Abstract
The isoforms of SH2-B, APS, and Lnk form a family of signaling proteins that have been described as activators, mediators, or inhibitors of cytokine and growth factor signaling. We now show that the three alternatively spliced isoforms of human SH2-B readily homodimerize in yeast two-hybrid and cellular transfections assays, and this is mediated specifically by a unique domain in its amino terminus. Consistent with previous reports, we further show that the SH2 domains of SH2-B and APS bind JAK2 at Tyr813. These findings suggested a model in which two molecules of SH2-B or APS homodimerize with their SH2 domains bound to two JAK2 molecules, creating heterotetrameric JAK2-(SH2-B)2-JAK2 or JAK2-(APS)2-JAK2 complexes. We further show that APS and SH2-B isoforms heterodimerize. At lower levels of SH2-B or APS expression, dimerization approximates two JAK2 molecules to induce transactivation. At higher relative concentrations of SH2-B or APS, kinase activation is blocked. SH2-B or APS homodimerization and SH2-B/APS heterodimerization thus provide direct mechanisms for activating and inhibiting JAK2 and other kinases from the inside of the cell and for potentiating or attenuating cytokine and growth factor receptor signaling when ligands are present.
SH2 domains were the first recognized of the modular domains that mediate intermolecular interactions (48). As a result, SH2 domains have received enormous attention in terms of biological importance, as well as biochemical mechanism and potential for targeting in drug discovery. Intermolecular recognition by SH2 domains is both sequence specific and phosphorylation dependent (10, 49, 64, 65). This is rationalized by solved three-dimensional structures (15, 72, 73). Of equal interest, SH2 domains also can regulate the associated catalytic domains. The phosphatase SHP2 (SH-PTP2) is probably the best characterized of enzymes that are regulated by their SH2 domains (14, 30, 50, 66). The mechanisms of inhibition of SHP2 by its SH2 domains and activation by phosphopeptide binding to the SH2 domains are also understood in terms of a determined three-dimensional structure (21). More recently, Carter-Su and coworkers indicated that the SH2 domain of the adapter protein SH2-B might possess a third, previously unrecognized mode of action. Their findings suggested that the isolated SH2 domain of SH2-B stimulates JAK signaling downstream from growth hormone receptors (29, 58). Based on this suggestion, we carefully analyzed SH2-B in terms of both structure and function in order to characterize this new mechanism.
SH2-B and the closely related adapter, APS, function in cytokine and growth factor signaling. The isoforms of SH2-B have potential roles in insulin, insulin growth factor 1 (IGF-1), platelet-derived growth factor (PDGF), nerve growth factor (NGF), fibroblast growth factor (FGF), growth hormone, and gamma interferon signaling (27, 28, 38, 52, 53, 56, 59, 61, 75), whereas APS appears to be involved in insulin, IGF-1, PDGF, stem cell factor, interleukin-3, interleukin-5, granulocyte-macrophage colony-stimulating factor, NGF, erythropoietin, and B-cell receptor signaling (1, 23, 37, 52, 71). APS appears to be phosphorylated most prominently at Tyr618, which serves as a docking site for c-Cbl and potentially other SH2 domain-containing proteins (32, 71, 82). c-Cbl binding may target coupled proteins to ubiquitin-mediated proteosomal degradation (71, 82). Alternatively, c-Cbl may serve as a positive signal mediator, as suggested for its role in insulin-stimulated Glut4 translocation via the CAP/Cbl pathway (32). Only one of the SH2-B isoforms contains tyrosine within a corresponding carboxyl-terminal segment, suggesting that SH2-B must be phosphorylated at alternative sites (42).
SH2-B and APS share a common domain organization, including carboxyl-terminal SH2 domains, central pleckstrin homology (PH) domains, and a conserved amino-terminal domain whose function has not been identified previously. Their PH domains presumably couple SH2-B and APS to cellular phosphatidylinositides, although this has not been formally demonstrated. Consistent with this, SH2-B is localized to plasma membranes (60). The SH2 domains of SH2-B and APS are clearly necessary for interactions with growth factor receptors and cytokine receptor-coupled kinases (JAKs) through typical sequence and phosphotyrosine-dependent mechanisms. However, activation of JAK signaling by SH2-B, as proposed by Carter-Su and coworkers, cannot be reconciled in terms of known functions for SH2 domains.
While probing the mechanism of its role in JAK activation, we identified a new type of protein domain in the amino terminus of SH2-B that mediates homodimerization. We have determined the high-resolution crystal structure of the corresponding domain in APS, which forms a four-helix bundle that is uniquely bonded by a phenylalanine zipper (9). Modeling studies indicate that SH2-B forms nearly identical structures. Dimerization via this domain is necessary for SH2-B's cellular functions and its ability to stimulate JAK2 autophosphorylation and substrate phosphorylation (57). SH2-B expression also enhances growth hormone-induced JAK2 and Stat phosphorylation, suggesting potential roles for SH2-B as a cytoplasmic activator of cytokine-induced signaling through JAK-Stat pathways. Dimerization via the newly identified domain, in addition to typical SH2 domain interactions, is critical to SH2-B action.
MATERIALS AND METHODS
Molecular biology.
cDNAs encoding three human SH2-B isoforms were obtained either from SmithKline Beecham (SH2-Bβ) or from skeletal muscle poly(A) RNA by using reverse transcription-PCR (RT-PCR) (SH2-Bα and SH2-Bγ). The nucleotide sequences for the three human SH2-B isoforms have been deposited at GenBank (accession numbers AAF73912, AAF73913, and AAF73914). The human APS cDNA was produced by RT-PCR from human skeletal muscle poly(A) RNA with flanking primers based on the published sequence (GenBank no. AB000520). Arginine residues within the critical, SH2 domain FLVR motifs of SH2-Bα, SH2-Bβ, SH2-Bγ, and APS were substituted with alanine by using a QuikChange site-directed mutagenesis kit (Stratagene). cDNAs encoding truncated forms of the proteins were generated by restriction enzyme digestion or by PCR. For Northern blot analyses the 3′ cDNA fragment of SH2-Bβ was amplified by PCR, labeled with [32P]dATP by the random hexamer method (Stratagene), and hybridized to multiple human tissue mRNA blots (Clontech). The membranes were washed at high stringency, and mRNA was identified by using a PhosphorImager (Molecular Dynamics).
Yeast-two hybrid and bridging trihybrid experiments.
Matchmaker LexA two-hybrid reagents were purchased from Clontech. Saccharomyces cerevisiae strain EGY48 (MATα trp1 his3 ura3 6LexAop-LEU2 LYS2), transformed with p8op-lacZ, was used as the host for interaction studies. EGY48/p8op-lacZ was sequentially transformed with plasmid constructs by using polyethylene glycol-lithium acetate according to the manufacturer's protocols. To determine protein-protein interactions as a function of leucine biosynthesis (LEU2), transformants were grown on synthetic dextrose (SD) agar plates for 3 days at 30°C. Four independent colonies were streaked on SD glucose agar plates, grown overnight, replica plated on synthetic galactose-raffinose agar plates, and regrown for 5 days at 30°C to induce expression of B42 fusion proteins. To determine β-galactosidase activity, colonies were replica plated on nitrocellulose filters (Nytran) and frozen in liquid nitrogen; the nitrocellulose filters were placed on four paper filters (Whatman 3MM) soaked with 5.0 ml of Z-buffer--X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside)-2-mercaptoethanol (100 mM sodium phosphate [pH 7.0] containing 100 mM KCl, 1.0 mM MgSO4, 0.27% [vol/vol] 2-mercaptoethanol, and 0.334% [wt/vol] X-Gal) and incubated at 37°C. The β-galactosidase activity was quantified by growing yeast colonies in liquid synthetic galactose-raffinose (GR) medium overnight with shaking at 30°C to induce the expression of B42 fusion proteins. Cultures were diluted to optical densities of 0.2 and incubated an additional 3 h at 30°C (to optical densities of 0.4 to 0.6). Yeast cells were harvested by centrifugation, suspended in Z-buffer, and lysed by three cycles of freezing in liquid nitrogen and thawing to 37°C. β-Galactosidase activity was measured by using the Galacton Star substrate; results were expressed as relative light units.
To create the bridging yeast trihybrid (Y3H) assay, we modified the existing LexA yeast two-hybrid method. The original Y2H system contains three plasmids: pLexA, pB42AD, and p8op-lacZ. To create the Y3H system, a different third plasmid called pDis was introduced in place of p8op-lacZ. S. cerevisiae EGY48 (p8op-lacZ−) cells were sequentially transformed as described above with pLexA expressing JAK2(1-1129), IRK(940-1343), or IGF1RK(929-1337) as bait, pB42AD expressing JAK2(1-1129), IRK(940-1343), or IGF1RK(929-1337) as prey, and various SH2-B constructs in the third, bridging pDis plasmid. pDis was derived from the p426:Gal1 plasmid (American Type Culture Collection) by replacing its multiple cloning region with another having a nuclear localization signal under Gal1 promoter control. Transformants were grown on the appropriate SD plates for 3 days at 30°C. Four independent colonies were streaked on SD plates, incubated overnight, and replica plated on GR plates. The plates were immediately replica cleaned, incubated overnight, replica cleaned, and incubated at 30°C for 5 days to induce expression of pDis and B42 fusion proteins.
Cell culture and heterologous gene expression.
