Key Points
Question
How do bone mineral density (BMD) and BMD z scores develop in transgender adults after a minimum of 9 years of gender-affirming hormone treatment with prior use of a gonadotropin-releasing hormone (GnRH) agonist in adolescence?
Findings
In this cohort study of 75 individuals diagnosed with gender dysphoria, among those assigned male at birth, BMD z scores were not different at long-term follow-up compared with the start of GnRH agonist treatment at the total hip and femoral neck, in contrast to the lumbar spine. In individuals assigned female at birth, BMD z scores caught up with pretreatment levels at the lumbar spine, total hip, and femoral neck.
Meaning
These findings suggest that treatment with a GnRH agonist followed by long-term gender-affirming hormones is safe regarding bone health in transgender persons receiving testosterone, but bone health in transgender persons receiving estrogen requires extra attention and further study.
Abstract
Importance
Bone mineral density (BMD) z scores in transgender adolescents decrease during puberty suppression with a gonadotropin-releasing hormone (GnRH) agonist. Previous research found that after short-term use of gender-affirming hormones (GAH), pretreatment z scores were not restored. Long-term follow-up studies are lacking.
Objective
To assess BMD after long-term GAH treatment in transgender adults who used puberty suppression in adolescence.
Design, Setting, and Participants
This single-center cohort study with follow-up duration of 15 years selected participants from a database containing all people visiting a gender identity clinic at an academic hospital in the Netherlands between 1972 and December 31, 2018. Recruitment occurred from March 1, 2020, to August 31, 2021. A total of 75 participants diagnosed with gender dysphoria who had used puberty suppression before age 18 years prior to receiving at least 9 years of long-term GAH were included.
Exposures
Puberty suppression with a GnRH agonist followed by GAH treatment.
Main Outcomes and Measures
Lumbar spine, total hip, and femoral neck BMD and z scores before the start of puberty suppression, at start of GAH, and at short- and long-term follow-up.
Results
Among 75 participants, 25 were assigned male at birth, and 50 were assigned female at birth. At long-term follow-up, the median (IQR) age was 28.2 (27.0-30.8) years in participants assigned male at birth and 28.2 (26.6-30.6) years in participants assigned female at birth. The median (IQR) duration of GAH treatment was 11.6 (10.1-14.7) years among those assigned male at birth and 11.9 (10.2-13.8) years among those assigned female at birth. The z scores decreased during puberty suppression. In individuals assigned male at birth, the mean (SD) z score after long-term GAH use was −1.34 (1.16; change from start of GnRH agonist: −0.87; 95% CI, −1.15 to −0.59) at the lumbar spine, −0.66 (0.75; change from start of GnRH agonist: −0.12; 95% CI, −0.31 to 0.07) at the total hip, and −0.54 (0.84; change from start of GnRH agonist: 0.01; 95% CI, −0.20 to 0.22) at the femoral neck. In individuals assigned female at birth, after long-term GAH use, the mean (SD) z score was 0.20 (1.05; change from start of GnRH agonist: 0.09; 95% CI, −0.09 to 0.27) at the lumbar spine, 0.07 (0.91; change from start of GnRH agonist: 0.10; 95% CI, −0.06 to 0.26) at the total hip, and −0.19 (0.94; change from start of GnRH agonist: −0.20; 95% CI, −0.26 to 0.06) at the femoral neck.
Conclusions and Relevance
In this cohort study, after long-term use of GAH, z scores in individuals treated with puberty suppression caught up with pretreatment levels, except for the lumbar spine in participants assigned male at birth, which might have been due to low estradiol concentrations. These findings suggest that treatment with GnRH agonists followed by long-term GAH is safe with regard to bone health in transgender persons receiving testosterone, but bone health in transgender persons receiving estrogen requires extra attention and further study. Estrogen treatment should be optimized and lifestyle counseling provided to maximize bone development in individuals assigned male at birth.
This cohort study assesses bone mineral density after long-term use of gender-affirming hormones among transgender adults who used puberty suppression in adolescence.
Introduction
Bone mass acquisition occurs during childhood and adolescence and reaches a peak at the end of the second decade of life. Inadequate acquisition of peak bone mass (PBM) may result in a greater risk of fractures and osteoporosis in adulthood.1 Peak bone mass and maintenance of bone mineral density (BMD) are influenced by a number of factors, of which sex steroids are a key factor.2 For example, treatment with a gonadotropin-releasing hormone (GnRH) agonist to suppress estrogen production in adults with endometriosis was found to reduce BMD.3 In children, GnRH agonists have mainly been used to treat central precocious puberty. Previous research4 in this field revealed a decrease in bone mineral accrual during treatment. However, after treatment cessation, accrual resumed and BMD was similar to peers by late adolescence.4
A relatively new field wherein GnRH agonists are used is the treatment of transgender adolescents. Transgender people perceive great distress due to a mismatch between their gender identity and sex assigned at birth, known as gender dysphoria. For some, the development of secondary sex characteristics during puberty imposes a substantial strain. Around the year 2000, treatment with GnRH agonists was introduced as a means of puberty suspension to lighten the burden of undesired physical changes while extending the time for exploration of treatment wishes with subsequent gender-affirming hormones (GAH). A decrease in BMD z scores (ie, a decrease compared with the mean BMD of age-, sex-, and ethnicity-matched persons) was seen during GnRH agonist treatment in transgender adolescents.5,6,7,8,9,10 However, z scores at age 22 years did not return to pretreatment scores after addition of GAH,11 which could indicate a permanent loss of bone mineral accrual potential due to postponement of puberty or might be a reflection of delayed achievement of PBM. Evidence on long-term bone health in transgender adolescents treated with a GnRH agonist and subsequent GAH is limited. Due to the possibly increased risk of osteoporosis and associated increased fracture risk in the case of lower PBM, it is important to gain more long-term insight into the bone health of transgender adolescents who were treated with a GnRH agonist. In this cohort study, we therefore investigated BMD development and BMD z scores in transgender adults after long-term GAH with prior use of a GnRH agonist in adolescence. Additionally, we investigated whether estradiol, testosterone, or vitamin D concentrations; BMI; and duration of mono GnRH agonist treatment were associated with z scores at follow-up.
