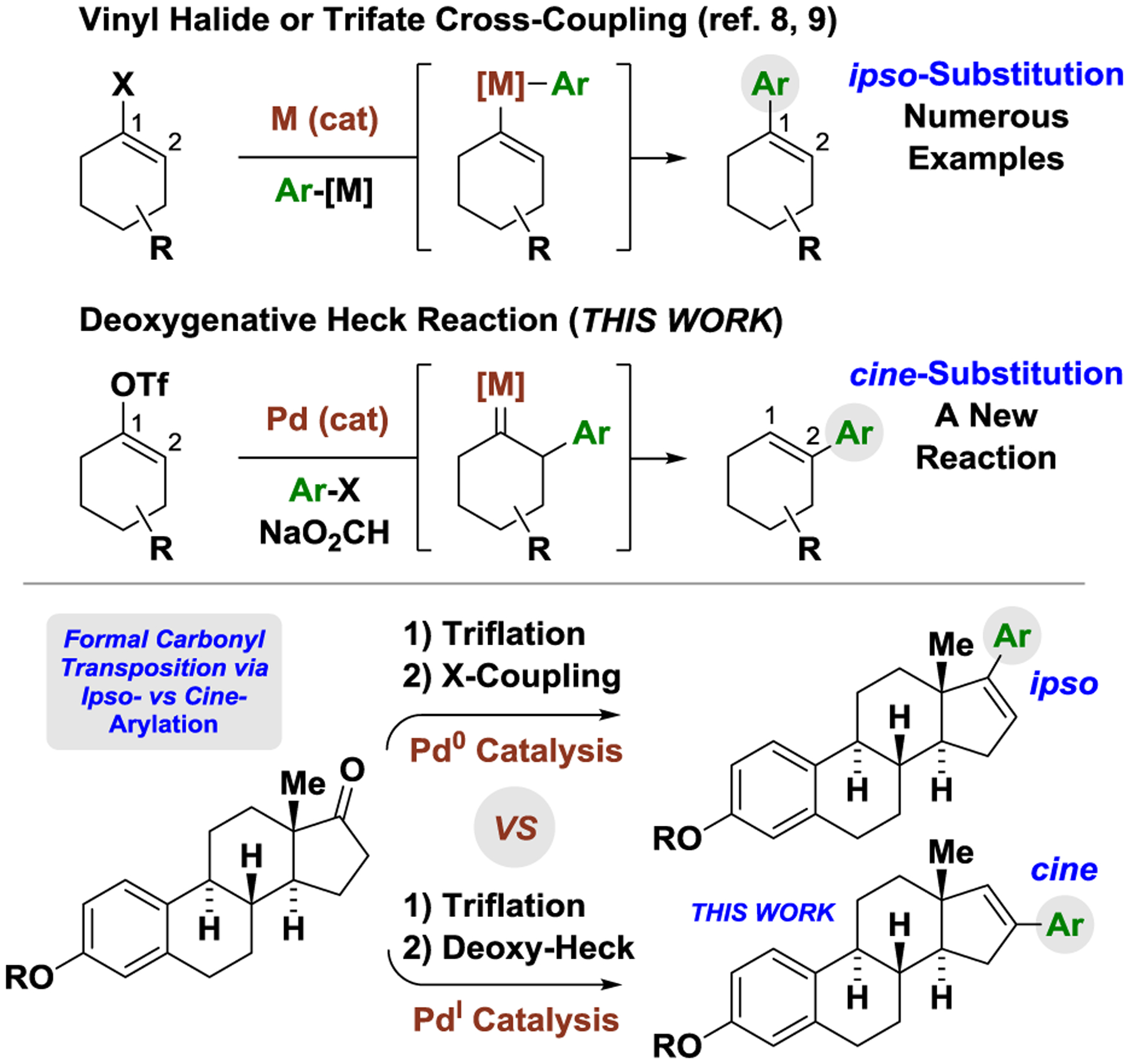
Cross-coupling with ipso- vs cine-substitution.
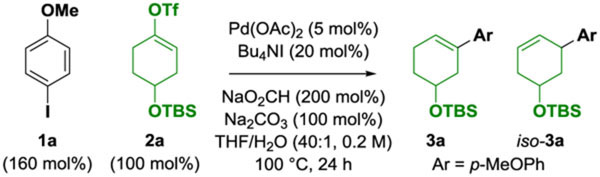
Optimal conditions identified for palladium(I)-iodide-catalyzed deoxygenative Heck reaction represent the culmination of numerous experiments. For the sake of brevity, key features of the catalytic system are highlighted by deviation from ideal conditions (Table 1). The most efficient conditions involved exposure of iodoanisole 1a (160 mol%) and vinyl triflate 2a (100 mol%) to Pd(OAc)2 (5 mol%) and Bu4NI (20 mol%) in the presence of NaO2CH (200 mol%) and Na2CO3 (100 mol%) in THF-H2O (0.2 M, 40:1) at 100 °C. The product of deoxygenative Heck reaction 3a was obtained in 88% yield along with a small quantity of iso-3a (Table 1, entry 1). The structural assignment of 3a was verified by its conversion to a reported compound (see Supporting Information). The presence of Bu4NI was found to be essential (Table 1, entries 2–4). As documented by Schoenebeck, iodide counterions stabilize palladium(I) species.13,14 Indeed, the unique influence of iodide counterions in ruthenium-catalyzed C-C coupling via hydrogen transfer is what led us to palladium(I) catalysis.14,15 The ammonium cation of Bu4NI is also necessary, as other iodide sources failed to animate the catalytic process (Table 1, entry 5). Bu4NI and Pd(OAc)2 form the dianionic iodide-bridged dimer [Pd2I6][NBu4]2, which, in the presence of NaO2CH, is a latent source of the palladium(I) complex [Pd2I4][NBu4]2 (vide supra), which is a competent catalyst for the reaction (Table 1, entry 6). Palladium(0) precatalysts diminish efficiency (Table 1, entry 7), and phosphine ligands completely suppress catalysis (Table 1, entries 8 and 9). Consistent with intervention of palladium(I) species, aryl iodides react with significantly greater efficiency than aryl bromides (Table 1, entry 10).11,12 Finally, water is required (Table 1, entry 11), presumably to solubilize NaO2CH and Na2CO3. Other enol derivatives (tosylates, phosphates, vinyl halides) were less efficient partners for C-C coupling (not shown).
