Abstract
Introduction
Retained products of conception (RPOC) generally result after first half of pregnancy termination and also may occur after vaginal or cesarean delivery. It frequently presents with irregular or continuous vaginal bleeding, lower abdominal and pelvic pain, and discharge per vaginum due to infection; it can also cause late complications like formation of intrauterine adhesions and subfertility. The diagnosis of the RPOC along with the symptoms is generally supported by ultrasonography with or without colour Doppler. The patient also undergoes uterine vasculature assessment to diagnose arteriovenous malformation (AVM). The management of RPOC has been conventionally done with blind dilation and suction curettage (D and C); however, expectant management, uterine artery embolization, and hysteroscopic resection of RPOC are safe and efficient alternatives.
Materials and methods
In this review, we analyse the current available evidence regarding the clinical presentation, diagnosis and treatment of RPOC comparing the sensitivity, specificity, outcomes, pros and cons of various methods.
Conclusion
RPOC is a common complication associated with early and late complications. The judicious use of antibiotics along with interventional radiology and hysteroscopy forms the backbone for the treatment of this condition.
Keywords: Retained products of conception, Hysteroscopy, Intrauterine adhesions, Uterine arteriovenous malformation
Introduction
The residual trophoblastic tissue remaining within the uterine cavity after termination of pregnancy is known as retained product of conception (RPOC). It can occur after a miscarriage or termination of pregnancy or after delivery.
Abortion: Burden and Legal status
Miscarriage and induced abortions are prevalent and annually, 73 million viable first trimester pregnancies are terminated globally [1]. It is further estimated that 10–25% of the pregnancies end in miscarriage. Moreover, WHO estimates that 56 million abortions take place globally every year [2] Traditionally, WHO has defined unsafe abortion as ‘a procedure for termination of a pregnancy done by an individual who does not have the necessary training or in an environment not confirming to minimal medical standards. Unsafe abortions have been attributed in 4.7% to 13.2% of the maternal deaths and many of these unsafe abortions present with RPOC and its complications. In 2017, WHO replaced this dichotomous classification of abortions into safe and unsafe abortions to three tier classification of safe, less safe, and least safe abortions [3].
Abortion is legal in India. Legal restrictions have acted as a barrier to access safe abortion care resulting in amendment in the abortion laws of country. The cost of abortion care is covered by the Govt under Ayushman Bharat & Employee State Insurance schemes. On an average ten women die every day due to complications of unsafe abortion in India. A combined study by Guttmacher Institute New York, IIPS Mumbai and Population Council New Delhi estimated that 15.6 million abortions took place in India in year 2015. Only 22% of these abortions were performed within healthcare facilities. The study calculated the abortion rate of 47 abortions per 1000 women of the age group 15 to 49 years. Considering the quantum of global load of abortion and its complication, WHO formulated Abortion Care Guidelines 2022 to standardize abortion care worldwide [3].
Incidence of RPOC
Historically, the reported incidence of RPOC (Fig. 1) is 1% after term pregnancy and 0.4 to 3.8% after early pregnancy loss. Most of the deliveries have been institutionalized under maternity care programs, however significant proportion of the abortions take place outside the health care facilities making RPOC common and underreported. Smorgick et al. reported the incidence of RPOC as high as 15% after abortions [4]. Infertility patients have higher incidence of RPOC. In a retrospective cohort study Vashnavi et al. [5] reported incidence of RPOC after manual vacuum aspiration (MVA) and medical management as 13% and 29.4%, respectively.
Fig. 1.