Methods
This study was submitted for review of ethical and legal aspects to the local medical ethics committee of Amsterdam University Medical Center (UMC), Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. The committee confirmed that the Medical Research Involving Human Subjects Act did not apply to our study owing to a lack of interventions.12 Hence, an official approval of our study was not required. All participants provided written informed consent. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Study Design and Population
This study was conducted at the same center as the study by Klink et al11 and contained some overlapping participants. However, the current study population was larger and had a markedly extended follow-up time. In this prospective follow-up cohort study, transgender persons were invited to participate if they had started medical transition with a GnRH agonist before the age of 18 years and had subsequently used GAH for at least 9 years. Individuals were selected from the Amsterdam Cohort of Gender Dysphoria,13 a retrospectively built database containing all people visiting the gender identity clinic of Amsterdam UMC from 1972 until December 31, 2018. People with disorders of sex development or precocious puberty were not included. Eligible participants were approached by mail, email, and/or telephone. Recruitment took place between March 31, 2020, and August 31, 2021.
During the study visit at the outpatient clinic of the Amsterdam UMC, clinical data (including ethnicity, age, Tanner stage, body mass index [BMI; calculated as weight in kilograms divided by height in meters squared] at the start of treatment, and whether gonadectomy had been performed), blood samples, and a dual-energy x-ray absorptiometry (DXA) scan were obtained. Ethnicity data were relevant to the study outcome because BMD z scores are ethnicity dependent.
To assess change over time, retrospective DXA scans were collected from the medical records. At our center, the medical protocol for transgender adolescents includes prescribed DXA as part of standard care at the start of GnRH agonist and GAH treatment and at ages 22 and 25 years.
The main outcomes were areal BMD and BMD z scores for 3 regions of interest at 4 time points. Additionally, we analyzed BMD T scores at follow-up (ie, compared with the mean BMD of young sex- and ethnicity-matched persons). T scores were not calculated at the start of GnRH agonist treatment because participants had not reached PBM yet.
Medical Treatment Protocol
If diagnosed with gender dysphoria (based on criteria from the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, Text Revision)14 and the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition),15 adolescents could start puberty suppression consisting of subcutaneous or intramuscular triptorelin (a GnRH agonist) 3.75 mg, every 4 weeks or 11.25 mg every 10 to 12 weeks. To start treatment, Tanner genital stage 2 to 3 was required for people assigned male at birth, and Tanner breast stage 2 was required for people assigned female at birth.
Provided that gender dysphoria persisted, puberty consistent with the gender identity was induced with GAH in incremental dosages when adolescents turned age 16 years. Over time, the protocol was adapted, enabling those aged 15 years to start GAH under certain conditions. Individuals assigned male at birth were usually given 5 μg/kg body weight oral 17β-estradiol, which was gradually increased to a daily maintenance dose of 2 to 4 mg. For those assigned female at birth, an intramuscular testosterone ester mixture starting at a dose of 25 mg/m2 body surface area every 2 weeks was used, building up to a maintenance dose of 250 mg every 3 to 4 weeks.
After at least 1 year of GAH and a minimum age of 18 years, persons could become eligible for gonadectomy. Treatment with GnRH agonists was discontinued if gonadectomy was performed or, in those assigned female at birth, when the full maintenance dose of testosterone was reached. Treatment with GAH is usually continued throughout life.
Bone Densitometry
Bone density was assessed by a densitometric system (Hologic Delphi; Hologic, Inc), which was updated in 2004. In February 2011, the appliance was replaced by a different system (Hologic Discovery; Hologic, Inc). The software on this device was updated in 2012 and 2015. A new device (Hologic Horizon; Hologic, Inc) was installed in December 2020. Cross-calibration with an imaging phantom allowed for direct comparison of BMD after updates and between all devices.
The DXA scans were performed between 2001 and 2018. Anatomical regions of interest were the lumbar spine, total hip, and femoral neck of the nondominant hip. All T scores and z scores were calculated at the time of study analyses. Although BMD z scores were calculated for both the sex assigned at birth and the experienced gender, the sex assigned at birth was used as reference in the analyses assessing z scores over time to allow comparisons with baseline values. T scores were calculated using the sex assigned at birth as well as the experienced gender. Bone mineral density values from the National Health and Nutrition Examination Survey were used as reference.16 In children and adolescents, osteoporosis is defined as a z score of −2 or lower in combination with a clinically meaningful fracture history. In adults, osteoporosis is diagnosed if the T score is −2.5 or lower.17
Biochemical Assays
Until January 2010, estradiol was measured using a radioimmunoassay (RIA; DiaSorin; lower limit of quantitation [LLOQ]: 4.90 pg/mL [to convert to picomoles per liter, multiply by 3.671]; interassay coefficient of variation [CV]: 10%). Between January 2010 and July 2014 a competitive immunoassay (Delfia; PerkinElmer Wallac Oy; LLOQ: 5.45 pg/mL; interassay CV: 10%-13%) was used for estradiol measurements. A conversion formula was applied to compare concentrations (Delfia concentration = 1.267 × [Diasorin concentration] – 28.870). After July 2014, this assay was replaced by liquid chromatography tandem mass spectrometry (LC-MS/MS; Amsterdam UMC; LLOQ: 5.45 pg/mL; interassay CV: <7%). Another formula (LC-MS/MS concentration = 1.60 × [Delfia concentration] – 29.00) was applied for converting measurements.
For testosterone, an RIA (Coat-A-Count; Siemens Medical Solutions; LLOQ: 28.82 ng/dL [to convert to nanomoles per liter, multiply by 0.0347]; interassay CV: 7%-20%) was used until January 2013. Thereafter, a competitive immunoassay (Architect; Abbott; LLOQ: 2.88 ng/dL; interassay CV: 6%-10%) was used. The formula applied to convert measurements depended on the testosterone concentration (testosterone <8 nmol/L: Architect concentration = 1.1 × [RIA concentration] + 0.2; testosterone >8 nmol/L: Architect concentration = 1.34 × [RIA concentration] – 1.65).
Between 2012 and 2015, 25-hydroxyvitamin D was measured using an LC-MS/MS method (LLOQ: 1.6 ng/mL [to convert to nanomoles per liter, multiply by 2.496]; interassay CV: 8%).18 The method was adjusted in 2015 without necessitating conversion of yielded concentrations.19 Luteinizing hormone (LH) was measured using an immunometric assay (Architect; Abbott; LLOQ: 2.0 mIU/mL [to convert to international units per liter, multiply by 1.0]; interassay CV: <6%).
Statistical Analysis
Continuous variables were reported as means with SDs for normally distributed data and as medians with IQRs for nonnormally distributed data. Dichotomous variables were reported as percentages.