Table 1.
Palladium(I)-iodide-catalyzed deoxygenative Heck reaction of aryl iodide 1a with vinyl triflate 2a: Deviation from optimal conditions.a
| |||
|---|---|---|---|
| Entry | Deviation from Optimal Conditions | 3a Yield (%) | 3a:iso-3a |
| →1 | None | 88 | 14:1 |
| 2 | Without Bu4Nl | <5 | --- |
| 3 | Bu4NCl vs Bu4Nl | <5 | --- |
| 4 | Bu4NBr vs Bu4Nl | <5 | --- |
| 5 | Nal vs Bu4Nl | <5 | --- |
| 6 | [Pd2|6]|TBA]2 (2.5 mol%) vs Pd(OAc)2 | 65 | >20:1 |
| 7 | Pd2(dba)3 (2.5 mol%) vs Pd(OAc)2 | 40 | 8:1 |
| 8 | [Pd(l)(tBu3P)]2 (2.5 mol%) vs Pd(OAc)2 | <5 | --- |
| 9 | tBu3P●HBF4 (5 mol%) | <5 | --- |
| 10 | Ar-Br vs Ar-I | 15 | >20:1 |
| 11 | Without H2O | <5 | --- |
Yields of isomeric mixtures isolated by silica gel chromatography.
To assess reaction scope, optimal conditions developed for formation of 3a were applied to a diverse combination of reactants (Table 2). Both para- and ortho-iodoanisoles 1a and 1b are competent partners for C-C coupling, as are the corresponding unprotected phenols 1c and 1d. Notably, as illustrated by the formation of 3f, aldehyde functional groups are tolerated under the conditions of formate-mediated reductive coupling. Other vinyl triflates, including the spirocyclic ketal-containing vinyl triflate 2b, and the steroidal vinyl triflates 2c and 2d, which are derived from estrone and cholestanone, respectively, engage in C-C coupling. Tolerance of acidic residues is evident in formation of salicylate adduct 3i. In the case of the estrone-derived vinyl triflate, the presence of a quaternary carbon center adjacent to the vinylic C-O bond does not preclude formation of adduct 3j. Finally, the N-heterocyclic fused vinyl triflate 2e, and bridged bicyclic triflate 2f derived from tropinone, deliver products of C-C coupling in good yield, as demonstrated by formation of adducts 3l-3n and 3o-3q, respectively. As shown in formation of 3m and 3p, Lewis basic nitrogen atoms are tolerated. The presence of unprotected NH indoles is demonstrated by formation of adducts 3n and 3q. Fully substituted vinyl triflates are not competent partners for the deoxygenative Heck reaction, presumably due to inefficient π-complexation/carbopalladation.
Table 2.
Palladium(I)-iodide-catalyzed deoxygenative Heck reaction of aryl iodides 1a-1j with vinyl triflates 2a-2f.a
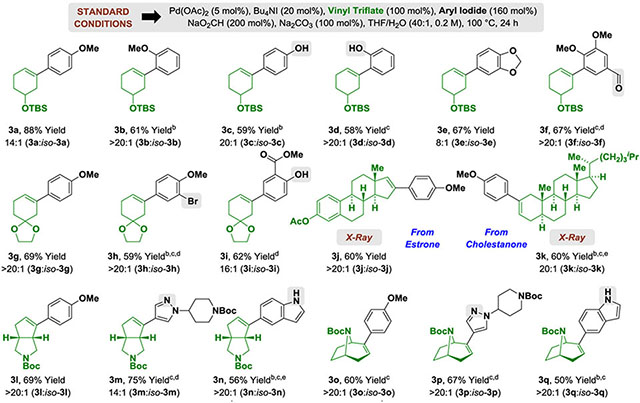
Yields of material isolated by silica gel chromatography. bPd(OAc)2 (10 mol%). c105 ⁰C. dArI (200 mol%). e48 Hr.