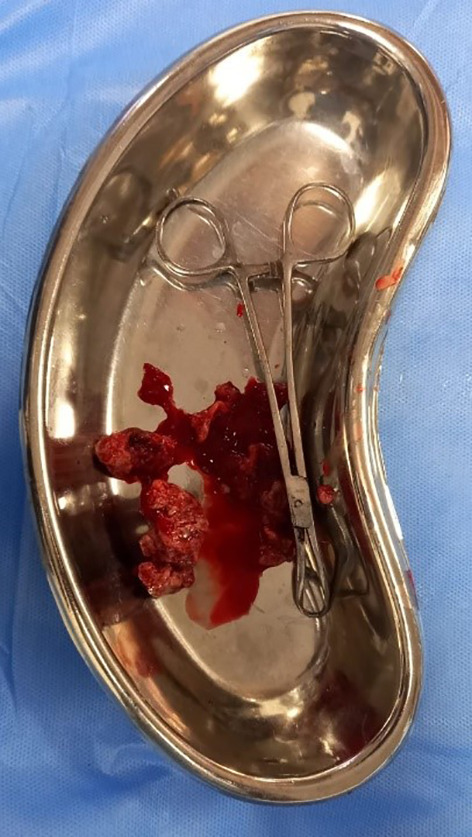
Gross image of RPOC
Diagnosis
Diagnosis of RPOC is a challenge. It is usually suspected clinically and aided by investigations like ultrasonography, color Doppler or hysteroscopy. Typical presentation of the case is post-abortion or post-partum vaginal bleeding with dilated cervical canal and ultrasonographic findings of intrauterine contents. An endometrial thickness of more than or equal to 15 mm after two weeks of induced abortion or early pregnancy loss has been used as an ultrasonographic criteria to define retained products of conception [6].
Two-dimensional ultrasonography is easily available and non-invasive tool for the diagnosis of RPOC; however, it has very high sensitivity with low specificity to diagnose RPOC. Transvaginal route of ultrasonography gives better image of uterine cavity, endometrial thickness as well as adnexa, however it is difficult to differentiate between necrotic decidual tissue and blood clots. Presence of intrauterine hyperechoic lesion in a case with vaginal bleeding has high sensitivity and negative predictive value. Addition of color doppler to ultrasonography has not added much to the diagnosis of RPOC. However, presence of blood flow helps in distinguishing RPOC from a hematoma (Fig. 2) [6]. Tinelli and Haimovich in 2017 described ultrasonographic patterns of RPOC which is known as Gutenberg classification of RPOC [7]. (Table 1).
Fig. 2.
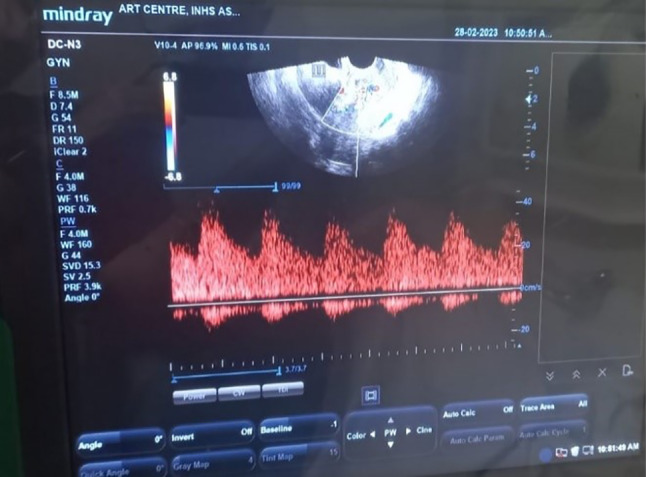
Highly vascularised mass with increased vascularity in the myometrium
Table 1.
Gutenberg Classification: Ultrasonographic patterns of RPOC
| Type | Lesion description |
|---|---|
| Type 0 | Hyperechogenic avascular mass |
| Type 1 | Different echoes with minimal or no vascularity |
| Type 2 | Highly vascularized mass confined to the cavity |
| Type 3 | Highly vascularized mass with highly vascularized myometrium |
Serum beta hCG Estimation
Serum hCG levels remain above non-pregnant levels of 5mIU/mL even after 4 weeks postpartum. Gestational trophoblastic disease (GTD) should be suspected whenever levels are very high. Hydatidiform mole accounts for 20% cases of GTD. Serum hCG levels are also raised in cases with placental site trophoblastic tumor (PSTT) [8].
Complications
RPOC are associated with both early and long-term complications. The early complication are bleeding and infection, while long-term complications are formation of intrauterine adhesions (IUA) and Asherman’s syndrome and subfertility. Rarely RPOC are associated with formation of polyps, enhanced myometrial vascularity (EMV)and arteriovenous malformation (AVM). Osseous metaplasia is a very rare complication associated with RPOC. Management of these long-term complications of RPOC like IUA and AVM is still a challenge [9].
Management
The aim of management of RPOC is to stop bleeding, to treat or prevent infection and to avoid long term complications. The three common management options are expectant management, medical management with use of anti-progestins and or prostaglandins; and surgical management in the form of vacuum aspiration or dilatation of cervix and curettage [10, 11].