Analyses were done separately for those assigned male at birth and those assigned female at birth. Linear mixed models were used to analyze areal BMD (in grams per centimeter squared) and BMD z scores. Repeated measures were nested within participants. A random intercept was included in the model. We did not add a random slope or adjustment covariates. In the first model, time was made into a categorical single-year variable between the start of GnRH agonist treatment and the start of GAH treatment and between the start of GAH treatment and follow-up. Scans made 6 years or more after the start of GAH were taken together in 2-year intervals to ensure appropriate group size. For most participants, DXA scans were made on the same days as the start of GnRH agonist treatment and the start of GAH treatment. Otherwise, a DXA scan made within a 3-month range before or after start of treatments was used for these time points. If people had used a GnRH agonist for more than 2 years before GAH initiation, the range was stretched to 6 months before and 3 months after the start of GAH.
A different linear mixed model was used to evaluate BMD z score development at 4 distinct time points. In this model, time was divided into 4 categories: start of GnRH agonist, start of GAH, short-term follow-up around age 22 years, and long-term follow-up. The criteria described in the previous paragraph were followed regarding which DXA scans were used at the start of treatments. For the DXA scan at short-term follow-up, scans made between ages 21 and 24 years were used. The long-term follow-up DXA scan was the scan prospectively made at the study visit after at least 9 years of GAH. A linear regression model was used to estimate the association of sex steroids, LH, and 25-hydroxyvitamin D concentrations; BMI; and duration of mono GnRH agonist treatment (in years) with long-term follow-up BMD z scores. All independent variables were analyzed separately. People who had not undergone gonadectomy and were still using a GnRH agonist were left out of the analysis regarding LH.
Stata software, version 15.1 (StataCorp LLC), was used for data analyses. Statistical significance was defined as a 95% CI excluding 0.
Results
Overall
In total, 143 individuals were eligible to participate. Of those, 30 individuals (21%) did not provide informed consent, 27 (19%) could not be reached, 6 (4%) discontinued GAH, 2 (1%) lived abroad, 2 (1%) had psychological reasons for nonparticipation, and 1 (1%) had insufficient data. The remaining 75 participants (52%; 25 assigned male at birth and 50 assigned female at birth) were included. The inclusion process and reasons for not participating are shown in the flowchart (eFigure in Supplement 1). Characteristics of the participants are provided in Table 1. The median (IQR) age at long-term follow-up was 28.2 (27.0-30.8) years in participants assigned male at birth and 28.2 (26.6-30.6) years in participants assigned female at birth. The median (IQR) duration of GAH treatment was 11.6 (10.1-14.7) years among those assigned female at birth and 11.9 (10.2-13.8) years among those assigned female at birth. Data on BMI, hormone concentrations, BMD T scores, and BMD z scores at long-term follow-up are shown in Table 2. Characteristics of persons not included are provided in eTable 1 in Supplement 1. People not included were similar to those included, except for age at the start of GAH in individuals assigned female at birth.
Table 1. Characteristics of Study Participants.
| Characteristic | Assigned male at birth (n = 25) | Assigned female at birth (n = 50) |
|---|---|---|
| At start of GnRH agonist treatment | ||
| Age, median (IQR), y | 14.5 (13.4-15.7) | 14.9 (13.0-16.4) |
| BMI, median (IQR) | 19.3 (18.0-21.9) | 20.0 (17.9-23.5) |
| European ethnicity, No. (%) | 19 (76) | 43 (86) |
| Tanner stage, No. (%) | ||
| B2-B3 | NA | 9 (18) |
| B4-B5 | NA | 40 (80) |
| G2-G3 | 5 (20) | NA |
| G4-G5 | 20 (80) | NA |
| Testicular volume, median (IQR), mL | 20 (13-20) | NA |
| Experienced menarche, No. (%) | NA | 38 (76) |
| LH, median (IQR), mIU/mL | 2.0 (1.7-3.5) | 2.9 (1.5-5.5) |
| Estradiol, median (IQR), pg/mL | 15.53 (6.27-22.61) | 38.95 (10.90-90.98) |
| Testosterone, median (IQR), ng/dL | 461.10 (317.00-576.37) | 37.46 (37.46-43.23) |
| Lumbar spine z score, mean (SD) | ||
| Male reference | −0.37 (1.07) | 0.83 (1.16) |
| Female reference | −1.07 (1.07) | 0.11 (0.91) |
| Lumbar spine z score <−2.0, No./total No. (%) | ||
| Male reference | 2/22 (9) | 0/47 |
| Female reference | 4/22 (18) | 1/47 (2) |
| Total hip z score, mean (SD) | ||
| Male reference | −0.53 (1.00) | −0.34 (0.90) |
| Female reference | −0.23 (1.04) | −0.05 (0.86) |
| Total hip z score <−2.0, No./total No. (%) | ||
| Male reference | 1/21 (5) | 1/47 (2) |
| Female reference | 1/21 (5) | 0/47 |
| Femoral neck z score, mean (SD) | ||
| Male reference | −0.58 (0.99) | −0.43 (0.88) |
| Female reference | −0.28 (1.01) | −0.11 (0.87) |
| Femoral neck z score <−2.0, No./total No. (%) | ||
| Male reference | 2/21 (10) | 0/47 |
| Female reference | 1/21 (5) | 0/47 |
| Age at start of GAH treatment, median (IQR), y | 16.0 (16.0-16.8) | 16.1 (16.0-17.6) |
| Age at short-term follow-up, median (IQR), y | 22.1 (21.7-22.6) | 22.1 (21.9-22.5) |
| Age at long-term follow-up, median (IQR), y | 28.2 (27.0-30.8) | 28.2 (26.6-30.6) |
| Duration of mono GnRH agonist treatment, median (IQR), y | 1.5 (0.7-2.6) | 1.5 (0.7-3.1) |
| Duration of GAH treatment, median (IQR), y | ||
| At short-term follow-up | 5.3 (4.3-6.3) | 5.7 (4.7-6.1) |
| At long-term follow-up | 11.6 (10.1-14.7) | 11.9 (10.2-13.8) |
| Gonadectomy, No. (%) | 24 (96) | 50 (100) |
| Age at gonadectomy, median (IQR), y | 19.1 (18.8-21.0) | 18.8 (18.3-19.5) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); GAH, gender-affirming hormones; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone; NA, not applicable.
SI conversion factors: To convert estradiol from picograms per milliliter to picomoles per liter, multiply by 3.671; to convert LH from milli-international units per milliliter to international units per liter, multiply by 1.0; to convert testosterone from nanograms per deciliter to nanomoles per liter, multiply by 0.0347.