A series of experiments and observations from the literature provide insight into the reaction mechanism (Scheme 1).. Exposure of aryl iodide 1a to standard reaction conditions in the absence of vinyl triflate followed by filtration through Celite with the aid of THF and diffusion of hexane into the liquor led to the formation of the crystalline dianionic iodide-bridged dimer [Pd2I6][NBu4]2 (Scheme 1, eq. 1).19 At longer reaction times under the conditions of formate-mediated reduction, we believe [Pd2I6][NBu4]2 slowly releases the palladium(I) complex [Pd2I4][NBu4]2. The formation of the palladium(I) complex [Pd2I4][NBu4]2 is corroborated by 31P NMR studies in which [Pd2I6][NBu4]2 is exposed to tBu3P•HBF4 in the presence of formate (Scheme 1, eq. 2). The signal corresponding to the known palladium(I) dimer [Pd2(tBu3P)2(I)2] is the only signal observed in the 31P NMR (See Supporting Information). In the absence of formate, the palladium(I) dimer [Pd2(tBu3P)2(I)2] is not observed. Similarly, exposure of Pd(OAc)2 to tBu3P•HBF4 in the presence formate and Bu4NI leads to clean formation of the palladium(I) dimer [Pd2(tBu3P)2(I)2] (Scheme 1, eq. 3). Again, in the absence of formate, [Pd2(tBu3P)2(I)2] is not observed. These data corroborate intervention of the dianionic palladium(I) complex [Pd2I4][NBu4]2 under catalytically relevant conditions. In the absence of tBu3P, [Pd2I4][NBu4]2 and [Pd2I6][NBu4]2 may exist in equilibrium with higher polynuclear palladium-iodide complexes (including nanoparticles)20 that may or may not be catalytically relevant. DFT calculations by Schoenebeck13b support the feasibility of aryl iodide oxidative addition by the dinuclear palladium(I) complex [Pd2(tBu3P)2(Br)2] in the catalytic conversion of aryl iodides to aryl bromides (Scheme 1, eq. 4). Finally, exposure of deuterio-2b to iodoanisole 1a under standard conditions provides deuterio-3g, demonstrating deuterium transfer from the vinylic position of the triflate to the vicinal vinylic carbon atom of the product (Scheme 1, eq. 5).
Scheme 1.
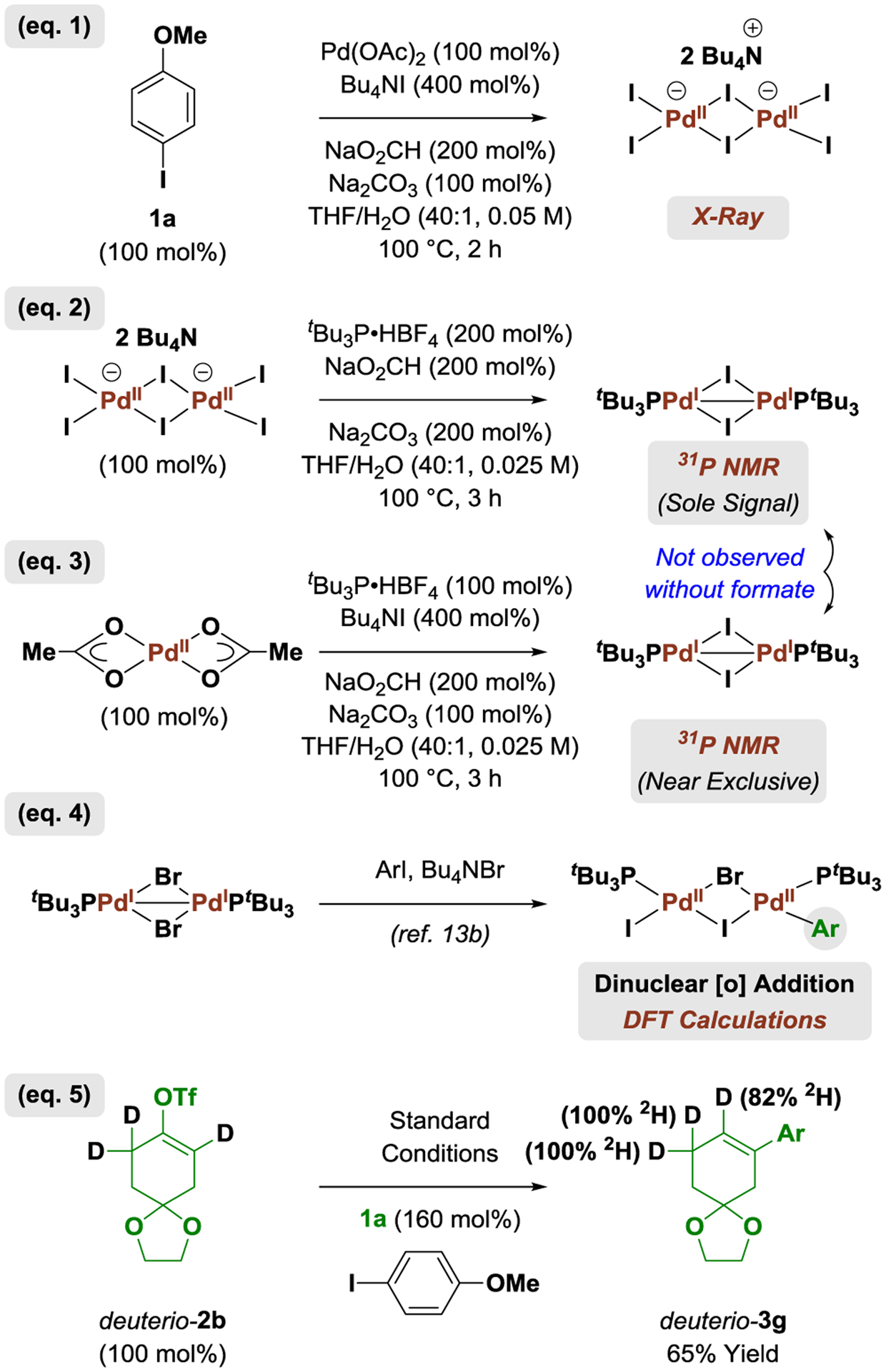
Mechanistic studies.a
aSee Supporting Information for experimental details.