Expectant & Conservative Management
Munros J et al. reported the rate of complete evacuation of the retained products when treated with expectant management between 47 and 81%, compared to 95%–97% with surgical management. When endometrial thickness is less than 10mm, there is no blood flow and EMV on ultrasonography, patient can be managed conservatively with 93% specificity and 90% PPV. Uterine contracting agents like misoprostol may be tried as conservative means of management [12, 13].
Surgical Management
When RPOC are suspected on ultrasonography, its surgical removal has been advised to stop vaginal bleeding and to prevent infection; and to avoid long-term complications like IUA and infertility. Most used surgical method of management are dilatation and curettage, suction & curettage or MVA. Vacuum aspiration is less traumatic than Dilation & Curettage. D & C is reported to be associated with an up to 30% chance of IUA formation [5]. A potential complication of vigorous curettage is abnormal embryonic implantation. It predisposed to abnormal placental development favoring morbidly adherent placenta with its potentially devastating obstetrical consequences. Both methods can also be performed under ultrasonographic guidance [14]. Evacuation of RPOC helps in treating and preventing infective complication. Antibiotics has both therapeutic and preventive role.
Hysteroscopy
Hysteroscopy for diagnosis and evacuation is another surgical alternative whenever facility is available. Hysteroscopic resection (HR) is a safe, effective, and minimally invasive modality as compared to blind and nonspecific D&C. Removal of RPOC under direct visualization avoids damage to adjacent endometrium and reduces trauma, inflammation and adhesion formation [15]. Smorgick et al. [4] in a meta-analysis concluded that hysteroscopy has low complication rates, lower incidence of adhesion formation and higher subsequent pregnancies. Ultrasonography is highly sensitive for exclusion of RPOC. Use of color Doppler along with ultrasonography helps in classifying RPOC into Type 0 to Type 3 as per Gutenberg Classification [7] which is further helpful in deciding the setting of hysteroscopic intervention, risk of bleeding and use of energy source. Use of monopolar energy source along with Gycine 1.5% as distension medium is frequently used in Type 2 and Type 3 RPOC, however bipolar energy with normal saline as distension medium is a safer option. Authors recommended that Hysteroscopy should be preferred over blind D&C [16].
Timing of Surgical Intervention and Infertility Outcome
Though, early surgical intervention was expected to be linked with lower long-term complications and improved fertility outcome however, there was no statistically significant difference after early or late surgical intervention in cases with pathologically confirmed RPOC. The conception rates, mean time to conception and development of new onset infertility were same in both groups [17].
IUA
Asherman’s syndrome was first described in 1948 by Joseph Asherman. Term IUA & Asherman have been used interchangeably. Basal layer of endometrium acts as regenerative reservoir of endometrium. Formation of IUA is multifactorial and the exact pathophysiological process has not been established though pregnancy is the main common factor in 91% of the cases. Multiple uterine surgeries, trauma, infection, and inflammation play a role. Destruction of basal layer of endometrium leads to formation of adhesions between the opposing endometrial and myometrial layers in susceptible cases [17].
Blind procedures like D&C have more chances of IUA formation than more specific procedure like hysteroscopic resection. Hooker et al. reported an incidence of 29.6% after D and C vs. 12.8% after hysteroscopic resection [17]. Repeated endometrial injury increases the chances of adhesion formation. Capmas et al. published rate of IUA after D and C from 17 to 30%. This rate increased up to 32% after three procedures which include > 50% of complex synechiae [9].
Clinically, IUA may present with menstrual irregularities, infertility abortion, ectopic pregnancies, fetal growth abnormalities, preterm labor and delivery, abnormal placentation, and postpartum hemorrhage. IUA have been managed with hysteroscopic adhesiolysis which may be combined with adhesion prevention strategies like use of mechanical barriers or hyaluronic gel, and estrogen therapy. Surgical technique with minimum endometrial injury, and use of bipolar cautery prevents future adhesion formation.
Placental Polyp and AVM
Placental Polyp
Placenta polyp has been described as retained placental tissue persistently existing in uterine cavity after an abortion or delivery. Placental polyp has organized villi and decidua along with regenerated endometrium. These components of polyp are firmly attached to uterine wall. The incidence of placental polyp is less than 0.25% of all pregnancies. Up to six percent of polyps are hyper vascular and have potential to cause massive haemorrhage [18].