Table 2. Body Mass Index, Biochemical Values, Bone Mineral Density T and z Scores, and Percentage With Osteoporosis at 3 Regions of Interest After Long-Term Use of Gender-Affirming Hormones.
| Variable | Assigned male at birth (n = 25) | Assigned female at birth (n = 50) |
|---|---|---|
| BMI, median (IQR) | 24.7 (21.1-28.7) | 24.3 (22.4-26.3) |
| LH, median (IQR), mIU/mL | 16.0 (12.5-20.5) | 3.7 (0.1-18.0) |
| Estradiol, median (IQR), pg/mL | 58.57 (34.32-89.08) | 20.43 (16.89-37.32) |
| Testosterone, median (IQR), ng/dL | 20.17 (14.41-25.94) | 533.14 (403.46-778.10) |
| 25-Hydroxyvitamin D, median (IQR), ng/mL | 25.64 (17.23-32.85) | 22.04 (15.22-26.84) |
| Lumbar spine | ||
| T score, mean (SD) | ||
| Male reference | −1.34 (1.16) | −0.24 (1.06) |
| Female reference | −0.94 (1.16) | 0.16 (1.06) |
| z Score, mean (SD) | ||
| Male reference | −1.34 (1.16) | −0.23 (1.05) |
| Female reference | −0.90 (1.15) | 0.20 (1.05) |
| T score <−2.5, No./total No. (%) | ||
| Male reference | 4/25 (16) | 0 |
| Female reference | 4/25 (16) | 0 |
| z Score <−2.0, No./total No. (%) | ||
| Male reference | 9/25 (36) | 2 |
| Female reference | 4/25 (16) | 0 |
| Total hip | ||
| T score, mean (SD) | ||
| Male reference | −0.70 (0.75) | −0.56 (0.74) |
| Female reference | −0.12 (0.92) | 0.06 (0.91) |
| z Score, mean (SD) | ||
| Male reference | −0.66 (0.75) | −0.51 (0.74) |
| Female reference | −0.10 (0.92) | 0.07 (0.91) |
| T score <−2.5, No./total No. (%) | ||
| Male reference | 0/23 | 0/50 |
| Female reference | 0/23 | 0/50 |
| z Score <−2.0, %, No./total No. (%) | ||
| Male reference | 0/23 | 2/50 (4) |
| Female reference | 0/23 | 1/50 (2) |
| Femoral neck | ||
| T score, mean (SD) | ||
| Male reference | −0.67 (0.85) | −0.80 (0.77) |
| Female reference | −0.09 (1.04) | −0.25 (0.94) |
| z Score, mean (SD) | ||
| Male reference | −0.54 (0.84) | −0.68 (0.77) |
| Female reference | −0.03 (1.04) | −0.19 (0.94) |
| T score <−2.5, No./total No. (%) | ||
| Male reference | 0/23 | 0/50 |
| Female reference | 0/23 | 0/50 |
| z Score <−2.0, No./total No. (%) | ||
| Male reference | 1/23 (4) | 4/50 (8) |
| Female reference | 0/23 | 2/50 (4) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); LH, luteinizing hormone.
SI conversion factors: To convert estradiol from picograms per milliliter to picomoles per liter, multiply by 3.671; to convert LH from milli-international units per milliliter to international units per liter, multiply by 1.0; to convert testosterone from nanograms per deciliter to nanomoles per liter, multiply by 0.0347; to convert 25-hydroxyvitamin D from nanograms per milliliter to nanomoles per liter, multiply by 2.496.
BMD Development in Participants Assigned Male at Birth
Areal BMD and BMD z score development over time estimated by the mixed-model analysis are shown in Figure 1. Results from the mixed-model analyses in which z scores were assessed at 4 specific time points (ie, start of GnRH agonist, start of GAH, short-term follow-up, and long-term follow-up) are shown in Figure 2.
Figure 1. Areal Bone Mineral Density (BMD) and BMD z Score Development Over Time at 3 Regions of Interest.
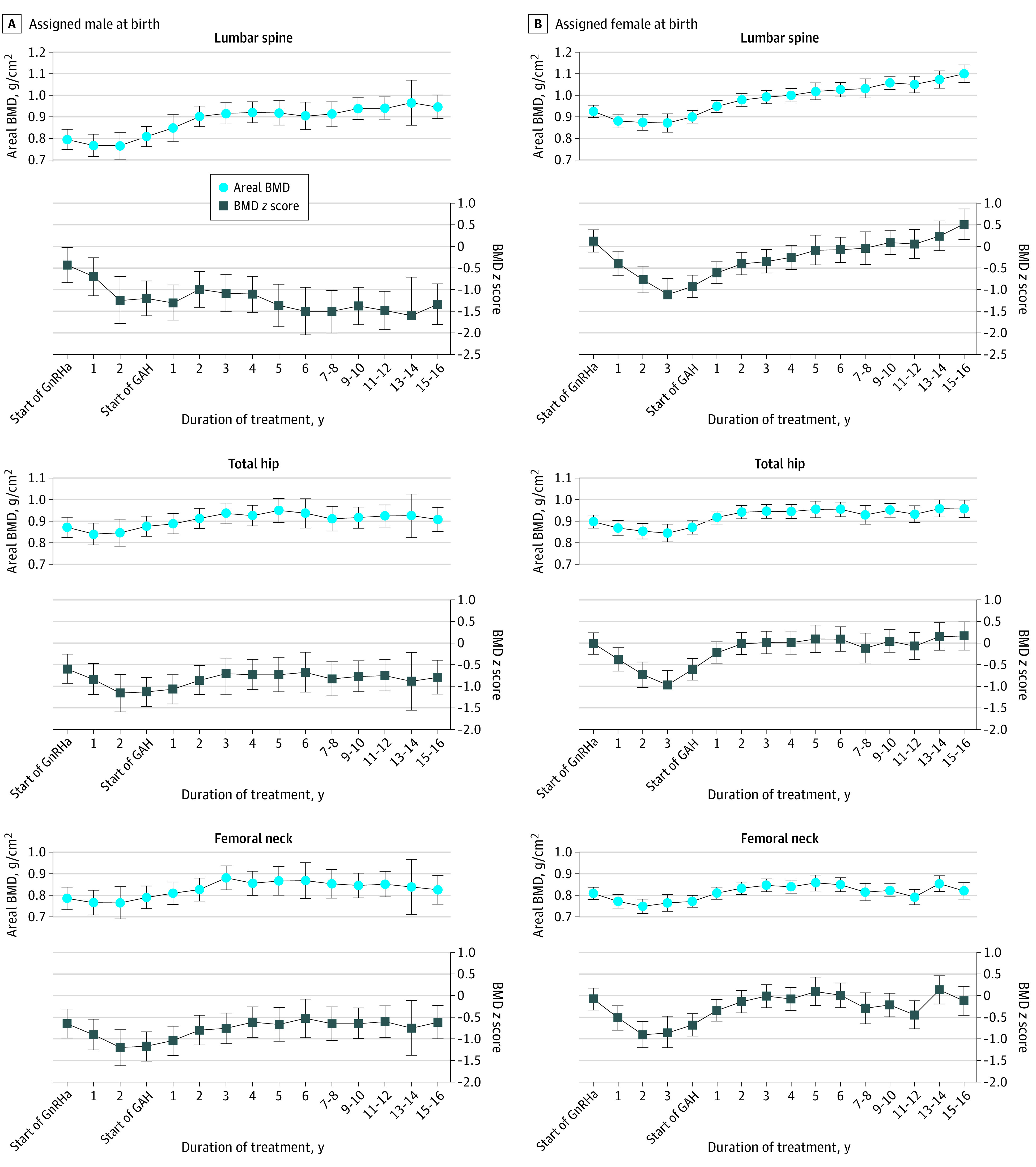
Estimated by mixed-model analyses. A mean (SD) of 7 (2) scans were available per participant. Whiskers indicate 95% CIs. GAH indicates gender-affirming hormones and GnRHa, gonadotropin-releasing hormone agonist.