Based on these data, the following mechanism for the deoxygenative Heck reaction of vinyl triflates is proposed (Scheme 2). Entry into the catalytic cycle occurs via conversion of Pd(OAc)2 to the iodide-bridged palladium(II) dimer [Pd2I6][NBu4]2. Formate-mediated reduction of [Pd2I6][NBu4]2 provides the palladium(I) dimer [Pd2I4][NBu4]2. Oxidative addition of aryl iodide generates the arylpalladium(II) complex [Pd2I5(Ar)][NBu4]2,13b which exists in equilibria with the monometallic complexes [PdI3][NBu4] and [PdI2(Ar)][NBu4]. The latter complex reversibly coordinates the vinyl triflate, which triggers migratory insertion. Carbopalladation occurs with concomitant elimination of triflate to form the palladium(IV) carbene.21 β-Hydride elimination followed by C-H reductive elimination releases the product and generates PdI2, which combines with [PdI3][NBu4] and Bu4NI to close the catalytic cycle. The absence of palladium(0) species is consistent with the requirement of aryl iodides (and tolerance of aryl bromides) in this process. This mechanism illustrates an important and distinctive feature of the bimetallic palladium(I) catalyst: conventional formate-mediated transfer hydrogenolysis of reactant C-I bonds (i.e. hydrodehalogenation)22 is suppressed as the hydride and aryl/vinyl moieties do not cohabit the metal.
Scheme 2.
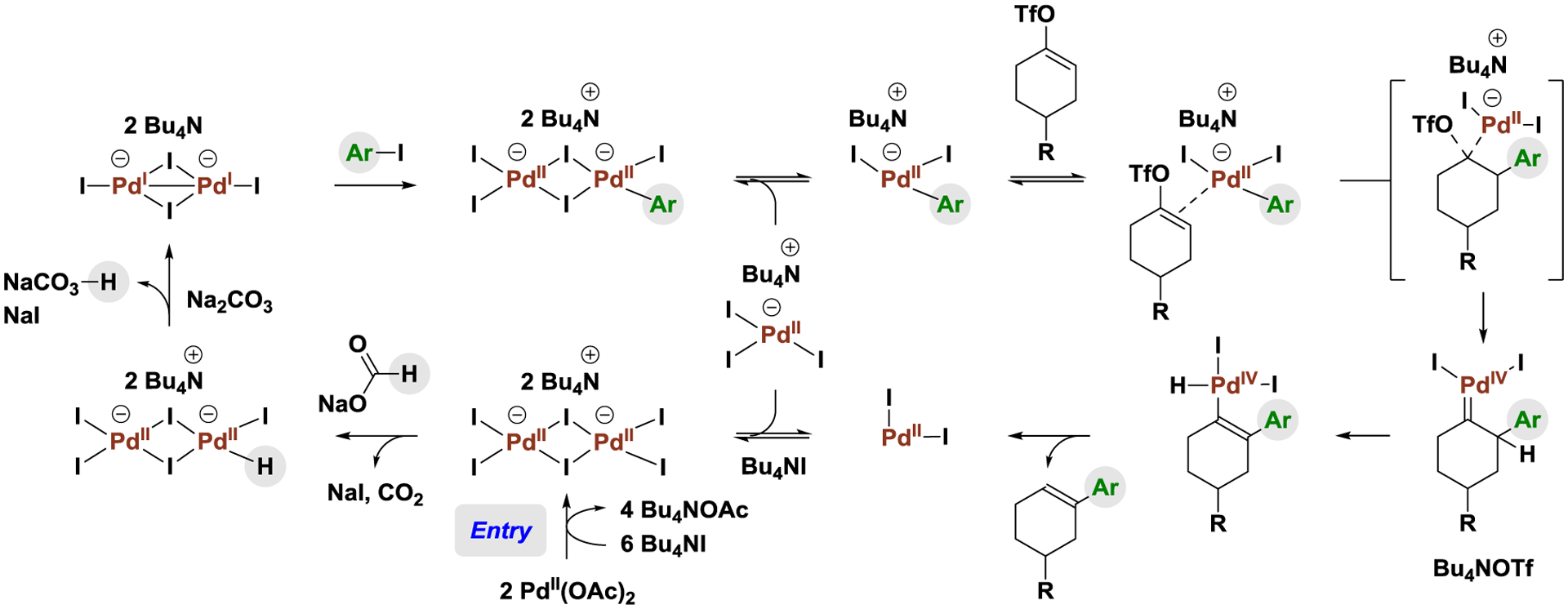
Proposed catalytic cycle for palladium(I)-catalyzed deoxygenative Heck reaction of vinyl triflates.
In conclusion, we report a new catalytic transformation; the deoxygenative Heck reaction of vinyl triflates. Notably, these processes represent the first efficient cross-electrophile reductive couplings mediated by an inexpensive feedstock reductant, sodium formate. Additionally, the ability to affect vinylic cross-coupling with cine-substitution unlocks access to products that would otherwise require more circumlocutious methods of preparation. Most importantly, the present data add to a growing body of work16 in which the distinctive structural-interactional features of dinuclear iodide-bridged palladium(I) complexes unlock unique catalytic pathways. Specifically, in the context of reductive coupling, the bimetallic nature of the palladium(I) species assists in suppressing competing hydrodehalogenation,22 as the aryl and hydride ligands do not simultaneously reside on the metal center. Reductive biaryl cross-couplings will be disclosed shortly.