AVM
An abnormal communication between arteries and veins without intervening capillaries is called arteriovenous malformation (AVM). AVM can be congenital or acquired. Congenital AVM is a result of abnormal embryonic vascular development. Acquired AVM are formed due to uterine trauma associated with RPOC, curettage procedure, delivery, gestational trophoblastic disease, or gynaecologic malignancies [19].
Hyper-vascular placental polyps and AVM can cause torrential life-threatening haemorrhage. History, clinical findings, beta hCG levels, ultrasound and Doppler imaging, CT or MRI with contrast is helpful in differentiating these conditions however, definitive diagnosis could not be made in all cases. Different management options like expectant management, medical management, hysteroscopic transcervical resection, uterine artery embolization have been tried depending upon the clinical presentation with different success rates. Placental polyps and AVM presenting with massive haemorrhage have also been managed with abdominal hysterectomy.
Uterine artery embolization is a safe and effective choice to control vaginal bleeding in post-abortal or postpartum cases with AVM or vascular polyps when facility is available and patient’s condition permit. Hysteroscopic transcervical resection (TCR) is procedure of choice for placental polyp without hypervascularity however not in cases with AVM [20, 21].
Conservative Management: Wait and See
Conservative or expectant management has been tried in asymptomatic and clinically stable cases who are available for the follow-up. Follow-up by simple observation is another potential choice of management in patients with few or no symptoms. Previous studies have reported that both placental polyp and uterine AVM can disappear without any medical intervention [22].
Medical Management
Anti-metabolite drug Methotrexate have been used to treat placental polyps. Hormonal preparation of estrogen and progesterone, danazol, depot gonadotropin releasing hormone preparations have been used in the management of AVM in asymptomatic and stable cases. The accurate diagnosis of the underlying condition is important to select appropriate medical treatment.
In a systematic review and meta-analysis Adam Rosen et al. reported overall 88% (106/121) success rate of medical management. After adjusting for the clustering effect success rate for progestins was 82.5%(95% CI, 70.1%-90.4%), GnRH-a 89.3%; (99% CI, 71.4%-96.5%) and methotrexate 90.0%; (99% CI, 55.8%-98.8%) were significantly different from the null hypothesis of 50% success. Progestins and GnRH-a had lowest complications. There were no predictors of success of medical management. Subsequently, twenty-six pregnancies have been described, with no reported AVM recurrences. Authors concluded that medical management is a reasonable approach in well selected cases, however data should be interpreted keeping publication bias in mind [23].
Surgical Management
A placental polyp is seen as a hypo- or anechoic tubular structure in the uterine cavity on grey scale USG (Fig. 3). Color Doppler USG scan provide significant information about the presence of blood flow, size of the vascularized mass and blood supply to the placental polyp (Fig. 4) [24].AVM is usually visualised as a heterogeneous myometrium with multiple hypo- or anechoic tubular structures. Color Doppler USG cannot always differentiate between AVM and vascular placental polyp [24].
Fig. 3.
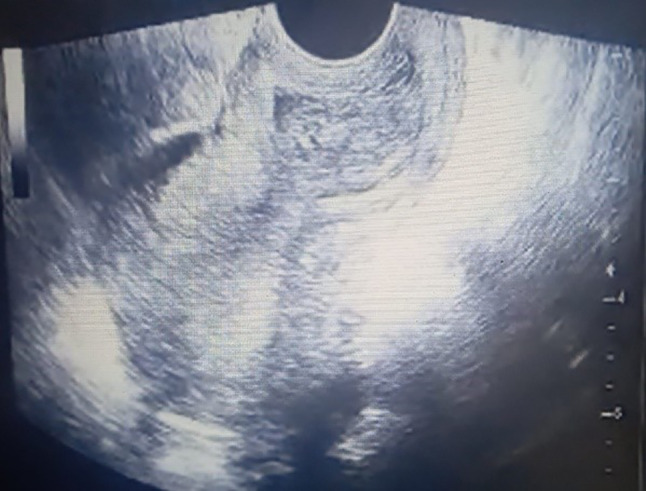
Placental polyp
Fig. 4.