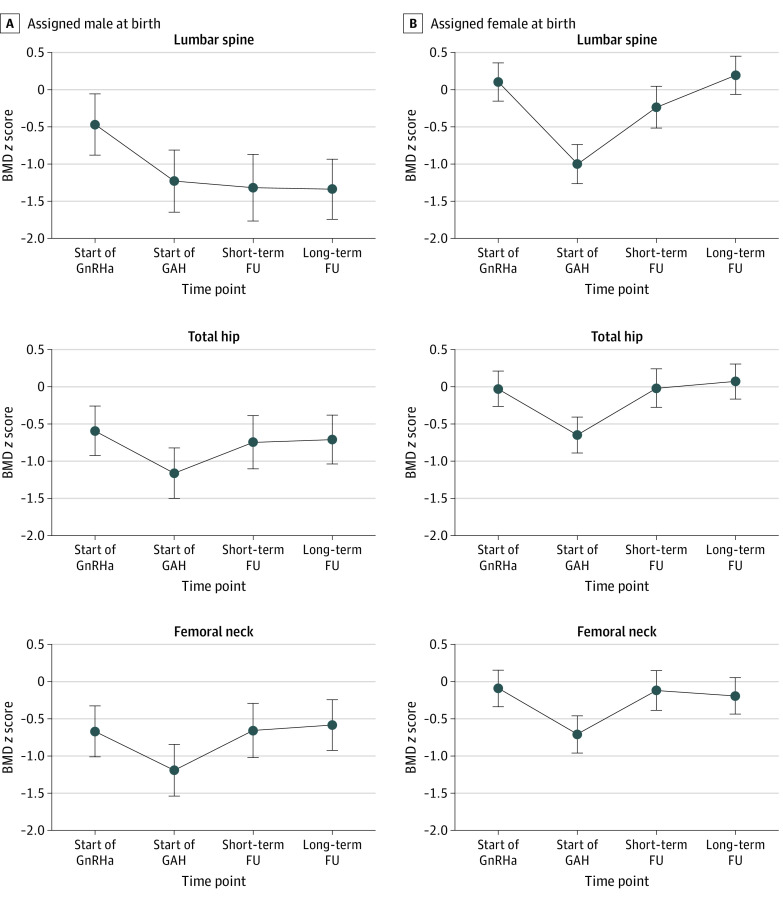
Figure 2. Bone Mineral Density (BMD) z Score Development and Mean Change at 4 Time Points and 3 Regions of Interest.
Estimated by mixed-model analyses. The median (IQR) duration of gender-affirming hormone (GAH) treatment at short-term follow-up (FU) was 5.3 (4.3-6.3) years in individuals assigned male at birth and 5.7 (4.7-6.1) years in individuals assigned female at birth. The median (IQR) duration of GAH treatment at long-term FU was 11.6 (10.1-14.7) years in individuals assigned male at birth and 11.9 (10.2-13.8) years in individuals assigned female at birth. At the start of gonadotropin-releasing hormone agonist (GnRHa) treatment, 3 individuals assigned male at birth and 2 individuals assigned female at birth had missing dual-energy x-ray absorptiometry (DXA) scans. At the start of GAH treatment, DXA scans were missing in 5 individuals assigned male at birth and 8 individuals assigned female at birth. At short-term FU, DXA scans were not available for 11 individuals assigned male at birth and 20 individuals assigned female at birth. No DXA scans were missing at long-term FU. Circles indicate areal BMD and whiskers, 95% CIs. Mean changes in BMD z scores between all 4 time points are listed in eTable 2.
In participants assigned male at birth, BMD remained stable during GnRH agonist treatment but increased during GAH (Figure 1). The z scores were already lower than 0 at the start of a GnRH agonist and further decreased during GnRH agonist treatment (Figure 2). During GAH treatment, the lumbar spine z score remained stable (mean [SD], −1.34 [1.16]), resulting in a decreased z score at long-term follow-up (−0.87; 95% CI, −1.15 to −0.59) compared with start of GnRH agonist treatment. The z scores at the total hip (mean [SD], −0.66 [0.75]) and femoral neck (mean [SD], −0.54 [0.84]) increased during GAH treatment, and the z score at follow-up was not different from the pretreatment z score (total hip: −0.12 [95% CI, −0.31 to 0.07]; femoral neck: 0.01 [95% CI, −0.20 to 0.22]). Mean changes in BMD z scores between all 4 time points are listed in eTable 2 in Supplement 1.
Hormone Concentrations, BMI, and Duration of Mono GnRH Agonist Treatment in Participants Assigned Male at Birth
The associations of estradiol, testosterone, LH and vitamin D concentrations; BMI; and duration of mono GnRH agonist treatment with long-term BMD z scores are shown in Figure 3. The BMD z scores at long-term follow-up were greater with increasing estradiol concentrations, although the score differences were not significant (lumbar spine: 0.27 per 27.24 pg/mL [95% CI, −0.01 to 0.55 per 27.24 pg/mL]; total hip: 0.18 per 27.24 pg/mL [95% CI, −0.02 to 0.37 per 27.24 pg/mL]; femoral neck: 0.16 per 27.24 pg/mL [95% CI, −0.06 to 0.38 per 27.24 pg/mL]). The LH and vitamin D concentrations and the duration of mono GnRH agonist treatment were not associated with long-term follow-up z scores. A positive association was found between BMI and z scores at follow-up at the total hip (0.05 per kg/m2; 95% CI, 0.01-0.10 per kg/m2) and at the femoral neck (0.08 per kg/m2; 95% CI, 0.04-0.13 per kg/m2) but not at the lumbar spine. Because testosterone was low in all participants assigned male at birth at follow-up (range, 14.41-31.70 ng/dL), this analysis was omitted.