Supplementary Material
ACKNOWLEDGMENT
The Robert A. Welch Foundation (F-0038) and the NIH-NIGMS (RO1-GM069445) are acknowledged for partial support of this research. We thank Serhii Vasylevskyi for the acquisition and interpretation of single crystal X-ray diffraction data.
Footnotes
Supporting Information. Experimental procedures and spectroscopic data for all new compounds (1H NMR, 13C NMR, IR, HRMS), including images of NMR spectra. Single-crystal X-ray diffraction data for [Pd2I6][NBu4]2 and compounds 3j and 3k.
Accession Codes. CCDC 2290442, 2293685 and 2289930 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
The authors declare no competing financial interest.
REFERENCES
- (1).For production volumes of hydrogen, 2-propanol and formic acid, see:; Weissermel K; Arpe HJ Industrial Organic Chemistry, 4th ed.; Wiley-VCH, 2003. [Google Scholar]
- (2).For selected reviews on metal-catalyzed C-C coupling via hydrogenation and transfer hydrogenation, see:; (a) Nguyen KD; Park BY; Luong T; Sato H; Garza VJ; Krische MJ Metal-Catalyzed Reductive Coupling of Olefin-Derived Nucleophiles: Reinventing Carbonyl Addition. Science 2016, 354, aah5133. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Doerksen RS; Meyer CC; Krische MJ Feedstock Reagents in Metal-Catalyzed Carbonyl Reductive Coupling: Minimizing Preactivation for Efficiency in Target-Oriented Synthesis. Angew. Chem. Int. Ed 2019, 58, 14055–14064. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Santana CG; Krische MJ From Hydrogenation to Transfer Hydrogenation to Hydrogen Auto-Transfer in Enantioselective Metal-Catalyzed Carbonyl Reductive Coupling: Past, Present and Future. ACS Catal. 2021, 11, 5572–5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).(a) Swyka RA; Zhang W; Richardson J; Ruble JC; Krische MJ Rhodium-Catalyzed Aldehyde Arylation via Formate-Mediated Transfer Hydrogenation: Beyond Metallic Reductants in Grignard/Nozaki-Hiyama-Kishi-Type Addition. J. Am. Chem. Soc 2019, 141, 1828–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Swyka RA; Shuler WG; Spinello BJ; Zhang W; Lan C; Krische MJ Conversion of Aldehydes to Branched or Linear Ketones via Regiodivergent Rhodium-Catalyzed Vinyl Bromide Reductive Coupling-Redox Isomerization Mediated by Formate. J. Am. Chem. Soc 2019, 141, 6864–6868. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Shuler WG; Swyka RA; Schempp TT; Spinello BJ; Krische MJ Vinyl Triflate-Aldehyde Reductive Coupling-Redox Isomerization Mediated by Formate: Rhodium-Catalyzed Ketone Synthesis in the Absence of Stoichiometric Metals. Chem. Eur. J 2019, 25, 12517–12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).For selected reviews on metal-catalyzed cross-electrophile reductive coupling, see:; (a) Gosmini C; Moncomble A Cobalt-Catalyzed Cross-Coupling Reactions of Aryl Halides. Isr. J. Chem 2010, 50, 568–576. [Google Scholar]; (b) Knappke CEI; Grupe S; Gärtner D; Corpet M; Gosmini C; Jacobi von Wangelin A Reductive Cross-Coupling Reactions between Two Electrophiles. Chem. Eur. J 2014, 20, 6828–6842. [DOI] [PubMed] [Google Scholar]; (c) Everson DA; Weix DJ Cross-Electrophile Coupling: Principles of Reactivity and Selectivity. J. Org. Chem 2014, 79, 4793–4798. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Wang X; Dai Y; Gong H Nickel-Catalyzed Reductive Couplings. Top. Curr. Chem 2016, 374, 61–89. [DOI] [PubMed] [Google Scholar]; (e) Poremba KE; Dibrell SE; Reisman SE Nickel-Catalyzed Enantioselective Reductive Cross-Coupling Reactions. ACS Catal. 2020, 10, 8237–8246. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Charboneau DJ; Hazari N; Huang H; Uehling MR; Zultanski SL Homogeneous Organic Electron Donors in Nickel-Catalyzed Reductive Transformations. J. Org. Chem 2022, 87, 7589–7609. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Yi L; Ji T; Chen K-Q; Chen X-Y; Rueping M Nickel-Catalyzed Reductive Cross-Couplings: New Opportunities for Carbon-Carbon Bond Formations through Photochemistry and Electrochemistry. CCS Chem. 2022, 4, 9–30. [Google Scholar]; (h) Liu Y; Li P; Wang Y; Qiu Y Electroreductive Cross-Electrophile Coupling (eXEC) Reactions. Angew. Chem. Int. Ed 2023, 62, e202306679. [DOI] [PubMed] [Google Scholar]