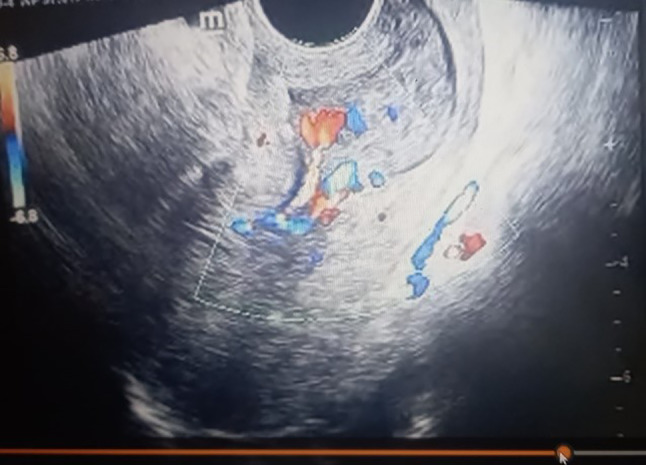
Placental polyp with arteriovenous malformation
CT angiography is useful investigation for preoperative anatomical conceptualization. Three-dimensional CT angiography is helpful in identifying feeding arteries of polyps and afferent and efferent arteries of AVM [24]. Placental polyps are usually visualized as high-intensity lesions in T2-weighted images and low-intensity lesions in T1-weighted images in MRI. In contrast-enhanced MRI, a flow void can be observed in the fundus of the polyp, implying the existence of rapid blood flow. A uterine AVM is typically visualized as a vascular tumor expanding and meandering within the myometrium. MRI and MR angiography are expensive and have longer acquisition times than CT angiography. Hence, TVS along with Doppler and CT angiography is reasonably appropriate option diagnosis in patients with suspected placental polyp or uterine AVM who have active vaginal bleeding.
Thus, no simple, distinguishing finding or characteristic is present for aiding the diagnosis of both these diseases. The combined use of USG, CT angiography, and MRI along with the results of serum hCG levels is critical for reaching the final diagnosis.
In cases with placental polyp or AVM (Fig. 5) severity of the bleeding dictates the management. When bleeding is severe, rapid medical intervention is required. Abdominal hysterectomy is curative. UAE should be considered especially in patients desirous of future pregnancy, however UAE itself has deleterious effect on fertility and reproductive outcome [23]. If vaginal bleeding remains uncontrollable even after UAE, TAH should be performed promptly to avoid fatal blood loss.
Fig. 5.
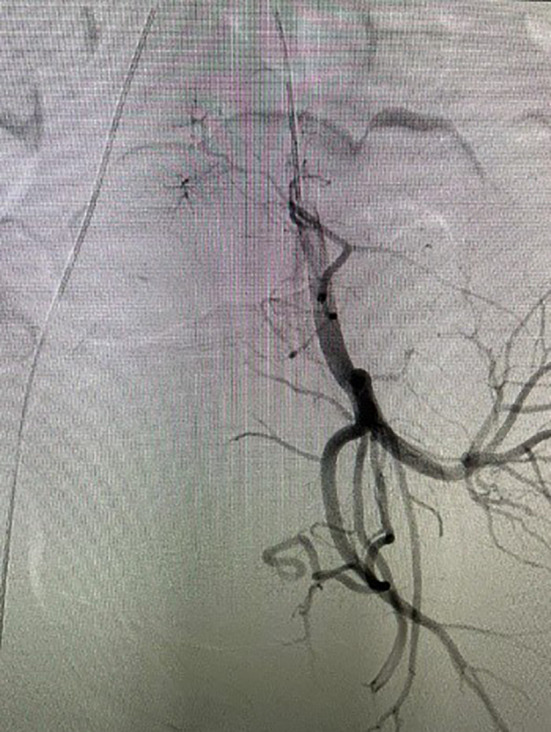
AVM on digital subtraction angiography
Osseous Metaplasia
Osseous metaplasia of the endometrium is a very rare cause of infertility which occurs in approximately 0.3 per 1000 women. It results from the transformation of non-osseous connective tissue into mature bone which interfere with implantation. A history of abortion or delivery is present in most patients with osseous metaplasia; however, the aetiology and pathogenesis of this condition are controversial. Inflammatory response to retained fetal bones following an abortion is the reason for endometrial ossification. Ultrasound and hysteroscopy are essential for the diagnosis and management. A hyperechogenic pattern on USG is strongly suggestive of osseous tissue. Ultrasound-guided hysteroscopy is preferred over dilatation and curettage for the removal of bone fragments [25].