HEK293 and BOSC23 cells were cultured in Dulbecco modified Eagle medium with 10% fetal bovine serum (HyClone) at 37°C and 7.5% CO2; 3T3-L1 fibroblasts were cultured similarly under 10% CO2. For retrovirus-mediated gene transduction, cDNA sequences encoding wild-type (wt) and mutated SH2-Bα, SH2-Bβ, SH2-Bγ, and APS sequences with C-terminal hemagglutinin (HA) tags were subcloned into pBabe-puro (28). Then, 10 μg of each plasmid was used to transfect BOSC23 cells by using the calcium phosphate coprecipitation method. At 2 days postinfection, cells were replated at low density in 2 μg of puromycin/ml, and resistant clones were recovered. Expression levels of the transduced genes in each clone were determined by anti-HA Western blotting. In additional experiments, cDNA sequences encoding full-length SH2-Bα, SH2-Bβ, SH2-Bγ, and APS, as well as corresponding FLVR mutants and truncated proteins, were subcloned into pCMV-Tag 2 (Stratagene) to create constructs with C-terminal Flag tags. Alternatively, cDNAs already containing C-terminal HA tags were subcloned into pCMV-Tag 2 to create constructs with C-terminal HA tags. A full-length mouse Jak2 cDNA, kindly provided by T. Gustafson, Metabolex, was subcloned into pCMV-Tag 2 to create a construct with an N-terminal Flag tag. Subconfluent HEK293 cells were transfected by using FuGENE (Roche) as recommended. Cells were harvested 2 days after transfection.
Immunoprecipitations and Western blotting.
3T3-L1 fibroblasts and transfected HEK293 cells were treated with ligands at 37°C, cooled to 4°C, washed with ice-cold phosphate-buffered saline (140 mM NaCl, 3 mM KCl, 6 mM Na2HPO4, 1 mM KH2PO4 [pH 7.4]), and solubilized with modified radioimmunoprecipitation assay buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1.0 mM EDTA, 1.0 mM NaF, 1.0 mM sodium vanadate, 1.0 mM phenylmethylsulfonyl fluoride, 2.0 μg of aprotinin/ml, 10 μg of leupeptin/ml, 1.0 μg of pepstatin/ml, 0.25% sodium deoxycholate, and 1.0% NP-40) for 30 min at 4°C. The cell lysates were clarified by centrifugation at 15,000 × g for 20 min at 4°C. Proteins were precipitated with anti-Flag or anti-HA antibodies bound to protein G-Sepharose or anti-JAK2 or anti-STAT5b antibodies (Santa Cruz) bound to protein A-Sepharose (Pharmacia), eluted from the washed pellets, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to a polyvinylidene difluoride membranes (Immobilon PVDF; Millipore) by electroblotting. Membranes were blocked with SuperBlock reagent (Pierce) for 1 h at 22°C and reacted with specific antibodies in TBST buffer (30 mM Tris [pH 7.4], 120 mM NaCl, 0.1% Tween 20) containing 5% bovine serum albumin for 2 h at 22°C. Proteins were identified after incubation with horseradish peroxidase-linked second antibody (Amersham) by using an enhanced chemiluminescence method (Pierce). In the indicated experiments, immunoblots were stripped with 2% SDS and 100 mM 2-mercaptoethanol in 62.5 mM Tris-HCl (pH 6.7) for 30 min at 50°C and then reblotted. Anti-pTyr (4G10) antibodies were from UBI, anti-HA antibodies were kindly provided by Hamid Band or purchased from Roche, and anti-Flag antibodies were from Stratagene.
In vitro reconstitution assays.
Mouse JAK2 cDNA encoding full-length JAK2 or JAK2(1-544) was subcloned into pcDNA6 V5 HisA (Invitrogen) to generate proteins with C-terminal V5 and His6 tags. HEK293 cells transfected with 20 μg of each plasmid by using FuGENE 6 according to the manufacturer's instructions were harvested after 36 h and homogenized in 50 mM Tris-HCl (pH 7.8), 150 mM NaCl, 0.5% NP-40, 1 μM phenylmethylsulfonyl fluoride, 1 μM aprotinin, 1 μM leupeptin, and 2 μM pepstatin A. JAK2 proteins were isolated from clarified lysates by using nickel-nitrilotriacetic acid (Ni-NTA) affinity resin (Qiagen). The human SH-2Bβ cDNA was subcloned into pET28a (Novagen). Protein was expressed in transformed Escherichia coli gold BL21(DE3) (Invitrogen), isolated from lysed bacteria by using Ni-NTA affinity resin, and further purified by ion exchange (MonoQ FPLC) and gel filtration (Superdex-200 FPLC) chromatography. In vitro reconstitution assays with various concentrations of JAK2 and SH2-Bβ were conducted for 10 min at 25°C in 150-μl final volumes containing 50 mM Tris-HCl (pH 7.5), 1.2 mM MgCl2, 1.0 mM dithiothreitol, 1.2 mM ATP, and 150 mM NaCl. Proteins were separated by SDS-PAGE and identified by immunoblotting with anti-pY and anti-JAK2 antibodies.
RESULTS
Cloning of human SH2-Bα, SH2-Bβ, and SH2-Bγ cDNAs and Northern analyses.
We have cloned cDNAs encoding three isoforms of human SH2-B, which arise from alternative splicing of a common precursor mRNA. The SH2-B 1-632 sequence is common and contains the conserved N-terminus, PH, and SH2 domains (Fig. 1A). In contrast, the C-terminal extensions diverge in both sequence and length: 124 residues for SH2-Bα, 40 residues for SH2-Bβ, and 52 residues for SH2-Bγ. Relative to the SH2-Bα mRNA, SH2-Bβ contains a 100-nucleotide insertion just after the encoded SH2 domain; SH2-Bγ contains an additional 53-nucleotide insertion at the same location. These insertions alter the reading frame so that the three sequences are unique after residue 633. The sequences of the C-terminal extensions do not contain recognizable domains or features, apart from Tyr753 at the extreme C terminus of SH2-Bα, which may correspond to a phosphorylation site in APS (38). The isoforms of human SH2-B are highly similar to related forms found in rodents (38, 46, 61, 83). We have deposited the nucleotide sequences for the three human SH2-B isoforms at GenBank (accession numbers AAF73912, AAF73913, and AAF73914).
FIG. 1.
Human SH2-B isoforms. (A) Sequences of human SH2-B isoforms and APS were aligned by using the program CLUSTAL W. The three SH2-B isoforms have identical sequences through residue 632; the C-terminal tails differ by sequence and length. Dimerization, PH, and SH2 domains are shaded gray. Residues buried at the interface between two molecules in the DDs are denoted with triangles. (B) Northern analyses of SH2-B show a 3.3-kb transcript. The probe hybridizes to a common region so all three isoforms are recognized. Tissue codes: HE, heart; BR, brain; PL, placenta; LU, lung; LI, liver; MU, skeletal muscle; KI, kidney; PA, pancreas; SP, spleen; TH, thymus; PR, prostate; TE, testis; OV, ovary; IN, small intestine; CO, colon; PB, peripheral blood leukocyte.
To verify that these cDNAs are biologically relevant, we performed RT-PCR on human skeletal muscle poly(A) RNA with common primers flanking the unique C termini of each SH2-B isoform. Three major cDNA products were obtained and sequenced (data not shown), confirming the existence of all three SH2-B variants in human muscle. Northern blots were used to determine the expression pattern of human SH2-B. The greatest amounts of a 3.3-kb transcript were detected in skeletal and cardiac muscle and ovary, but the presence of the transcript in all human tissues suggests ubiquitous expression (Fig. 1B). The alternatively spliced mRNAs encoding the three SH2-B isoforms are insufficiently resolved in these blots, so the data do not address potential differences in the tissue distribution of the variants.
Tyrosine phosphorylation of human SH2-B is stimulated by GH, PDGF, IGF-1, serum, and pervanadate.
As part of our initial analyses of the human SH2-B proteins, we stably transfected 3T3-L1 fibroblasts with retrovirus vectors expressing either wt HA-tagged SH2-Bβ or a mutant whose SH2 domain was rendered incompetent (R555A). Transformants were stimulated with growth hormone, EGF, PDGF, serum or pervanadate, the cells were lysed, and SH2-Bβ was immunoprecipitated with anti-HA antibodies. After electrophoretic separation and transfer to membranes, phosphorylated proteins were detected by immunoblotting with anti-phosphosphotyrosine antibodies (Fig. 2). Human SH2-Bβ was tyrosine phosphorylated after stimulation under all five conditions. Of the polypeptide ligands, PDGF stimulated the greatest increase in SH2-Bβ phosphorylation; this was possibly due to the large number of endogenous PDGF receptors expressed by these cells (67). Fetal bovine serum, which contains many different growth factors, hormones, and cytokines, and pervanadate, a nonspecific inhibitor of protein-tyrosine phosphatases, both stimulated high levels of SH2-Bβ phosphorylation. In every case, a competent SH2 domain was necessary for the efficient phosphorylation of SH2-Bβ, a finding consistent with direct SH2 domain-mediated binding to the responsible kinase, presumably JAK2 in the case of growth hormone stimulation and the PDGF receptor following PDGF stimulation. The human isoforms thus behave much like their rodent counterparts as substrates of receptor tyrosine kinases.
FIG. 2.
Tyrosine phosphorylation of SH2-Bβ requires a functional SH2 domain. HA-tagged SH2-Bβ (wt) and SH2-Bβ R555A (RA) were stably expressed in 3T3-L1 fibroblasts by retrovirus infection. Cultures were stimulated with GH, EGF, PDGF, serum, or pervanadate (VO4). SH2-Bβ was immunoprecipitated and analyzed by Western blotting with antiphosphotyrosine and anti-HA antibodies. M, transfected with empty vector.
Yeast two-hybrid experiments show that SH2-B isoforms bind JAK2 via their SH2 domains.
Since SH2-Bβ was tyrosine phosphorylated in cells after growth hormone stimulation and this required a functional SH2 domain, we sought to determine whether direct physical interactions occurred between SH2-B isoforms and JAK2. Full-length JAK2 was expressed as bait in the LexA Y2H system, and the three SH2-B isoforms were expressed as prey. All three isoforms and APS bound JAK2 (Fig. 3). The SH2 domain of each protein mediated the interaction, since binding was abolished by R555A or R445A mutations in the FLVR motifs known to be critical for phosphotyrosine binding. Tyrosine phosphorylation was required, since kinase-defective JAK2 with a K882E mutation in its ATP-binding site no longer bound SH2-B or APS. Furthermore, a specific site within JAK2 (Tyr813) has been shown to mediate binding to SH2-Bβ (29). Substitution (Y813F) in Y2H experiments similarly blocked binding to human SH2-Bα, SH2-Bβ, SH2-Bγ, and APS, a finding consistent with high specificity via a typical mode of SH2 domain-mediated binding between JAK2 and these adaptor proteins. To test this further Y2H experiments were performed with the carboxyl-terminal tail of each SH2-B isoform, which contains its SH2 domain and unique extension. JAK2 bound each of the SH2 domain-containing tails (Fig. 3). SH2 domain-phosphotyrosine (Tyr813) interactions are thus necessary and sufficient for SH2-B mediated interactions with JAK2, exactly as we have found for APS (9) and consistent with previous findings (61).