Figure 3. Regression Coefficients for the Associations of Biochemical Values, Body Mass Index (BMI), and Duration of Mono Gonadotropin-Releasing Hormone Agonist (GnRHa) Treatment With Bone Mineral Density (BMD) z Scores at Follow-up at 3 Regions of Interest.
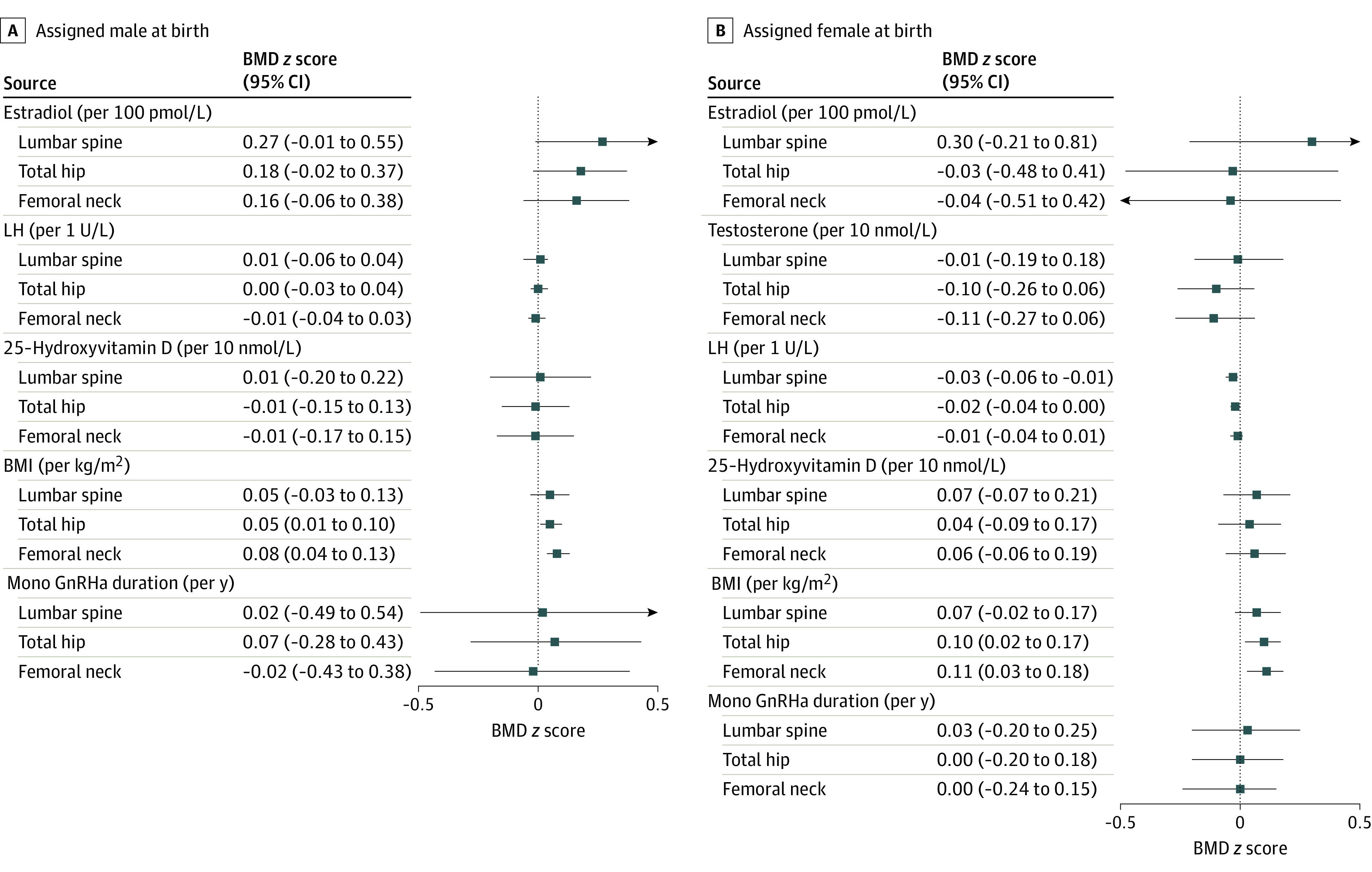
Estimated by regression analyses. The association between testosterone and BMD z scores in individuals assigned male at birth was not assessed because testosterone was low (range, 0.5-1.1 nmol/L) in all individuals assigned male at birth at follow-up. Squares, triangles, and circles represent regression coefficients (β); whiskers represent 95% CIs. SI conversion factors: To convert estradiol from picomoles per liter to picograms per milliliter, divide by 3.671; to convert LH from international units per liter to milli-International units per milliliter, divide by 1.0; to convert testosterone from nanomoles per liter to nanograms per deciliter, divide by 0.0347; to convert 25-hydroxyvitamin D from nanomoles per liter to nanograms per milliliter, divide by 2.496. BMI is calculated as weight in kilograms divided by height in meters squared. LH indicates luteinizing hormone.
BMD Development in Participants Assigned Female at Birth
In participants assigned female at birth, BMD increased over time at all 3 regions (Figure 1). The BMD z scores decreased after the start of GnRH agonist treatment (Figure 2). However, during GAH treatment, the z scores increased, resulting in a similar z score compared with the start of GnRH agonist treatment. After long-term GAH, the mean (SD) z score was 0.20 (1.05; change from start of GnRH agonist: 0.09; 95% CI, −0.09 to 0.27) at the lumbar spine, 0.07 (0.91; change from start of GnRH agonist: 0.10; 95% CI, −0.06 to 0.26) at the total hip, and −0.19 (0.94; change from start of GnRH agonist: −0.20; 95% CI, −0.26 to 0.06) at the femoral neck. Mean changes in BMD z scores between all 4 time points are listed in eTable 2 in Supplement 1.