Summary
RPOC are common and more so after abortions. Retained products of conception are associated with both early complications (like vaginal bleeding and infection) and late complications (like IUA, placental polyps, AVM) and their consequences (massive bleeding, infertility, morbidity, and mortality). Global abortion rates are very high. Awareness, availability, and acceptance of contraceptive methods prevent unwanted pregnancy and therefore, induced abortion and its complications. There is a need to standardize abortion care practices worldwide so as to select best abortion method; and to prevent, diagnose and timely treatment of complication to limit its sequel. In a clinically suspected case diagnosis is aided by ultrasound and Doppler. D&C is the commonly performed procedure, however its blind, traumatic and has higher risk of IUA formation. S&E or MVA is easier and safer option. Expectant management can be used when products are limited and avascular, however follow-up is required. Hysteroscopy has an advantage of removing the products under vision and has lower complication rates. Removal of RPOC under ultrasound guidance is more specific and complete. Prophylactic and therapeutic use of antibiotics helps in the management of infection. Classification of the RPOC as per Gutenberg classification aids in counseling and selecting management options.
Presence of active or life-threatening bleeding determines the choice intervention for achieving haemostasis. When available, UAE can be the first choice of treatment, followed by observation in cases where bleeding is controlled. In cases with uncontrollable bleeding hysterectomy is required. Serum beta hCG level, CT angiography and MRI angiography may help in differentiating between vascular polyps and AVM. Avascular or polypoidal lesions with low vascularity can be managed with TCR or curettage. In case of vascular polyp UAE should be done before TCR. Multiple procedures may be performed when mass is large. TCR should not be done when AVM is suspected.
Alternatively, expectant, and medical management may be tried in in asymptomatic and cases with minimal symptoms. Preventing injury to endometrium prevents IUA formation. Hysteroscopic adhesiolysis with bipolar cautery combined with barriers like hyaluronic gel may be helpful in reoccurrence of IUA.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Intstitute G. Induced Abortion Worldwide Fact Sheet. New York: Guttmacher Intstitute; 2016. [Google Scholar]
- 2.Abortion care guideline ISBN 978-92-4-003948-3 (electronic version) ISBN 978-92-4-003949-0 (print version) © World Health Organization 2022
- 3."MTP ACT, 1971 | Ministry of Health and Family Welfare | GOI". Ministry of Health and Family Welfare, Government of India. 10 August 1971. Archived from the original on 6 August 2022. Retrieved 23 July 2021
- 4.Smorgick N, Mittler A, Ben-Ami I, et al. Retained products of conception: What is the risk for recurrence on subsequent pregnancies? Eur J Obst Gynecol Reprod Biol. 2018;24:1–5. doi: 10.1016/j.ejogrb.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Purshottaman V, Rosen EM, et al. Retained products of conception after early pregnancy loss: a closer look. Univ North Carol ASRM Abstr. 2020;114(3):e188. [Google Scholar]
- 6.Hame CC, van Wessel S, Carnegy A, et al. Systematic review diagnostic criteria for retained products of conception—a scoping review. Acta Obstet Gynecol Scand. 2021;100:2135–2143. doi: 10.1111/aogs.14229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tinelli AP, Haimovich S. Hysteroscopy. New York: Springer; 2017. [Google Scholar]
- 8.Smorgick N, Segal H, Eisenberg N, Dovev MN, Dvash S, Rabinovich I. Serum β-HCG level in women diagnosed as having retained products of conception: a prospective cohort study. J Minim Invasive Gynecol. 2022;29(3):424–428. doi: 10.1016/j.jmig.2021.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Capmas P, Lobersztajn A, Duminil L, et al. Operative hysteroscopy for retained products of conception: efficacy and subsequent fertility. J Gynecol Obstet Hum Reprod. 2019;48:151. doi: 10.1016/j.jogoh.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Fei Z, Xin X, Fei He, Yuechong C. Meta-analysis of the use of hyaluronic acid gel to prevent intrauterine adhesions after miscarriage. Eur J Obstet Gynecol Reprod Biol. 2020;244:1–4. doi: 10.1016/j.ejogrb.2019.