FIG. 3.
Yeast two-hybrid studies show that JAK2/SH2-B binding requires tyrosine phosphorylation of JAK2 and an intact SH2 domain in the SH2-B isoforms. Full-length wt JAK2 or kinase-deficient (KD; K882E) or Y813F variants were used as bait, and the indicated wt, mutated, or truncated versions of human SH2-Bα, SH2-Bβ, SH2-Bγ, or APS were used as prey. Y2H interactions between JAK2 and SH2-B were determined by growth of transformants in medium lacking leucine (for the reporter Leu2) and by filter lift color assays (for the reporter LacZ).
Yeast two-hybrid experiments reveal a dimerization domain (DD) in SH2-B.
Our early studies indicated that, in addition to binding JAK2, SH2-B proteins might oligomerize as had been suggested previously (51). Y2H studies were used to test this possibility. Although wt SH2-B isoforms autoactivate when used as bait in the LexA system, this problem was circumvented by the removal of four internal residues (Δ493-496). wt isoforms were used as prey. SH2-Bγ binds itself in the assay (homodimerizes) and to SHB-2α and SH2-Bβ (Fig. 4). The SH2 domain-incompetent (R555A) forms of SH2-Bα, SH2-Bβ, and SH2-Bγ retained binding, demonstrating that dimerization does not depend on phosphotyrosine-SH2 domain interactions.
FIG. 4.
SH2-B dimerization. Y2H studies were further used to define a mechanism for SH2-B dimerization. Human SH2-Bγ was used as bait, and wt, mutated, or truncated human SH2-Bα, SH2-Bβ, or SH2-Bγ were used as prey. Homodimeric interactions were determined by growth of transformants in medium lacking leucine (Leu2) and by filter lift color assays (LacZ).
The mechanism is therefore distinct from some others, such as the Stat proteins, whose dimerization is mediated by its SH2 domains (31). The carboxyl-terminal tails of SH2-Bα(497-756), SH2-Bβ(497-671) and SH2-Bγ(497-683) do not bind SH2-Bγ, indicating that something amino-terminal to the SH2 domains mediates dimerization. Serial truncations showed that dimerization was mediated by a fragment amino-terminal to the PH domain (Fig. 4). SH2-B's DD could be pared down to a fragment as short as but no shorter than residues 24 to 85. This precisely matches the 63-residue DD we recently identified in APS (9).
SH2-B and APS proteins homodimerize in HEK293 cells.
To determine whether these proteins homodimerize in the more natural setting of a mammalian cell, as opposed to yeast nuclei, full-length and truncated SH2-B isoforms and APS were transiently expressed in HEK293 cells. Each protein was expressed in the same cells with two distinct epitope tags: C-terminal influenza virus HA or C-terminal Flag tags. To detect homodimers, HA-tagged proteins were immunoprecipitated with anti-HA antibodies, and the precipitates were immunoblotted with anti-Flag antibodies or vice versa. Homodimerization was detected for each of the proteins—SH2-Bα, SH2-Bβ, SH2-Bγ, and APS—when both of the coexpressed proteins were full length (Fig. 5A) and when one partner was full length and the other was a shorter fragment corresponding to the DD (Fig. 5B). Homodimerization was also observed when HA- and Flag-tagged DDs alone were coexpressed (Fig. 5C). These data demonstrate that SH2-B and APS proteins homodimerize in mammalian cells and that this interaction is mediated by their amino-terminal “dimerization domains,” thus confirming and extending the results obtained from the Y2H experiments.
FIG. 5.
Dimerization of SH2-B and APS in cultured HEK293 cells. (A) HA- and Flag-tagged forms of each full-length protein were coexpressed by transient transfection in HEK293 cells. SH2-Bα-HA and SH2-Bα-Flag (lane 1), SH2-Bβ-HA and SH2-Bβ-Flag (lane 2), SH2-Bγ-HA and SH2-Bγ-Flag (lane 3), or APS-HA and APS-Flag (lane 4) proteins were coexpressed. Proteins were immunoprecipitated with anti-HA antibodies and immunoblotted with anti-Flag antibodies. (B) Flag-tagged, full-length proteins were coexpressed in HEK293 cells, along with an HA-tagged DD. Proteins were immunoprecipitated with anti-HA antibodies and immunoblotted with anti-Flag antibodies. (C) HA- and Flag-tagged APS DDs were coexpressed in 293 cells. Proteins were immunoprecipitated with anti-Flag antibodies and immunoblotted with anti-HA antibodies.
Heterodimerization between SH2-B and APS.
The finding that SH2-B and APS have highly homologous DDs suggested that, in addition to homodimerizing, the two proteins might bind each other. This too was tested by using the Y2H method. SH2-Bα, SH2-Bβ, and SH2-Bγ as prey all bound to APS as bait (Fig. 6A). Their SH2 domains were not responsible, since C-terminal fragments of the SH2-Bs did not bind to APS. In contrast, all amino-terminal fragments of SH2-B that contained its DD (residues 24 to 85) bound to APS, whereas shorter fragments having partial DDs did not. Similar studies were conducted with SH2-Bγ as bait and APS as prey. Again, the full-length proteins bound, the APS SH2 domain did not bind, and fragments of APS containing an intact DD (residues 21 to 85) bound. In yeast two-hybrid experiments SH2-B or APS homodimerization and SH2-B/APS heterodimerization were indistinguishable. Although yeast two-hybrid results of this nature are more qualitative than quantitative, the findings are consistent with the potential for heterodimerization in cells.
FIG. 6.
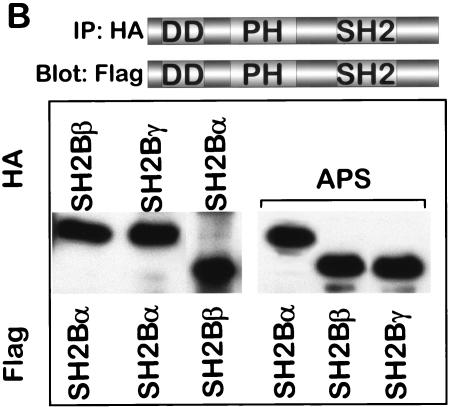
SH2-B and APS heterodimerization by yeast two-hybrid analysis. APS or SH2-Bγ were used as bait, and wt, mutated, and truncated APS, SH2-Bα, SH2-Bβ, and SH2-Bγ were used as prey. Interactions were determined independently using Leu2 and LacZ reporters. (B) HA- and Flag-tagged forms of SH2-B and APS were coexpressed in HEK293 cells, as described above, although in this case different proteins were coexpressed. For example, for the first lane SH2-Bβ-HA and SH2-Bα-Flag proteins were coexpressed, for the second lane SH2-Bγ-HA and SH2-Bα-Flag proteins were coexpressed, etc. Proteins were immunoprecipitated with anti-HA antibodies and immunoblotted with anti-Flag antibodies.
Cellular experiments similar to those described above to look at homodimerization in HEK293 cells were thus devised to look at heterodimerization between APS and SH2-B isoforms. For these experiments two different full-length proteins—one bearing the HA epitope and the other bearing the Flag tag—were coexpressed. We looked first at dimerization between two different isoforms of SH2-B. The SH2-B isoforms—SH2-Bα, SH2-Bβ, and SH2-Bγ—bound to each other regardless of which was HA tagged or Flag tagged (Fig. 6B). Similarly, when coexpressed, APS and each of the SH2-B isoforms coimmunoprecipitated, a finding consistent with a shared mechanism for dimerization. These findings raise the possibility that distinct SH2-B isoforms might heterodimerize with themselves and with APS in cells. We do not know which of the endogenous proteins are present within given subcellular compartments, but if two different proteins were present, then our findings clearly demonstrate that the possibility exists for heterodimerization as has been seen in cultured cortical neurons (51).
Molecular model of the SH2-B DD.
Since sequences of the APS and SH2-B DDs are 51% identical, we reasoned that they are probably quite similar in terms of both structure and function. Our recently determined X-ray crystallographic structure of the APS DD (Fig. 7A) (9) was used, along with the program MODELLER, to build a model of the SH2-B domain (Fig. 7B). In the APS structure, two protein molecules are closely associated (Fig. 7A). Each protomer forms a U or V shape comprising two α helices connected by a β turn. The open ends of the U's interdigitate to form a four-helix bundle. This topology was recognized by DeGrado and coworkers in 1998 and is referred to as a bisecting U motif (19, 20). The dimerization interface of APS is distinct, however, from any known since it is composed almost entirely of aromatic residues. The most conspicuous feature is a stack of 10 phenylalanine side chains (side chains of Phe38, Phe42, Phe65, Phe69, and Phe73 from each protomer) (Fig. 7A). We coined the term “phenylalanine zipper” to refer to this new motif due to its resemblance to the leucine zipper. Identical residues stack at the core of the modeled SH2-B domain (Phe41, Phe45, Phe68, Phe73, and Phe76) (Fig. 7B). In addition to the phenylalanine zipper, which runs along the interface between two C-terminal helices, Phe26 and Phe45 in APS interact at both ends of the domain at the interface between the apex of each U and the amino terminus of the N-terminal helix. There are also two grooves on the surfaces of each N-terminal helix where they are crossed by the C-terminal helices. Side chains of APS residues Ala31, Ala35, and Ala39 line the surface of the groove (Fig. 7A). All of these uniquely interesting features of APS are identically reproduced in the SH2-B model. In fact, of 24 residues from each protomer that are buried at the dimerization interface of APS (48 residues in the dimer), all 24 (100%) are identical in SH2-B. In contrast, only six residues on the DD surface (15%) are identical, strongly supporting common functions and mechanisms for the two domains.