Hormone Concentrations, BMI, and Duration of Mono GnRH Agonist Treatment in Participants Assigned Female at Birth
At follow-up, there was no association of estradiol, testosterone, or vitamin D concentrations or duration of mono GnRH agonist treatment with BMD z scores at any of the regions of interest in participants assigned female at birth (Figure 3). The LH was negatively associated with the lumbar spine z score at follow-up (−0.03 per 1.0 mIU/mL; 95% CI, −0.06 to −0.01 per 1.0 mIU/mL). Similar to participants assigned male at birth, BMI in those assigned female at birth was positively associated with z scores at the total hip (0.10 per kg/m2; 95% CI, 0.02-0.17 per kg/m2) and femoral neck (0.11 kg/m2; 95% CI, 0.03-0.18 per kg/m2) but not at the lumbar spine.
Discussion
This cohort study found that BMD z scores declined during GnRH agonist treatment but caught up with pretreatment levels at long-term follow-up at all regions of interest except for the lumbar spine in participants assigned male at birth. At the total hip and femoral neck, BMI was positively associated with z scores at long-term follow-up in both participants assigned male at birth and assigned female at birth. In those assigned female at birth, a negative association between LH and z scores at long-term follow-up was found.
Our study provided evidence that bone mineral accrual is temporarily suspended by the use of puberty suppression but, due to an increase during GAH treatment, BMD catches up with pretreatment levels at long-term follow-up, except for the lumbar spine in individuals assigned male at birth. Because the median age at long-term follow-up was 28.2 years, and PBM is generally reached around this age, it was not possible to assess whether PBM was decreased or achievement was delayed.
Research has consistently found that bone mineral accrual decreases during GnRH agonist treatment.5,6,7,8,9,10,11 Accrual generally increases during GAH use, but after short-term treatment, z scores do not catch up to levels similar to pre-GnRH agonist initiation at every region of interest.8,9,11 Klink et al11 found that the lumbar spine z score at age 22 years in both individuals assigned male at birth and those assigned female at birth was still significantly lower compared with the z score at the start of GnRH agonist treatment. This decrease was not seen when comparing the z score at the start of GnRH agonist treatment and age 22 years at the femoral neck in individuals assigned either male at birth or female at birth.11
In individuals assigned male at birth, z scores were already lower than 0 at the start of GnRH agonist treatment. The z scores might have a tendency to decline over time due to, for example, lifestyle factors being different from peers. This hypothesis is in line with findings of low pretreatment z scores in adult transgender women.20 However, information on the natural course of BMD development in transgender people is not available.
A relatively low estradiol dosage in the first period of GAH treatment might play a role in the lack of improvement in the lumbar spine z score in individuals assigned male at birth. This possibility is supported by the pattern we found for z scores at long-term follow-up to be greater with higher estradiol concentrations and is consistent with the fact that the spine is particularly sensitive to sex steroids. At follow-up, LH was in the higher range in the majority of participants assigned male at birth, which may also indicate insufficient estrogen supplementation.
Interestingly, in contrast to testosterone concentrations, which varied widely due to variation in time since last testosterone injection, LH was negatively associated with long-term follow-up lumbar spine z score in participants assigned female at birth. This finding might imply that a higher dose of testosterone leading to more suppression of LH might be beneficial for BMD sustainment in those assigned female at birth, as has also been reported in adults.20 Body mass index was positively associated with higher z scores at the total hip and femoral neck but not the lumbar spine. This association is likely due to the fact that the spine mainly consists of trabecular bone, whereas the hip contains a greater part of cortical bone. Cortical bone is known to be affected by mechanical loading more than trabecular bone.21
At follow-up, when participants were in their late 20s (around 28 years), the majority had z scores within the normal range when using reference data of the affirmed gender, as is recommended by the International Society for Clinical Densitometry.17 However, because the lumbar spine z score in participants assigned male at birth did not catch up, extra attention should be paid to maximize bone mineral accrual by optimizing mechanical loading of the bone (eg, by motivating to play sports) and furthermore by advising against smoking, ensuring adequate calcium intake or supplementation and sunlight exposure or vitamin D supplementation in this group, and optimizing estrogen treatment. Given the results of our study, monitoring of BMD using DXA scans is not necessary in individuals assigned female at birth who started treatment in late puberty, but follow-up with DXA remains important in those assigned male at birth.
To our knowledge, this study is the first to report on long-term bone health in transgender adolescents treated with a GnRH agonist and subsequent GAH treatment. With a median follow-up of almost 12 years of GAH treatment, this study yields new and important data. While treatment with puberty suppression for adolescents diagnosed with gender dysphoria has become controversial, especially because of questions about the long-term safety, we presented evidence that GnRH agonist treatment is generally safe regarding bone health. In future studies, it would be informative to assess the association between estradiol and BMD in a larger study population. Additionally, the natural course of BMD development in transgender people should be studied because BMD z scores before the start of GnRH agonist treatment were already decreased in participants assigned male at birth. This research would help clarify to what extent the finding that follow-up measurements did not catch up with pretreatment levels at the lumbar spine can be attributed to the hormonal treatment and to what extent to other factors.
Limitations
There are some limitations to this study. Overall, 19% of eligible participants could not be reached. Another 21% did not provide informed consent for various reasons. The DXA data were not available for all participants at every time point. By using mixed-model analyses, we tried to have the least possible bias due to missing data.22 Additionally, we were unable to stratify participants for puberty stage at the start of GnRH agonist treatment because so few were in early puberty at the start of treatment. Individuals starting puberty suppression in late puberty have inherently gained more bone mass before the start of treatment compared with individuals starting in early puberty. It can be speculated that this might be beneficial for long-term bone health, although a previous study8 found that those starting puberty suppression in early puberty actually had similar or higher BMD z scores after 3 years of GAH compared with those starting in late puberty. Furthermore, the association of sex steroids and vitamin D concentrations with BMD z scores could not be assessed over time because these variables were only sufficiently available at short- and long-term follow-up. Ideally, to account for seasonal fluctuation, multiple measurements of vitamin D would have been used per participant. Likewise, the testosterone concentration depends on the time since last administration in individuals assigned female at birth who are receiving intramuscular testosterone.