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Hamel C, Coppus S, van den Berg J, et al. Mifepristone followed by misoprostol compared with placebo followed by misoprostol as medical treatment for early pregnancy loss (the Triple M trial): a double-blind placebo-controlled randomised trial. EClinicalMedicine. 2021;32:100716. doi: 10.1016/j.eclinm.2020.100716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munros J, Gracia M, Nonell R, et al. Delayed hysteroscopic removal of retained products of conception is associated with spontaneous expulsion. SRL Reprod Med Gynecol. 2017;3:24–28. [Google Scholar]
- 13.Vyas S, Choi HH, Whetstone S, Jha P, Poder L, Shum DJ. Ultrasound features help identify patients who can undergo non-invasive management for suspected retained products of conception: a single institutional experience. Abdom Radiol (NY) 2021;46(6):2729–2739. doi: 10.1007/s00261-020-02948-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamel CC, van Wessel S, Carnegy A, Coppus SFPJ, Snijders MPML, Clark J, Emanuel MH. Diagnostic criteria for retained products of conception-A scoping review. Acta Obstet Gynecol Scand. 2021;100(12):2135–2143. doi: 10.1111/aogs.14229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alonso Pacheco L, Timmons D, Saad Naguib M, Carugno J. Hysteroscopic management of retained products of conception: a single center observational study. Facts Views Vis Obgyn. 2019;11(3):217–222. [PMC free article] [PubMed] [Google Scholar]
- 16.Melcer Y, Smorgick N, Schneider D, Pansky M, Halperin R, Ben-Ami I. Infertility following retained products of conception: Does the timing of surgical intervention matter? Isr Med Assoc J. 2016;18(10):605–608. [PubMed] [Google Scholar]
- 17.Hooker AB, de Leeuw RA, Emanuel MH, et al. The link between intrauterine adhesions and impaired reproductive performance: a systematic review of the literature. BMC Pregnancy Childbirth. 2022;22:837. doi: 10.1186/s12884-022-05164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishihara T, Kanasaki H, Oride A, Hara T, Kyo S. Differential diagnosis and management of placental polyp and uterine arteriovenous malformation: case reports and review of the literature. Womens Health (Lond) 2016;12(6):538–543. doi: 10.1177/1745505717692590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calzolari S, Cozzolino M, Castellacci E, et al. Hysteroscopic management of uterine arteriovenous malformation. JSLS. 2017;21(2):e2016.00109. doi: 10.4293/JSLS.2016.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karadag B, Erol O, Ozdemir O, et al. Successful treatment of uterine arteriovenous malformation due to uterine trauma. Case Rep Obstet Gynecol. 2016;1890650:3. doi: 10.1155/2016/1890650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosen A, Chan WV, Matelski J, et al. Medical treatment of uterine arteriovenous malformation: a systematic review and meta-analysis. Fertil Steril. 2021;116(4):1107–1116. doi: 10.1016/j.fertnstert.2021.05.095. [DOI] [PubMed] [Google Scholar]
- 22.Takeda A, Koyama K, Imoto S, et al. Computed tomographic angiography in diagnosis and management of placental polyp with neovascularization. Arch Gynecol Obstet. 2010;281(5):823–828. doi: 10.1007/s00404-009-1161-6. [DOI] [PubMed] [Google Scholar]
- 23.Nakashololo T, Khan N, Dunn Z, Snyman L, Ismail SMHI. Uterine arteriovenous malformations, clinical and radiological considerations: a report of two cases. Radiol Case Rep. 2021;16(7):1924–1929. doi: 10.1016/j.radcr.2021.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura Y, Osuga K, Nagai K, Hongyo H, Tanaka K, Ono Y, Higashihara H, Matsuzaki S, Endo M, Kimura T, Tomiyama N. The efficacy of uterine artery embolization with gelatin sponge for retained products of conception with bleeding and future pregnancy outcomes. CVIR Endovasc. 2020;3(1):13. doi: 10.1186/s42155-020-00107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horo G, Aka K, Toure A, Koffi A, Seni K, Kone M. Endoscopy management of endometrial ossification associated with secondary infertility: a case report and review of literature. J Clin Gynecol Obstet. 2016;5(1):45–49. doi: 10.14740/jcgo385w. [DOI] [Google Scholar]