FIG. 7.
Molecular models of DDs. (A) The X-ray crystal structure of the APS DD (9) is detailed on the left. (B) A molecular model of the SH2-B domain was generated by using the program MODELLER and the coordinates from the APS domain structure. For both models, one molecule in the dimer is colored green and the other is red. Amino acids are numbered and colored accordingly.
Additional Y2H experiments tested the relevance of the model and the importance of individual residues for dimerization. Substitutions within the phenylalanine zipper abolished binding, including F29A, F41A, F68A, and F72A (Table 1), even when only one protomer was mutated. The F45A substitution did not affect binding, even when the residue was substituted in both protomers. Therefore, F45 at the extreme ends of the zipper is not a necessary component of the SH2-B dimerization interaction. Due to methodological difficulties we were unable to substitute Phe76. However, paired aromatic residues outside of the zipper, located at both ends of the DD, are critical to the interaction, since F29A and Y48A substitutions both abolished binding. Y48 at the apex of each U corresponds to F45 of APS, whereas F29 at the extreme amino termini of the opposite protomers corresponds to APS F26.
TABLE 1.
Mutational scan of the dimerization domaina
| SH2-Bγ | Growth as determined with:
|
|||||
|---|---|---|---|---|---|---|
| Indicated prey
|
Indicated bait
|
|||||
| wt SH2-Bγ | Same-site mutant | wt APS | JAK2 | IRK | IGF1RK | |
| Wild type | + | + | + | + | + | + |
| Aromatics | ||||||
| F29A | − | − | − | + | + | + |
| F41A | − | − | − | + | + | + |
| F45A | + | + | − | + | + | + |
| Y48A | − | − | − | + | + | + |
| F68A | − | − | − | + | + | + |
| F72A | − | − | − | + | + | + |
| Alanines | ||||||
| A34D | − | − | − | + | + | + |
| A38D | − | − | − | + | + | + |
| A42D | + | − | − | + | + | + |
| Surface | ||||||
| S32A | + | + | + | + | + | + |
| A63D | + | + | + | + | + | + |
| SH2 domain: R555A | + | + | + | − | − | − |
Aromatics in the zipper or at the ends of the domain were substituted with alanine; native alanines were substituted with aspartic acid. Y2H studies were conducted with substituted variants of SH2-Bγ as bait and wt SH2-Bγ, same-site mutants of SH2-Bγ, or wt APS as prey. Additional Y2H studies used substituted variants of SH2-Bγ as prey and JAK2, IRK, or IGF1RK as bait. Symbols: +, growth; −, no growth (no interaction).
Similar substitution studies were conducted with the alanine residues of the groove, except these were substituted with aspartic acid. A34D and A38D substitutions abolished dimerization, even when only one protomer was substituted (Table 1). A42D abolished binding only when both protomers were substituted, suggesting that, although A42 is important, substitutions at this position are less deleterious in terms of binding energy. Two additional substitutions served as negative controls. In general, residues on the domain surface would not be expected to perturb binding, providing they do not disrupt the global fold. As predicted by the molecular model, the S32A and A63D substitutions, on the domain surface, have no affect whatsoever on either SH2-B homodimerization or heterodimerization with APS. In summary, aromatic residues throughout the phenylalanine zipper and at the ends of the domain are critical for dimerization. Alanine residues that create a shallow trough at the helical intersections are also critical, whereas the substitution of surface residues away from the dimerization interface does not alter function.
Global function was retained by the “dimerization domain” mutants, as evidenced by the fact that each retained the capacity to bind various kinases in yeast two-hybrid system, including JAK2 and the insulin and IGF-1 receptors (insulin receptor kinase [IRK] and IGF-1 receptor kinase [IGF1RK]) (Table 1). In contrast, an R555A mutation in the FLVR motif of the SH2 domain of SH2-B blocked binding to all three kinases and yet had no effect on either SH2-B/SH2-B homodimerization or SH2-B/APS heterodimerization.
Y3H assay.
Additional experiments were designed to test whether SH2-B proteins and kinases create heterotetrameric complexes as a potential mechanism for activating kinases (57). We hypothesized that two SH2-B proteins would homodimerize via their DDs and that each would bind a kinase via its SH2 domain. We developed a bridging yeast trihybrid (Y3H) assay to test this possibility. Y3H is similar to Y2H, except a third protein is expressed from an extra plasmid (pDis). Since two JAK2 proteins do not bind one another, under Y2H conditions with JAK2 expressed as both bait and prey there was no growth, a finding consistent with no interaction. There is similarly no interaction using Y3H with pDis as an empty vector and JAK2 expressed as bait and prey (Fig. 8). However, the coexpression of SH2-Bα, SH2-Bβ, or SH2-Bγ led to coupling of JAK2-bait and JAK2-prey, as indicated by growth on Leu− medium. SH2-B dimerization and SH2 domain binding to JAK2 were both required, since either removal of the DD or a mutation in the FLVR motif of the SH2 domain inhibited growth. Identical results obtained with SH2-Bα, SH2-Bβ, SH2-Bγ, and APS (9) demonstrate that this mechanism is common to the protein family. Moreover, identical results obtained with the IRK and IGF1RK verify that this mechanism extends to other classes of receptor tyrosine kinases that have been shown to interact physiologically with SH2-B. In contrast, the EGF receptor, which does not interact with SH2-B under physiological conditions, was not dimerized by SH2-B in the Y3H assay (Fig. 8). Additional control experiments demonstrated that EGF receptor was active in yeast and able to interact with alternative SH2 domain-containing proteins (data not shown).
FIG. 8.
Bridging Y3H assays show that heterotetramers form between SH2-B isoforms and either JAK2 or insulin or IGF-1 receptors. The bridging Y3H system was developed as a modification of the pLexA yeast two-hybrid method. Either full-length JAK2, the IRK, or the IGF1RK was expressed as both bait (pLexA) and prey (pB42AD). wt and variants of SH2-Bα, SH2-Bβ, and SH2-Bγ were expressed from a third (pDis) plasmid. Growth on synthetic dextrose plates indicates an interaction (+) between bait and prey.
The bridging Y3H method provides an additional opportunity to test whether specific substitutions within the DD blocked JAK2, IRK, or IGF1RK dimerization. The same substitutions used in the Y2H experiments (Table 1) were used to test the dimerization model. Substitution of individual phenylalanines within the phenylalanine zipper (F41A, F42A, F68A, and 72A) or at the ends of the domain (F29A and Y48A) or of individual alanine residues within the alanine trough (A34D, A38D, and A42D) all disrupted JAK2, IRK, or IGF1RK dimerization in parallel with their ability to disrupt SH2-B dimerization (Fig. 8). In contrast, control substitutions either at the domain surface (S32A and A63D) or elsewhere (F45A) that failed to block SH2-B dimerization similarly failed to inhibit kinase dimerization. These data provide a consistent mechanism for interactions between SH2-B and JAK2, IRK, or IGF1RK in which two SH2-B molecules dimerize and their SH2 domains bind the kinases. This “dimerizes” the kinases, which we hypothesize would promote activation through transphosphorylation. The model predicts that when kinase is present in large molar excess over SH2-B (or APS), increases in SH2-B (or APS) concentrations would activate the kinase and/or potentiate kinase signaling. The model also predicts the converse, that at higher concentrations SH2-B or APS might interfere with kinase signaling.
In vitro activation of JAK2.
A reconstitution assay was established to test whether SH2-B alters JAK2 activity in vitro. Adding ATP to recombinant JAK2 leads to low-level autophosphorylation (Fig. 9A and C). Adding recombinant SH2-B to the mix increases JAK2 autophosphorylation by >3-fold (Fig. 9A and B). Activation by SH2-B is concentration dependent. JAK2 autophosphorylation increased with increasing SH2-B concentrations. At 14 pM JAK2, 10−9 M SH2-B stimulated JAK2 phosphorylation the greatest (Fig. 9A and B). At a higher JAK2 concentration (118 pM) maximal phosphorylation was with 10−8 M SH2-B (Fig. 9C and D). Consistent with the model, further increasing the SH2-B concentration at both JAK2 concentrations reduced rather than increased JAK2 phosphorylation (Fig. 9).
FIG. 9.
In vitro reconstitution showing JAK2 activation and inhibition by SH2-B. Recombinant JAK2 and recombinant SH2-B (indicated concentrations) were incubated with ATP (1.2 mM). Experiments were conducted with JAK2 at (A and B) 14 pM or (C and D) 118 pM concentrations. In panels A and C, JAK2 was visualized immunoblots with anti-pY and anti-JAK2 antibodies; in panels B and D, the results from three separate experiments were quantified by scanning densitometry and combined (mean ± the standard error of the mean; ✽, P < 0.05).
JAK2 activation in cultured cells.
The following experiments tested whether wt SH2-B or APS proteins activated or inhibited JAK2 signaling according to the model in cellular systems. SH2-Bβ and JAK2 were transiently coexpressed in HEK293 cells, and the phosphorylation states of these proteins were assessed. In the absence of JAK2 expression, SH2-Bβ phosphorylation was not detectable. However, with increasing levels of JAK2 expression, SH2-Bβ phosphorylation increased markedly (Fig. 10A). Not surprisingly, JAK2 phosphorylation increased incrementally with JAK2 expression (Fig. 10B). However, the level of JAK2 phosphorylation also increased substantially due to the presence of SH2-Bβ. Therefore, the coexpression of SH2-Bβ and JAK2—even in the absence of activating cytokines or growth factors—activates JAK2 and leads to the tyrosine phosphorylation of both proteins. Parallel experiments were conducted with each of the human SH2-B isoforms and human APS (Fig. 10C). In every case the low level of JAK2 phosphorylation seen in the absence of SH2-B or APS expression increased dramatically upon their expression.