- (5).Metal-catalyzed cross-electrophile reductive couplings of organic halides mediated by tetrakis(dimethylamino)ethylene (TDAE) have been described, however, this compound remains quite expensive. For selected examples, see:; (a) Anka-Lufford LL; Huihui KMM; Gower NJ; Ackerman LKG; Weix DJ Nickel-Catalyzed Cross-Electrophile Coupling with Organic Reductants in Non-Amide Solvents. Chem. Eur. J 2016, 22, 11564–11567. [DOI] [PubMed] [Google Scholar]; (b) Shu W; García-Domínguez A; Quirós MT; Mondal R; Cárdenas DJ; Nevado C Ni-Catalyzed Reductive Dicarbofunctionalization of Nonactivated Alkenes: Scope and Mechanistic Insights. J. Am. Chem. Soc 2019, 141, 13812–13821. [DOI] [PubMed] [Google Scholar]
- (6).Metal-catalyzed cross-electrophile reductive couplings of organic halides via photoredox catalysis enables use of tertiary amines as terminal reductants. For selected examples, see:; (a) Duan Z; Li W; Lei A Nickel-Catalyzed Reductive Cross-Coupling of Aryl Bromides with Alkyl Bromides: Et3N as the Terminal Reductant. Org. Lett 2016, 18, 4012–4015. [DOI] [PubMed] [Google Scholar]; (b) Dewanji A; Bülow RF; Rueping M Photoredox/Nickel Dual-Catalyzed Reductive Cross Coupling of Aryl Halides Using an Organic Reducing Agent. Org. Lett 2020, 22, 1611–1617. [DOI] [PubMed] [Google Scholar]
- (7).Schwartz LA; Spielmann K; Swyka RA; Xiang M; Krische MJ Formate-Mediated Cross-Electrophile Reductive Coupling of Aryl Iodides and Bromopyridines. Isr. J. Chem 2021, 61, 298–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).For seminal reports of palladium-catalyzed cross-couplings of vinyl triflates, see:; (a) Scott WJ; Crisp GT Stille JK Palladium-Catalyzed Coupling of Vinyl Triflates with Organostannanes. A Short Synthesis of Pleraplysillin-1. J. Am. Chem. Soc 1984, 106, 4630–4632. [Google Scholar]; (b) Scott WJ; Stille JK Palladium-Catalyzed Coupling of Vinyl Triflates with Organostannanes. Synthetic and Mechanistic Studies. J. Am. Chem. Soc 1986, 108, 3033–3040. [Google Scholar]; (c) Oh-e T; Miyaura N; Suzuki A Palladium-Catalyzed Cross-Coupling Reaction of Aryl or Vinylic Triflates wtih Organoboron Compounds. Synlett 1990, 221–223. [Google Scholar]
- (9).For reviews on palladium-catalyzed cross-couplings of vinyl triflates, see:; (a) Scott WJ; McMurry JE Olefin Synthesis via Organometallic Coupling Reactions of Enol Triflates. Acc. Chem. Res 1988, 21, 47–54. [Google Scholar]; (b) Ritter K Synthetic Transformations of Vinyl and Aryl Triflates. Synthesis 1993, 735–762. [Google Scholar]
- (10).For selected reviews on reductive Heck reactions, see:; (a) Namyslo JC; Storsberg J; Klinge J; Gärtner C; Yao M-L; Ocal N; Kaufmann DE The Hydroarylation Reaction—Scope and Limitations. Molecules 2010, 15, 3402–3410. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ghosh T Reductive Heck Reaction: An Emerging Alternative in Natural Product Synthesis. ChemistrySelect 2019, 4, 4747–4755. [Google Scholar]; (c) Oxtoby LJ; Gurak JA; Wisniewski SR; Eastgate MD; Engle KM Palladium-Catalyzed Reductive Heck Coupling of Alkenes. Trends Chem. 2019, 1, 572–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).For selected reviews on cine-substitution, see:; (a) Suwiński J; Świerczek K Cine- and Tele-Substitution Reactions. Tetrahedron 2001, 57, 1639–1662. [Google Scholar]; (b) Peng Y; Li W-DZ Cine Substitution and the Cu Effect in Stille Cross-Coupling Reactions: Mechanistic Perspectives and Synthetic Utility. Eur. J. Org. Chem 2010, 2010, 6703–6718. [Google Scholar]; (c) Suwiński J Cine- and Tele-Substitution Reactions: Review of Work from 2002–2016. Arkivoc 2017, 402–435. [Google Scholar]
- (12).For selected examples of cine-substition in palladium-catalyzed cross-coupling, see:; (a) Ennis DS; Gilchrist TL Abnormal Products of Palladium Catalysed Coupling Reactions of (1-Bromovinyl)trimethylsilane. Tetrahedron Lett. 1989, 30, 3735–3736. [Google Scholar]; (b) Ikenaga K; Matsumoto S; Kikukawa K; Matsuda T A First Palladium-Catalyzed Aryldegermylation of Styryltrimethylgermanes. Chem. Lett 1990, 185–188. [Google Scholar]; (c) Hatanaka Y; Goda K.