Conclusions
This cohort study found that, after long-term use of GAH, z scores in individuals treated with puberty suppression caught up with pretreatment levels, except for the lumbar spine in participants assigned male at birth, possibly due to low estradiol concentrations. These findings suggest that use of a GnRH agonist followed by long-term GAH is safe regarding bone health in transgender persons receiving testosterone, but bone health in transgender persons receiving estrogen requires extra attention and further study. Estrogen treatment should be optimized and lifestyle counseling provided to maximize bone development in individuals assigned male at birth.
eFigure. Flowchart of Study Participation
eTable 1. Characteristics of Individuals Not Included
eTable 2. Mean Change in BMD Z-Scores Between All Four Time Points, at Three Regions
Data Sharing Statement
References
- 1.Bonjour JP, Chevalley T. Pubertal timing, bone acquisition, and risk of fracture throughout life. Endocr Rev. 2014;35(5):820-847. doi: 10.1210/er.2014-1007 [DOI] [PubMed] [Google Scholar]
- 2.Gordon CM, Zemel BS, Wren TA, et al. The determinants of peak bone mass. J Pediatr. 2017;180:261-269. doi: 10.1016/j.jpeds.2016.09.056 [DOI] [PubMed] [Google Scholar]
- 3.Surrey ES, Hornstein MD. Prolonged GnRH agonist and add-back therapy for symptomatic endometriosis: long-term follow-up. Obstet Gynecol. 2002;99(5 Pt 1):709-719. doi: 10.1097/00006250-200205000-00008 [DOI] [PubMed] [Google Scholar]
- 4.Antoniazzi F, Zamboni G, Bertoldo F, Lauriola S, Tatò L. Bone development during GH and GnRH analog treatment. Eur J Endocrinol. 2004;151(suppl 1):S47-S54. doi: 10.1530/eje.0.151s047 [DOI] [PubMed] [Google Scholar]
- 5.Carmichael P, Butler G, Masic U, et al. Short-term outcomes of pubertal suppression in a selected cohort of 12 to 15 year old young people with persistent gender dysphoria in the UK. PLoS One. 2021;16(2):e0243894. doi: 10.1371/journal.pone.0243894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joseph T, Ting J, Butler G. The effect of GnRH analogue treatment on bone mineral density in young adolescents with gender dysphoria: findings from a large national cohort. J Pediatr Endocrinol Metab. 2019;32(10):1077-1081. doi: 10.1515/jpem-2019-0046 [DOI] [PubMed] [Google Scholar]
- 7.Navabi B, Tang K, Khatchadourian K, Lawson ML. Pubertal suppression, bone mass, and body composition in youth with gender dysphoria. Pediatrics. 2021;148(4):e2020039339. doi: 10.1542/peds.2020-039339 [DOI] [PubMed] [Google Scholar]
- 8.Schagen SEE, Wouters FM, Cohen-Kettenis PT, Gooren LJ, Hannema SE. Bone development in transgender adolescents treated with GnRH analogues and subsequent gender-affirming hormones. J Clin Endocrinol Metab. 2020;105(12):e4252-e4263. doi: 10.1210/clinem/dgaa604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoffers IE, de Vries MC, Hannema SE. Physical changes, laboratory parameters, and bone mineral density during testosterone treatment in adolescents with gender dysphoria. J Sex Med. 2019;16(9):1459-1468. doi: 10.1016/j.jsxm.2019.06.014 [DOI] [PubMed] [Google Scholar]
- 10.Vlot MC, Klink DT, den Heijer M, Blankenstein MA, Rotteveel J, Heijboer AC. Effect of pubertal suppression and cross-sex hormone therapy on bone turnover markers and bone mineral apparent density (BMAD) in transgender adolescents. Bone. 2017;95:11-19. doi: 10.1016/j.bone.2016.11.008 [DOI] [PubMed] [Google Scholar]
- 11.Klink D, Caris M, Heijboer A, van Trotsenburg M, Rotteveel J. Bone mass in young adulthood following gonadotropin-releasing hormone analog treatment and cross-sex hormone treatment in adolescents with gender dysphoria. J Clin Endocrinol Metab. 2015;100(2):E270-E275. doi: 10.1210/jc.2014-2439 [DOI] [PubMed] [Google Scholar]
- 12.Government of the Netherlands. Wet Medisch-Wetenschappelijk Onderzoek Met Mensen. Published online January 1, 2020. Accessed September 25, 2023. https://wetten.overheid.nl/BWBR0009408/2020-01-01
- 13.Wiepjes CM, Nota NM, de Blok CJM, et al. The Amsterdam Cohort of Gender Dysphoria Study (1972-2015): trends in prevalence, treatment, and regrets. J Sex Med. 2018;15(4):582-590. doi: 10.1016/j.jsxm.2018.01.016 [DOI] [PubMed] [Google Scholar]
- 14.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, Text Revision). American Psychiatric Publishing; 2000. [Google Scholar]
- 15.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition). American Psychiatric Association; 2013. [Google Scholar]
- 16.Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4(9):e7038. doi: 10.1371/journal.pone.0007038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shuhart CR, Yeap SS, Anderson PA, et al. Executive summary of the 2019 ISCD Position Development Conference on monitoring treatment, DXA cross-calibration and least significant change, spinal cord injury, peri-prosthetic and orthopedic bone health, transgender medicine, and pediatrics. J Clin Densitom. 2019;22(4):453-471. doi: 10.1016/j.jocd.2019.07.001 [DOI] [PubMed] [Google Scholar]
- 18.Heijboer AC, Blankenstein MA, Kema IP, Buijs MM. Accuracy of 6 routine 25-hydroxyvitamin D assays: influence of vitamin D binding protein concentration. Clin Chem. 2012;58(3):543-548. doi: 10.1373/clinchem.2011.176545 [DOI] [PubMed] [Google Scholar]
- 19.Dirks NF, Vesper HW, van Herwaarden AE, et al. Various calibration procedures result in optimal standardization of routinely used 25(OH)D ID-LC-MS/MS methods. Clin Chim Acta. 2016;462:49-54. doi: 10.1016/j.cca.2016.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiepjes CM, de Jongh RT, de Blok CJ, et al. Bone safety during the first ten years of gender-affirming hormonal treatment in transwomen and transmen. J Bone Miner Res. 2019;34(3):447-454. doi: 10.1002/jbmr.3612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozaki E, Matsukawa M, Mano I, et al. Growth of cortical bone thickness and trabecular bone density in Japanese children. Bone. 2020;141:115669. doi: 10.1016/j.bone.2020.115669 [DOI] [PubMed] [Google Scholar]
- 22.Twisk J, de Boer M, de Vente W, Heymans M. Multiple imputation of missing values was not necessary before performing a longitudinal mixed-model analysis. J Clin Epidemiol. 2013;66(9):1022-1028. doi: 10.1016/j.jclinepi.2013.03.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Flowchart of Study Participation
eTable 1. Characteristics of Individuals Not Included
eTable 2. Mean Change in BMD Z-Scores Between All Four Time Points, at Three Regions
Data Sharing Statement