FIG. 10.
SH2-B and APS activate JAK2 in cultured cells. HA-tagged SH2-Bβ (0.0 or 1.0 μg of pCMV-Tag2-SH2-Bβ DNA) and Flag-tagged JAK2 (0.0 to 1.0 μg of pCMV-Tag2-JAK2 DNA) were coexpressed in HEK293 cells. Proteins were immunoprecipitated with anti-HA (A) or anti-Flag (B) antibodies and immunoblotted with anti-pY antibodies. (C) Experiments similar to those described in panel B were conducted with APS and the three isoforms of SH2-B. HA-tagged SH2-Bα, SH2-Bβ, SH2-Bγ, or APS (0.0 or 1.0 μg of pCMV-Tag2 DNA) and JAK2 (1.0 μg of pCMV-Tag2-JAK2 DNA) were coexpressed in 293 cells. Proteins were immunoprecipitated with antibodies to JAK2 and immunoblotted with pY antibodies.
SH2-B and APS isoforms enhance JAK2 and Stat5 phosphorylation.
The direct binding between JAK2 and SH2-B seen in yeast two-hybrid experiments and the growth hormone-stimulated SH2-Bβ phosphorylation found in 3T3-L1 fibroblasts indicated that human SH2-B isoforms are involved in signaling via the growth hormone receptor/JAK2/Stat5 axis, as has been documented for rodent forms of the proteins (57, 61). To test this possibility, we infected 3T3-L1 fibroblasts with retroviruses to stably express wt or SH2 domain-defective SH2-B isoforms or APS or the corresponding carboxyl-terminal, SH2 domain-containing fragments (SH2+CT). JAK2 and Stat5 were strongly tyrosine phosphorylated by growth hormone in 3T3-L1 fibroblasts. Expression of wt SH2-Bα increased JAK2 phosphorylation ∼2-fold compared to cells infected with empty vector and led to a small increase in Stat5 phosphorylation (Fig. 11A). In contrast, expression of SH2-Bα (R/A) decreased JAK2 phosphorylation by three- to fourfold and Stat5 phosphorylation by about twofold. Expression of SH2-Bα SH2+CT similarly decreased JAK2 and Stat5 phosphorylation, although to a lesser extent than SH2-Bα (R/A). The dominant inhibitory effects of SH2 domain-incompetent SH2-Bα (R/A) and the truncated SH2+CT protein indicated that these modified proteins interfered with normal cellular signaling, whereas the wt protein promoted growth hormone signaling.
FIG. 11.
(A) Activation or inhibition of endogenous JAK2 and Stat5b by expression of wt or dominant inhibitory forms of SH2-B or APS, respectively. 3T3-L1 fibroblasts were infected with retrovirus vectors expressing wt SH2-B or APS proteins, SH2 domain-incompetent, full-length proteins (R/A; SH2-Bα R555A, SH2-Bβ R555A, and APS R455A), or the carboxyl-terminal segments of SH2-Bα(497-756) or SH2-Bβ(497-671) containing the SH2 domains and unique tails (SH2). Cells were stimulated (+) or not (−) with GH (200 ng/ml for 10 min) and lysed. Proteins were immunoprecipitated with the indicated antibodies, separated by SDS-PAGE, and identified by immunoblotting with antibodies to pTyr, JAK2, or STATb.
Similar results were obtained after expression of wt and mutated forms of SH2-Bβ or APS. Expression of wt SH2-Bβ increased JAK2 phosphorylation two- to threefold and led to a slight increase in Stat5 phosphorylation, whereas expression of SH2-Bβ (R/A) or the carboxyl-terminal, SH2 domain-containing fragment decreased JAK2 phosphorylation by three- to fourfold (Fig. 11B). Similar results were obtained with APS: the wt protein increased JAK2 phosphorylation, whereas APS (R/A) diminished JAK2 phosphorylation (Fig. 11C). In every case effects on Stat5 phosphorylation were in the same direction as those of JAK2 phosphorylation but of smaller magnitude. Presumably, SH2-Bα (R/A), SH2-Bβ (R/A), and APS (R/A) are dominant inhibitory due to their abilities to dimerize with endogenous APS or SH2-B proteins. These complexes would be inactive, since only the endogenous proteins' SH2 domains would bind JAK2 and transphosphorylation could not occur. Dominant inhibition by the SH2+CT proteins presumably occurs through their capacity to compete for binding at Tyr813, which would block binding to JAK2 by the endogenous APS or SH2-B proteins. Although SH2-Bγ was not studied under these conditions, we presume that all results would be comparable. In conclusion, each of the wt proteins promoted growth hormone signaling, whereas the SH2 domain-incompetent proteins or carboxyl-terminal fragments were dominant inhibitors.
DDs are dominant inhibitors of JAK2 activation.
Our model predicts that the DD of SH2-B or APS is critical for juxtaposing the kinase domains of two JAK2 molecules for subsequent transphosphorylation. To test the importance of SH2-B or APS-mediated dimerization during JAK2 activation, we overexpressed the isolated domain in cells. We reasoned that an excess of DD would compete for SH2-B and APS self-association and thereby inhibit SH2-B- or APS-mediated JAK2 activation. Previous experiments showed that in HEK293 cells the levels of JAK2 phosphorylation increased significantly with the coexpression of either SH2-B or APS (Fig. 10B and C). In this experiment we expressed equivalent levels of SH2-Bβ in 293 cells, along with variable amounts of JAK2, with or without the DDs of SH2-B or APS. As expected, in the presence of SH2-Bβ, JAK2 phosphorylation was enhanced, and it increased in parallel with increasing levels of JAK2 protein expression (Fig. 12A). Notably, coexpression of the DDs of SH2-B or APS diminished JAK2 phosphorylation. The APS DD appeared to be a more effective inhibitor of JAK2 phosphorylation than the SH2-B domain, but this variance could be due to differences in expression efficiency or protein stability (quantitation of the DD concentration has been difficult due to its small size). Nevertheless, these experiments clearly demonstrated that isolated DDs act as dominant inhibitors of JAK2 phosphorylation in cells coexpressing SH2-Bβ and demonstrate that dimerization of SH2-B (or APS) is required for the activation of JAK2 by these proteins.
FIG. 12.
(A) The DD dominantly inhibits JAK2 phosphorylation. Proteins were coexpressed in 293 cells: JAK2 was expressed in variable amounts (0.0, 0.1, 0.3, and 0.5 μg of pCMV-Tag2-JAK2 DNA), HA-tagged SH2-Bβ was expressed in each experiment (0.7 μg of pCMV-Tag2-SH2-Bβ DNA), and the DDs from SH2-B (residues 24 to 85; 3 μg of pCMV-Tag2-SH2-B/DD DNA) or APS (residues 21 to 85; 3 μg of pCMV-Tag2-APS/DD DNA) were expressed as indicated (S or A, respectively). (B) Full-length SH2-Bβ (0.0 or 0.7 μg of pCMV-Tag2-SH2-Bβ DNA), JAK2 (1.0 μg of pCMV-Tag2-JAK2 DNA), and the APS DD (residues 21 to 85; 0.0 or 3.0 μg of pCMV-Tag2-APS/DD DNA) were coexpressed in 293 cells. Proteins were immunoprecipitated with anti-JAK2 antibodies and immunoblotted with antibodies to pY or JAK2. Cell lysates were blotted with antibodies to HA to detect SH2-Bβ or the APS DD.
The dominant inhibitory effects of SH2 domain-incompetent forms of SH2-B and APS in 3T3-L1 fibroblasts (Fig. 11) strongly support a role for the endogenous proteins in growth hormone-dependent JAK2 activation, but these experiments do not directly test whether dimerization is important. At higher transfection levels JAK2 was activated in HEK293 cells even in the absence of coexpressed SH2-B or APS (Fig. 10B and C). We wondered whether this, too, would be inhibited by the DDs; thus, we wondered whether endogenous SH2-B or APS dimers are involved in the activation of transfected JAK2. To investigate this issue, JAK2 and the APS DD were coexpressed in HEK293 cells, with or without coexpression of SH2-Bβ (Fig. 12B). When SH2-Bβ was omitted, JAK2 phosphorylation was nevertheless reduced by DD expression, indicating that endogenous APS/SH2-B dimers were involved in the spontaneous phosphorylation of transfected JAK2. SH2-Bβ coexpression increased JAK2 phosphorylation and, as expected, this, too, was inhibited by DD expression. These results strongly support a role for the SH2-B- and/or APS-mediated dimerization in biological signaling through JAK2.
DISCUSSION
Dimerization, a common theme in signal transduction, is required for the activation of a wide variety of transcription factors, cell surface proteins, and intracellular trafficking proteins (26). Particularly relevant to our current findings, extracellular ligand binding induces the dimerization of receptor tyrosine kinases, which stimulates intrinsic catalytic activity and autophosphorylation (78). We now provide evidence for an alternative or additional mechanism for promoting dimerization and activation, one that is mediated instead from within the cell. At the outset we had attempted to figure out how the SH2 domain of SH2-B might activate JAK signaling, as had been suggested by the Carter-Su lab (29, 58). Although we reproduced many of their findings, including SH2 domain-mediated binding to JAK2 specifically at Tyr813 and activation of JAK2 by SH2-B (29, 57), we were unable to show that the SH2 domain itself activated JAK2. The mechanism we identified relies on the seminal findings of those studies but differs substantially in terms of the proposed molecular mechanism. Instead of activating JAK2, in our studies the SH2 domain of SH2-B expressed alone inhibits JAK2. The SH2 domain of SH2-B behaves in our hands just like other SH2 domains: for well over a decade it has been recognized that SH2 domain overexpression or microinjection in cells is inhibitory. Many investigators have used this feature of SH2 domains to establish biological roles for SH2 domain proteins, including Grb2, PLC-γ, phosphatidylinositol 3-kinase p85, SHP2, Shc, Grb10, Nck, Zap70, Syk, and Cbl. Each has been shown to be a dominant inhibitor (4-8, 17, 18, 24, 33-35, 39-41, 43, 47, 54, 55, 62, 63, 68, 74, 76, 77, 80, 81). Under crystallization conditions of very high concentration, the SH2 domains of APS dimerize (22). This may provide an explanation for the alternative findings with SH2-B (29, 58).