-i. Hiyama T On the Regioselectivity of Palladium Catalyzed Cross-Coupling Reactions of Alkenylsilanes: Participation of β-Cationic Organosilicate-Palladium Species during the Transmetallation. J. Organomet. Chem 1994, 465, 97–100. [Google Scholar]; (d) Anderson JC; Anguille S; Bailey R The Direct Use of Phenyldimethylsilanes in Silicon Assisted Palladium Catalysed Cross Coupling. Chem. Commun 2002, 2018−2019. [DOI] [PubMed] [Google Scholar]; (e) Enguehard C; Allouchi H; Gueiffier A; Buchwald SL Ipso- or Cine-Substitutions of 6-Haloimidazo[1,2-a]pyridine Derivatives with Different Azoles Depending on the Reaction Conditions. J. Org. Chem 2003, 68, 5614–5617. [DOI] [PubMed] [Google Scholar]; (f) Willis MC; Chauhan J; Whittingham WG A New Reactivity Pattern for Vinyl Bromides: Cine-Substitution via Palladium Catalysed C–N Coupling/Michael Addition Reactions. Org. Biomol. Chem 2005, 3, 3094–3095. [DOI] [PubMed] [Google Scholar]; (g) Kanoh N; Ohno Y; Itagaki T; Fukuda H; Iwabuchi Y On the Origin of cine-Substitution in the Stille Coupling of Trisubstituted Iodoalkene and trans-Vinylstannane. Synlett 2013, 24, 2660–2664. [Google Scholar]; (h) Tsukada N; Abe T; Inoue Y Formation of cine-Substitution Products in the Suzuki-Miyaura Cross-Coupling Reaction Catalyzed by Dinuclear Palladium Complexes. Helv. Chim. Acta 2013, 96, 1093–1102. [Google Scholar]
- (13).(a) Aufiero M; Proutiere F; Schoenebeck F Redox Reactions in Palladium Catalysis: On the Accelerating and/or Inhibiting Effects of Copper and Silver Salt Additives in Cross-Coupling Chemistry Involving Electron-rich Phosphine Ligands. Angew. Chem. Int. Ed 2012, 51, 7226–7230. [DOI] [PubMed] [Google Scholar]; (b) Bonney KJ; Proutiere F; Schoenebeck F Dinuclear Pd(I) Complexes–Solely Precatalysts? Demonstration of Direct Reactivity of a Pd(I) Dimer with an Aryl Iodide. Chem. Sci 2013, 4, 4434–4439. [Google Scholar]; (c) Kalvet I; Bonney KJ; Schoenebeck F Kinetic and Computational Studies on Pd(I) Dimer-Mediated Halogen Exchange of Aryl Iodides. |J. Org. Chem 2014, 79, 12041–12046. [DOI] [PubMed] [Google Scholar]; (d) Yin G; Kalvet I; Schoenebeck F Trifluoromethylthiolation of Aryl Iodides and Bromides Enabled by a Bench-Stable and Easy-To-Recover Dinuclear Palladium(I) Catalyst. Angew. Chem. Int. Ed 2015, 54, 6809 –6813. [DOI] [PubMed] [Google Scholar]
- (14).For selected reviews on halide counterion effects in transition metal catalysis, see:; (a) Maitlis PM; Haynes A; James BR; Catellani M; Chiusoli GP Iodide Effects in Transition Metal Catalyzed Reactions. Dalton Trans. 2004, 3409–3419. [DOI] [PubMed] [Google Scholar]; (b) Fagnou K; Lautens M Halide Effects in Transition Metal Catalysis. Angew. Chem. Int. Ed 2002, 41, 26–47. [DOI] [PubMed] [Google Scholar]
- (15).(a) Ortiz E; Shezaf JZ; Chang Y-H; Goncçalves TP; Huang K-W; Krische MJ Understanding Halide Counterion Effects in Enantioselective Ruthenium-Catalyzed Carbonyl (α-Aryl)allylation: Alkynes as Latent Allenes and Trifluoroethanol-Enhanced Turnover in the Conversion of Ethanol to Higher Alcohols via Hydrogen Auto-Transfer. J. Am. Chem. Soc 2021, 143, 16709–16717. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Shezaf JZ; Santana CG; Saludares C; Briceno ES; Sakata K; Krische MJ Chiral-at-Ruthenium-SEGPHOS Catalysts Display Diastereomer-Dependent Regioselectivity: Enantioselective Isoprene-Mediated Carbonyl tert-Prenylation via Halide Counterion Effects. J. Am. Chem. Soc 2023, 145, 18676–18683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).For a review on the emerging field of palladium(I) catalysis, see:; Fricke C; Sperger T; Mendel M; Schoenebeck F Catalysis with Palladium(I) Dimers. Angew. Chem. Int. Ed 2021, 60, 3355–3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).For selected examples of photochemically promoted reactions that occur by way of palladium(I) intermediates, see:; (a) Parasram M; Chuentragool P; Sarkar D; Gevorgyan V Photoinduced Formation of Hybrid Aryl Pd-Radical Species Capable of 1,5-HAT: Selective Catalytic Oxidation of Silyl Ethers into Silyl Enol Ethers. J. Am. Chem. Soc 2016, 138, 6340–6343. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Parasram M; Chuentragool P; Wang Y; Shi Y; Gevorgyan V General, Auxiliary-Enabled Photoinduced Pd-Catalyzed Remote Desaturation of Aliphatic Alcohols. J. Am. Chem. Soc 2017, 139, 14857–14860. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Chuentragool P; Parasram M; Shi Y; Gevorgyan V General, Mild, and Selective Method for Desaturation of Aliphatic Amines. J. Am. Chem. Soc 2018, 140, 2465–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Cheung KPS; Kurandina D; Yata T; Gevorgyan V Photoinduced Palladium-Catalyzed Carbofunctionalization of Conjugated Dienes Proceeding via Radical-Polar Crossover Scenario: 1,2-Aminoalkylation and Beyond. J. Am. Chem. Soc 2020, 142, 9932–9937. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Zhao G; Yao W; Mauro JN; Ngai M-Y Excited-State Palladium-Catalyzed 1,2-Spin-Center Shift Enables Selective C-2 Reduction, Deuteration, and Iodination of Carbohydrates. J. Am. Chem. Soc 2021, 143, 1728–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Yao W; Zhao G; Wu Y; Zhou L; Mukherjee U; Liu P; Ngai M-Y Excited-State Palladium-Catalyzed Radical Migratory Mizoroki-Heck Reaction Enables C2-Alkenylation of Carbohydrates. J. Am. Chem. Soc 2022, 144, 3353–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Ceong KPS; Fang J; Mukherjee K; Mihranyan A; Gevorgyan V Asymmetric Intermolecular Allylic C–H Amination of Alkenes with Aliphatic Amines. Science 2022, 378, 1207–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Palladium(I) complexes have found use as precatalysts in conventional cross-coupling reactions of premetallated nucleophiles. For selected examples, see:; (a) Stambuli JP; Kuwano R; Hartwig JF Unparalleled Rates for the Activation of Aryl Chlorides and Bromides: Coupling with Amines and Boronic Acids in Minutes at Room Temperature. Angew. Chem., Int. Ed 2002, 41, 4746–4748. [DOI] [PubMed] [Google Scholar]; (b) Hama T; Liu X; Culkin DA; Hartwig JF Palladium-Catalyzed α-Arylation of Esters and Amides under More Neutral Conditions. J. Am. Chem. Soc 2003, 125, 11176–11177. [DOI] [PubMed] [Google Scholar]; (c) Bercot EA; Caille S; Bostick TM; Ranganathan K; Jensen R; Faul MM Diastereoselective Palladium-Catalyzed α-Arylation of 4-Substituted Cyclohexyl Esters. Org. Lett 2008, 10, 5251–5254. [DOI] [PubMed] [Google Scholar]; (d) Proutiere F; Aufiero M; Schoenebeck F Reactivity and Stability of Dinuclear Pd(I) Complexes: Studies on the Active Catalytic Species, Insights into Precatalyst Activation and Deactivation, and Application in Highly Selective Cross-Coupling Reactions. J. Am. Chem. Soc 2012, 134, 606–612. [DOI] [PubMed] [Google Scholar]; (e) Pirkl N; Del Grosso A; Mallick B; Doppiu A; Gooßen LJ Dihalogen-Bridged NHC–Palladium(I) Dimers: Synthesis, Characterisation and Applications in Cross-Coupling Reactions. Chem. Commun 2019, 55, 5275–5278. [DOI] [PubMed] [Google Scholar]
- (19).The bimetallic palladium complex [Pd2I6][NBu4]2 has previously been prepared:; (a) Chan S; Lee S-M; Lin Z; Wong W-T Syntheses, Structures and Reactivities of [Os6Pd(CO)18(bipy)] and [{(bipy)Pd}2Os3(CO)12]: Crystal and Molecular Structures of [{(bipy)Pd}2(μ-H)(μ-CO)][H3Os4(CO)12] and [(C4H9)4N]2[Pd2I6]. J. Organomet. Chem 1996, 510, 219–231. [Google Scholar]; (b) Linnenberg O; Mayerl L; Monakhov KY The Heck Reaction as a Tool to Expand Polyoxovanadates towards Thiol-Sensitive Organic–Inorganic Hybrid Fluorescent Switches. Dalton Trans. 2018, 47, 14402–14407. [DOI] [PubMed] [Google Scholar]
- (20).For formation of palladium nanoparticles from Pd(OAc)2 and KI, see:; Latocheski E; Marques MV; Albuquerque BL; Schuh TJ; Signori AM; Oliveira DC; Pal T; Domingos JB On the Formation of Palladium (II) Iodide Nanoparticles: An In Situ SAXS/XAS Study and Catalytic Evaluation on an Aryl Alkenylation Reaction in Water Medium. ChemCatChem 2019, 11, 684–688. [Google Scholar]
- (21).For selected reviews encompassing discussion of palladium(IV) carbenes, see:; (a) Reiser O Cyclopropanation and Other Reactions of Palladium-Carbene (and Carbyne) Complexes. In Handbook of Organopalladium Chemistry for Organic Synthesis; Negishi E, Ed.; Wiley-Interscience: New York, NY, USA, 2002; pp. 1561–1577. [Google Scholar]; (b) Albéniz AC Reactive Palladium Carbenes: Migratory Insertion and Other Carbene–Hydrocarbyl Coupling Reactions on Well-Defined Systems. Eur. J. Inorg. Chem 2018, 3693–3705. [Google Scholar]; Also see; (c) Cámpora J; Palma P; del Río D; López JA; Valerga P Reactivity of an Anionic Pd(II) Metallacycle with CH2X2 (X = Cl, Br, I): Formal Insertion of Methylene into a Pd–Caryl Bond. Chem. Commun 2004, 1490–1491. [DOI] [PubMed] [Google Scholar]
- (22).For selected reviews encompassing palladium-catalyzed hydrogenolysis of aryl C-X bonds (Hydrodehalogenation), see:; (a) Pinder AR The Hydrogenolysis of Organic Halides. Synthesis 1980, 425–452. [Google Scholar]; (b) Urbano FJ; Marinas JM Hydrogenolysis of Organohalogen Compounds over Palladium Supported Catalysts. J. Mol. Catal. A Chem 2001, 173, 329–345. [Google Scholar]; (c) Alonso F; Beletskaya IP; Yus M Metal-Mediated Reductive Hydrodehalogenation of Organic Halides. Chem. Rev 2002, 102, 4009–4092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