We now show that SH2-B and APS bind each other and to kinases such as JAK2 to form activated tetrameric complexes. This can stimulate downstream signaling in the absence of extracellular ligand activation and potentiates signaling during ligand activation. The Tyr318 docking site on JAK2 must be phosphorylated for SH2-B or APS to bind. Although this is promoted by cytokine binding, phosphorylation occurs at a slower rate even in its absence. This can be demonstrated with pervanadate to inhibit cellular phosphatases, which promotes kinase phosphorylation in the absence of bound ligands (e.g., Fig. 2). Presumably, SH2-B-mediated activation of the insulin receptor occurs similarly, with docking at Tyr1158 within the kinase activation loop as demonstrated for APS (22).
Although we utilized the newly cloned human isoforms of SH2-B, findings undoubtedly extend to the rodent proteins (>90% identity) and presumably other species of SH2-B and APS. Cellular and yeast two-hybrid data show that the human SH2-B isoforms bind JAK2 via their SH2 domains, a result in good agreement with findings for rat SH2-Bβ (32). The SH2-B isoforms readily dimerize through a 63-residue amino-terminal domain. The DD we identified in SH2-B is highly similar to the corresponding domain of APS, whose crystal structure we recently solved in order to identify the molecular mechanism for APS dimerization (9). Modeling studies indicated that mechanisms for APS and SH2-B dimerization are virtually identical. In fact the various SH2-B and APS isoforms are so similar that they readily heterodimerize in yeast and transfected cells, providing an additional potential level of combinatorial complexity that needs to be considered in future studies.
Of the numerous molecular mechanisms known to mediate dimerization, the phenylalanine zipper within a bisecting U appears to be unique. To our knowledge, the symmetrical stacking of Phe side chains to form an antiparallel zipper-like motif has not been described previously. In contrast, leucine or alanine residues are known to interact at the interfaces between α helices in proteins containing Leu zippers or Alacoils, respectively (16, 44). As a structural motif, the bisecting U was first recognized in 1998 by DeGrado and coworkers in α2D, a designed four-helix bundle (19). In place of the stacked phenylalanines of APS/SH2-B, the equivalent interface in α2D resembles an antiparallel Leu zipper with interdigitated Leu residues shielded by side chains of hydrophilic residues. The bisecting U was subsequently found to be relatively common in both monomeric and dimeric proteins (20). The two helices in each protomer of the APS DD are connected by a well-ordered β turn containing the sequence NPXY. We and others have shown previously that this motif can be involved in receptor endocytosis (2, 3, 12) and PTB domain recognition (13, 25, 69, 79, 84). To our knowledge, there is as yet no biological support for such functions for the corresponding HPXY sequence in SH2-B.
So what are the in vivo functions of the APS, SH2-B, and LNK proteins and how important is dimerization for these functions? APS-deficient mice have an insulin-sensitizing phenotype (36). Fasting and post-glucose challenge blood glucose and insulin levels were reduced in the Aps−/− mice relative to controls, despite their having increased adiposity and circulating leptin levels. These findings are potentially consistent with APS having a negative, inhibitory role in insulin signaling and suggest that SH2-B may not compensate for the loss of APS in this pathway. Targeted disruption of the SH2-B locus yields viable mice that show slight growth retardation at early ages (11, 45). The mice become insulin resistant due to decreased levels of insulin receptor autophosphorylation (11). These findings strongly support a role for SH2-B in activating insulin action in vivo. Consistent with an important role for these proteins in cytokine and growth factor signaling, the targeted deletion of Lnk has serious consequences (70). Lnk is a more distant relative of the APS/SH2-B family whose expression is restricted to hematological cells. Its domain structure is similar to APS and SH2-B, including homologous PH, SH2, and DDs (Fig. 1A). However, its DD is sufficiently distinct, having a leucine and tyrosine in place of two phenylalanines in the zipper, so that it does not heterodimerize with APS or SH2-B in yeast (data not shown). Along with its tissue specificity, this suggests that neither APS nor SH2-B could compensate in Lnk−/− mice, which may account for the more pronounced phenotype.
In conclusion, we have identified a new domain in SH2-B that mediates dimerization. Dimerization of SH2-B increases JAK2 phosphorylation through the formation of [JAK2]2[SH2-B]2 heterotetramers. Presumably, bringing two kinases into proximity facilitates transphosphorylation and activation. Similar mechanisms likely occur as well with other kinases.
Acknowledgments
These studies were funded by NIH grants DK43123 and DK45943 (S.E.S.), the Helen and Morton Adler Chair (S.E.S.), and DK36836 (Joslin Diabetes Center DERC). M.N. was supported by the Sankyo Foundation. E.D.W. and J.D.F. were recipients of NIH Fellowships DK09393 and DK09908, respectively.
Footnotes
This study is dedicated to the memory of our friend and colleague, Valerie Fanikos.
REFERENCES
- 1.Ahmed, Z., B. J. Smith, K. Kotani, P. Wilden, and T. S. Pillay. 1999. APS, an adapter protein with a PH and SH2 domain, is a substrate for the insulin receptor kinase. Biochem. J. 341:665-668. [PMC free article] [PubMed] [Google Scholar]
- 2.Backer, J. M., S. E. Shoelson, M. A. Weiss, Q. X. Hua, B. Cheatham, E. Haring, D. C. Cahill, and M. F. White. 1992. The insulin receptor juxtamembrane region contains two independent tyrosine/β-turn internalization signals. J. Cell Biol. 118:831-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bansal, A., and L. M. Gierasch. 1991. The NPXY internalization signal of the LDL receptor adopts a reverse-turn conformation. Cell 67:1195-1201. [DOI] [PubMed] [Google Scholar]
- 4.Briddon, S. J., S. K. Melford, M. Turner, V. Tybulewicz, and S. P. Watson. 1999. Collagen mediates changes in intracellular calcium in primary mouse megakaryocytes through Syk-dependent and -independent pathways. Blood 93:3847-3855. [PubMed] [Google Scholar]
- 5.Carroll, D. J., D. T. Albay, M. Terasaki, L. A. Jaffe, and K. R. Foltz. 1999. Identification of PLCγ-dependent and -independent events during fertilization of sea urchin eggs. Dev. Biol. 206:232-247. [DOI] [PubMed] [Google Scholar]
- 6.Carroll, D. J., C. S. Ramarao, L. M. Mehlmann, S. Roche, M. Terasaki, and L. A. Jaffe. 1997. Calcium release at fertilization in starfish eggs is mediated by phospholipase Cγ. J. Cell Biol. 138:1303-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, P., H. Xie, M. C. Sekar, K. Gupta, and A. Wells. 1994. Epidermal growth factor receptor-mediated cell motility: phospholipase C activity is required, but mitogen-activated protein kinase activity is not sufficient for induced cell movement. J. Cell Biol. 127:847-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng, Y., S. Bhattacharya, O. R. Swamy, R. Tandon, Y. Wang, R. Janda, and H. Riedel. 2003. Growth factor receptor-binding protein 10 (Grb10) as a partner of phosphatidylinositol 3-kinase in metabolic insulin action. J. Biol. Chem. 278:39311-39322. [DOI] [PubMed] [Google Scholar]
- 9.Dhe-Paganon, S., E. D. Werner, M. Nishi, L. Hansen, Y. I. Chi, and S. E. Shoelson. 2004. A phenylalanine zipper mediates APS dimerization. Nat. Struct. Mol. Biol. 11:968-974. [DOI] [PubMed] [Google Scholar]
- 10.Domchek, S. M., K. R. Auger, S. Chatterjee, T. R. Burke, and S. E. Shoelson. 1992. Inhibition of SH2 domain-phosphoprotein association by a nonhydrolyzable phosphonopeptide. Biochemistry 31:9865-9870. [DOI] [PubMed] [Google Scholar]
- 11.Duan, C., H. Yang, M. F. White, and L. Rui. 2004. Disruption of the SH2-B gene causes age-dependent insulin resistance and glucose intolerance. Mol. Cell. Biol. 24:7435-7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eberle, W., C. Sander, W. Klaus, B. Schmidt, K. von Figura, and C. Peters. 1991. The essential tyrosine of the internalization signal in lysosomal acid phosphatase is part of a β-turn. Cell 67:1203-1209. [DOI] [PubMed] [Google Scholar]
- 13.Eck, M. J., S. Dhe-Paganon, T. Trub, R. Nolte, and S. E. Shoelson. 1996. Structure of the IRS-1 PTB domain bound to the juxtamembrane region of the insulin receptor. Cell 85:695-705. [DOI] [PubMed] [Google Scholar]
- 14.Eck, M. J., S. Pluskey, T. Trub, S. C. Harrison, and S. E. Shoelson. 1996. Spatial constraints on the recognition of phosphoproteins by the tandem SH2 domains of the phosphatase SH-PTP2. Nature 379:277-280. [DOI] [PubMed] [Google Scholar]
- 15.Eck, M. J., S. E. Shoelson, and S. C. Harrison. 1993. Recognition of a high-affinity phosphotyrosyl peptide by the Src homology-2 domain of p56lck. Nature 362:87-91. [DOI] [PubMed] [Google Scholar]
- 16.Gernert, K. M., M. C. Surles, T. H. Labean, J. S. Richardson, and D. C. Richardson. 1995. The Alacoil: a very tight, antiparallel coiled-coil of helices. Protein Sci. 4:2252-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gotoh, N., K. Muroya, S. Hattori, S. Nakamura, K. Chida, and M. Shibuya. 1995. The SH2 domain of Shc suppresses EGF-induced mitogenesis in a dominant-negative manner. Oncogene 11:2525-2533. [PubMed] [Google Scholar]
- 18.Haruta, T., A. J. Morris, D. W. Rose, J. G. Nelson, M. Mueckler, and J. M. Olefsky. 1995. Insulin-stimulated GLUT4 translocation is mediated by a divergent intracellular signaling pathway. J. Biol. Chem. 270:27991-27994. [DOI] [PubMed] [Google Scholar]
- 19.Hill, R. B., and W. F. DeGrado. 1998. Solution structure of α2D, a native-like de novo designed protein. J. Am. Chem. Soc. 120:1138-1145. [Google Scholar]
- 20.Hill, R. B., D. P. Raleigh, A. Lombardi, and W. F. DeGrado. 2000. De novo design of helical bundles as models for understanding protein folding and function. Acc. Chem. Res. 33:745-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hof, P., S. Pluskey, S. Dhe-Paganon, M. J. Eck, and S. E. Shoelson. 1998. Crystal structure of the tyrosine phosphatase SHP-2. Cell 92:441-450. [DOI] [PubMed] [Google Scholar]
- 22.Hu, J., J. Liu, R. Ghirlando, A. R. Saltiel, and S. R. Hubbard. 2003. Structural basis for recruitment of the adaptor protein APS to the activated insulin receptor. Mol. Cell 12:1379-1389. [DOI] [PubMed] [Google Scholar]
- 23.Iseki, M., S. Takaki, and K. Takatsu. 2000. Molecular cloning of the mouse APS as a member of the Lnk family adaptor proteins. Biochem. Biophys. Res. Commun. 272:45-54. [DOI] [PubMed] [Google Scholar]
- 24.Jhun, B. H., D. W. Rose, B. L. Seely, L. Rameh, L. Cantley, A. R. Saltiel, and J. M. Olefsky. 1994. Microinjection of the SH2 domain of the 85-kilodalton subunit of phosphatidylinositol 3-kinase inhibits insulin-induced DNA synthesis and c-fos expression. Mol. Cell. Biol. 14:7466-7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kavanaugh, W. M., C. W. Turck, and L. T. Williams. 1995. PTB domain binding to signaling proteins through a sequence motif containing phosphotyrosine. Science 268:1177-1179. [DOI] [PubMed] [Google Scholar]
- 26.Klemm, J. D., S. L. Schreiber, and G. R. Crabtree. 1998. Dimerization as a regulatory mechanism in signal transduction. Annu. Rev. Immunol. 16:569-592. [DOI] [PubMed] [Google Scholar]
- 27.Kong, M., C. S. Wang, and D. J. Donoghue. 2002. Interaction of fibroblast growth factor receptor 3 and the adapter protein SH2-B: a role in STAT5 activation. J. Biol. Chem. 277:15962-15970. [DOI] [PubMed] [Google Scholar]
- 28.Kotani, K., P. Wilden, and T. S. Pillay. 1998. SH2-Bα is an insulin-receptor adapter protein and substrate that interacts with the activation loop of the insulin-receptor kinase. Biochem. J. 335:103-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurzer, J. H., L. S. Argetsinger, Y. J. Zhou, J. L. Kouadio, J. J. O'Shea, and C. Carter-Su. 2004. Tyrosine 813 is a site of JAK2 autophosphorylation critical for activation of JAK2 by SH2-Bβ. Mol. Cell. Biol. 24:4557-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lechleider, R. J., S. Sugimoto, A. M. Bennett, A. S. Kashishian, J. A. Cooper, S. E. Shoelson, C. T. Walsh, and B. G. Neel. 1993. Activation of the SH2-containing phosphotyrosine phosphatase SH-PTP2 by its binding site, phosphotyrosine 1009, on the human platelet-derived growth factor receptor B. J. Biol. Chem. 268:21478-21481. [PubMed] [Google Scholar]
- 31.Levy, D. E., and J. E. Darnell, Jr. 2002. Stats: transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 3:651-662. [DOI] [PubMed] [Google Scholar]
- 32.Liu, J., A. Kimura, C. A. Baumann, and A. R. Saltiel. 2002. APS facilitates c-Cbl tyrosine phosphorylation and GLUT4 translocation in response to insulin in 3T3-L1 adipocytes. Mol. Cell. Biol. 22:3599-3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehlmann, L. M., G. Carpenter, S. G. Rhee, and L. A. Jaffe. 1998. SH2 domain-mediated activation of phospholipase Cγ is not required to initiate Ca2+ release at fertilization of mouse eggs. Dev. Biol. 203:221-232. [DOI] [PubMed] [Google Scholar]
- 34.Melford, S. K., M. Turner, S. J. Briddon, V. L. Tybulewicz, and S. P. Watson. 1997. Syk and Fyn are required by mouse megakaryocytes for the rise in intracellular calcium induced by a collagen-related peptide. J. Biol. Chem. 272:27539-27542. [DOI] [PubMed] [Google Scholar]
- 35.Milarski, K. L., and A. R. Saltiel. 1994. Expression of catalytically inactive Syp phosphatase in 3T3 cells blocks stimulation of mitogen-activated protein kinase by insulin. J. Biol. Chem. 269:21239-21243. [PubMed] [Google Scholar]
- 36.Minami, A., M. Iseki, K. Kishi, M. Wang, M. Ogura, N. Furukawa, S. Hayashi, M. Yamada, T. Obata, Y. Takeshita, Y. Nakaya, Y. Bando, K. Izumi, S. A. Moodie, F. Kajiura, M. Matsumoto, K. Takatsu, S. Takaki, and Y. Ebina. 2003. Increased insulin sensitivity and hypoinsulinemia in APS knockout mice. Diabetes 52:2657-2665. [DOI] [PubMed] [Google Scholar]
- 37.Moodie, S. A., J. Alleman-Sposeto, and T. A. Gustafson. 1999. Identification of the APS protein as a novel insulin receptor substrate. J. Biol. Chem. 274:11186-11193. [DOI] [PubMed] [Google Scholar]
- 38.Nelms, K., T. J. O'Neill, S. Li, S. R. Hubbard, T. A. Gustafson, and W. E. Paul. 1999. Alternative splicing, gene localization, and binding of SH2-B to the insulin receptor kinase domain. Mamm. Genome 10:1160-1167. [DOI] [PubMed] [Google Scholar]
- 39.Noguchi, T., T. Matozaki, K. Horita, Y. Fujioka, and M. Kasuga. 1994. Role of SH-PTP2, a protein-tyrosine phosphatase with Src homology 2 domains, in insulin-stimulated Ras activation. Mol. Cell. Biol. 14:6674-6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Northrop, J. P., M. J. Pustelnik, A. T. Lu, and J. R. Grove. 1996. Characterization of the roles of SH2 domain-containing proteins in T-lymphocyte activation by using dominant negative SH2 domains. Mol. Cell. Biol. 16:2255-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Brien, K. B., L. S. Argetsinger, M. Diakonova, and C. Carter-Su. 2003. YXXL motifs in SH2-Bβ are phosphorylated by JAK2, JAK1, and platelet-derived growth factor receptor and are required for membrane ruffling. J. Biol. Chem. 278:11970-11978. [DOI] [PubMed] [Google Scholar]
- 43.O'Neill, T. J., D. W. Rose, T. S. Pillay, K. Hotta, J. M. Olefsky, and T. A. Gustafson. 1996. Interaction of a GRB-IR splice variant (a human GRB10 homolog) with the insulin and insulin-like growth factor I receptors. Evidence for a role in mitogenic signaling. J. Biol. Chem. 271:22506-22513. [DOI] [PubMed] [Google Scholar]
- 44.O'Shea, E. K., R. Rutkowski, and P. S. Kim. 1989. Evidence that the leucine zipper is a coiled coil. Science 243:538-542. [DOI] [PubMed] [Google Scholar]
- 45.Ohtsuka, S., S. Takaki, M. Iseki, K. Miyoshi, N. Nakagata, Y. Kataoka, N. Yoshida, K. Takatsu, and A. Yoshimura. 2002. SH2-B is required for both male and female reproduction. Mol. Cell Biol. 22:3066-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Osborne, M. A., S. Dalton, and J. P. Kochan. 1995. The yeast tribrid system-genetic detection of trans-phosphorylated ITAM-SH2-interactions. Biotechnology 13:1474-1478. [DOI] [PubMed] [Google Scholar]
- 47.Ota, Y., L. O. Beitz, A. M. Scharenberg, J. A. Donovan, J. P. Kinet, and L. E. Samelson. 1996. Characterization of Cbl tyrosine phosphorylation and a Cbl-Syk complex in RBL-2H3 cells. J. Exp. Med. 184:1713-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pawson, T. 2004. Specificity in signal transduction: from phosphotyrosine-SH2 domain interactions to complex cellular systems. Cell 116:191-203. [DOI] [PubMed] [Google Scholar]
- 49.Piccione, E., R. D. Case, S. M. Domchek, P. Hu, M. Chaudhuri, J. M. Backer, J. Schlessinger, and S. E. Shoelson. 1993. PI 3-kinase p85 SH2 domains specificity defined by direct phosphopeptide/SH2 domain binding. Biochemistry 32:3197-3202. [DOI] [PubMed] [Google Scholar]
- 50.Pluskey, S., T. J. Wandless, C. T. Walsh, and S. E. Shoelson. 1995. Potent stimulation of SH-PTP2 phosphatase activity by simultaneous occupancy of both SH2 domains. J. Biol. Chem. 270:2897-2900. [DOI] [PubMed] [Google Scholar]
- 51.Qian, X., and D. D. Ginty. 2001. SH2-B and APS are multimeric adapters that augment TrkA signaling. Mol. Cell. Biol. 21:1613-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qian, X., A. Riccio, Y. Zhang, and D. D. Ginty. 1998. Identification and characterization of novel substrates of Trk receptors in developing neurons. Neuron 21:1017-1029. [DOI] [PubMed] [Google Scholar]
- 53.Riedel, H., N. Yousaf, Y. Zhao, H. Dai, Y. Deng, and J. Wang. 2000. PSM, a mediator of PDGF-BB-, IGF-I-, and insulin-stimulated mitogenesis. Oncogene 19:39-50. [DOI] [PubMed] [Google Scholar]
- 54.Rivard, N., F. R. McKenzie, J. M. Brondello, and J. Pouyssegur. 1995. The phosphotyrosine phosphatase PTP1D, but not PTP1C, is an essential mediator of fibroblast proliferation induced by tyrosine kinase and G protein-coupled receptors. J. Biol. Chem. 270:11017-11024. [DOI] [PubMed] [Google Scholar]
- 55.Roche, S., J. McGlade, M. Jones, G. D. Gish, T. Pawson, and S. A. Courtneidge. 1996. Requirement of phospholipase C gamma, the tyrosine phosphatase Syp and the adaptor proteins Shc and Nck for PDGF-induced DNA synthesis: evidence for the existence of Ras-dependent and Ras-independent pathways. EMBO J. 15:4940-4948. [PMC free article] [PubMed] [Google Scholar]
- 56.Rui, L., and C. Carter-Su. 1998. Platelet-derived growth factor (PDGF) stimulates the association of SH2-Bβ with PDGF receptor and phosphorylation of SH2-Bβ. J. Biol. Chem. 273:21239-21245. [DOI] [PubMed] [Google Scholar]
- 57.Rui, L., and C. Carter-Su. 1999. Identification of SH2-Bβ as a potent cytoplasmic activator of the tyrosine kinase Janus kinase 2. Proc. Natl. Acad. Sci. USA 96:7172-7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rui, L., D. R. Gunter, J. Herrington, and C. Carter-Su. 2000. Differential binding to and regulation of JAK2 by the SH2 domain and N-terminal region of SH2-Bβ. Mol. Cell. Biol. 20:3168-3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rui, L., J. Herrington, and C. Carter-Su. 1999. SH2-B is required for nerve growth factor-induced neuronal differentiation. J. Biol. Chem. 274:10590-10594. [DOI] [PubMed] [Google Scholar]
- 60.Rui, L., J. Herrington, and C. Carter-Su. 1999. SH2-B, a membrane-associated adapter, is phosphorylated on multiple serines/threonines in response to nerve growth factor by kinases within the MEK/ERK cascade. J. Biol. Chem. 274:26485-26492. [DOI] [PubMed] [Google Scholar]
- 61.Rui, L., L. S. Mathews, K. Hotta, T. A. Gustafson, and C. Carter-Su. 1997. Identification of SH2-Bβ as a substrate of the tyrosine kinase JAK2 involved in growth hormone signaling. Mol. Cell Biol. 17:6633-6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sharma, P. M., K. Egawa, Y. Huang, J. L. Martin, I. Huvar, G. R. Boss, and J. M. Olefsky. 1998. Inhibition of phosphatidylinositol 3-kinase activity by adenovirus-mediated gene transfer and its effect on insulin action. J. Biol. Chem. 273:18528-18537. [DOI] [PubMed] [Google Scholar]
- 63.Shearer, J., C. De Nadai, F. Emily Fenouil, C. Gache, M. Whitaker, and B. Ciapa. 1999. Role of phospholipase Cγ at fertilization and during mitosis in sea urchin eggs and embryos. Development 126:2273-2284. [DOI] [PubMed] [Google Scholar]
- 64.Songyang, Z., S. E. Shoelson, M. Chaudhuri, G. Gish, T. Pawson, F. King, T. Roberts, S. Ratnofsky, R. J. Lechleider, B. G. Neel, R. B. Birge, J. E. Fajardo, M. M. Chou, H. Hanafusa, B. Schaffhausen, and L. C. Cantley. 1993. SH2 domains recognize specific phosphopeptide sequences. Cell 72:767-778. [DOI] [PubMed] [Google Scholar]
- 65.Songyang, Z., S. E. Shoelson, J. McGlade, J. P. Olivier, T. Pawson, X. R. Bustelo, M. Barbacid, H. Sabe, H. Hanafusa, T. Yi, R. Ren, D. Baltimore, S. Ratnofsky, R. A. Feldman, and L. C. Cantley. 1994. Specific motifs recognized by the SH2 domains of Csk 3BP2, fps/fes, Grb-2, HCP, SHC, Syk, and Vav. Mol. Cell. Biol. 14:2777-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sugimoto, S., T. Wandless, S. E. Shoelson, B. G. Neel, and C. T. Walsh. 1994. Activation of the SH2-containing protein tyrosine phosphatase, SH-PTP2, by phosphotyrosine-containing peptides derived from IRS-1. J. Biol. Chem. 269:13614-13622. [PubMed] [Google Scholar]
- 67.Summers, S. A., E. L. Whiteman, H. Cho, L. Lipfert, and M. J. Birnbaum. 1999. Differentiation-dependent suppression of platelet-derived growth factor signaling in cultured adipocytes. J. Biol. Chem. 274:23858-23867. [DOI] [PubMed] [Google Scholar]
- 68.Taylor, J. A., J. L. Karas, M. K. Ram, O. M. Green, and C. Seidel-Dugan. 1995. Activation of the high-affinity immunoglobulin E receptor Fc epsilon RI in RBL-2H3 cells is inhibited by Syk SH2 domains. Mol. Cell. Biol. 15:4149-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trub, T., W. E. Choi, G. Wolf, E. Ottinger, Y. Chen, M. A. Weiss, and S. E. Shoelson. 1995. Specificity of the PTB domain of Shc for β turn-forming pentapeptide motifs amino-terminal to phosphotyrosine. J. Biol. Chem. 270:18205-18208. [DOI] [PubMed] [Google Scholar]
- 70.Velazquez, L., A. M. Cheng, H. E. Fleming, C. Furlonger, S. Vesely, A. Bernstein, C. J. Paige, and T. Pawson. 2002. Cytokine signaling and hematopoietic homeostasis are disrupted in Lnk-deficient mice. J. Exp. Med. 195:1599-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wakioka, T., A. Sasaki, K. Mitsui, M. Yokouchi, A. Inoue, S. Komiya, and A. Yoshimura. 1999. APS, an adaptor protein containing Pleckstrin homology (PH) and Src homology-2 (SH2) domains inhibits the JAK-STAT pathway in collaboration with c-Cbl. Leukemia 13:760-767. [DOI] [PubMed] [Google Scholar]
- 72.Waksman, G., and J. Kuriyan. 2004. Structure and specificity of the SH2 domain. Cell 116:S45-S48. [DOI] [PubMed] [Google Scholar]
- 73.Waksman, G., S. E. Shoelson, N. Pant, D. Cowburn, and J. Kuriyan. 1993. Binding of a high-affinity phosphotyrosyl peptide to the Src SH2 domain: crystal structures of the complexed and peptide-free forms. Cell 72:779-790. [DOI] [PubMed] [Google Scholar]
- 74.Wang, J., H. Dai, N. Yousaf, M. Moussaif, Y. Deng, A. Boufelliga, O. R. Swamy, M. E. Leone, and H. Riedel. 1999. Grb10, a positive, stimulatory signaling adapter in platelet-derived growth factor BB-, insulin-like growth factor I-, and insulin-mediated mitogenesis. Mol. Cell. Biol. 19:6217-6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang, J., and H. Riedel. 1998. Insulin-like growth factor-I receptor and insulin receptor association with a Src homology-2 domain-containing putative adapter. J. Biol. Chem. 273:3136-3139. [DOI] [PubMed] [Google Scholar]
- 76.Wang, Z., S. Gluck, L. Zhang, and M. F. Moran. 1998. Requirement for phospholipase C-gamma1 enzymatic activity in growth factor-induced mitogenesis. Mol. Cell. Biol. 18:590-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang, Z., and M. F. Moran. 1996. Requirement for the adaptor protein GRB2 in EGF receptor endocytosis. Science 272:1935-1939. [DOI] [PubMed] [Google Scholar]
- 78.Weiss, A., and J. Schlessinger. 1998. Switching signals on or off by receptor dimerization. Cell 94:277-280. [DOI] [PubMed] [Google Scholar]
- 79.Wolf, G., T. Trub, E. Ottinger, L. Groninga, A. Lynch, M. White, M. Miyazaki, J. Lee, and S. E. Shoelson. 1995. The PTB domains of IRS-1 and Shc have distinct but overlapping binding specificities. J. Biol. Chem. 270:27407-27410. [DOI] [PubMed] [Google Scholar]
- 80.Xiao, S., D. W. Rose, T. Sasaoka, H. Maegawa, T. R. Burke, Jr., P. P. Roller, S. E. Shoelson, and J. M. Olefsky. 1994. Syp (SH-PTP2) is a positive mediator of growth factor-stimulated mitogenic signal transduction. J. Biol. Chem. 269:21244-21248. [PubMed] [Google Scholar]
- 81.Yamauchi, K., K. L. Milarski, A. R. Saltiel, and J. E. Pessin. 1995. Protein-tyrosine-phosphatase SHPTP2 is a required positive effector for insulin downstream signaling. Proc. Natl. Acad. Sci. USA 92:664-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yokouchi, M., T. Wakioka, H. Sakamoto, H. Yasukawa, S. Ohtsuka, A. Sasaki, M. Ohtsubo, M. Valius, A. Inoue, S. Komiya, and A. Yoshimura. 1999. APS, an adaptor protein containing PH and SH2 domains, is associated with the PDGF receptor and c-Cbl and inhibits PDGF-induced mitogenesis. Oncogene 18:759-767. [DOI] [PubMed] [Google Scholar]
- 83.Yousaf, N., Y. Deng, Y. Kang, and H. Riedel. 2001. Four PSM/SH2-B alternative splice variants and their differential roles in mitogenesis. J. Biol. Chem. 276:40940-40948. [DOI] [PubMed] [Google Scholar]
- 84.Zhou, M.-M., K. S. Ravichandran, E. T. Olejniczak, A. M. Petros, R. P. Meadows, J. E. Harlan, W. S. Wade, S. J. Burakoff, and S. W. Fesik. 1995. Structure and ligand recognition of the phosphotyrosine binding domain of Shc. Nature 378:584-592. [DOI] [PubMed] [Google Scholar]