Abstract
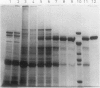
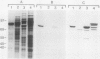
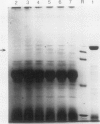
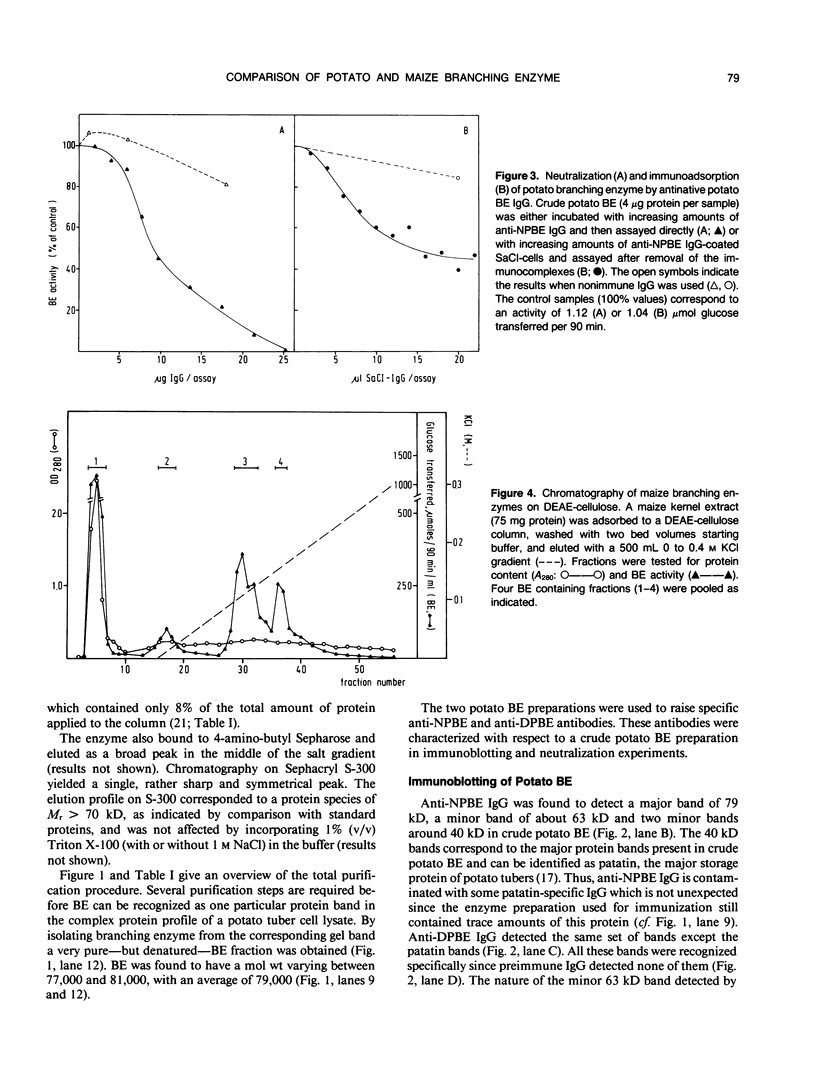
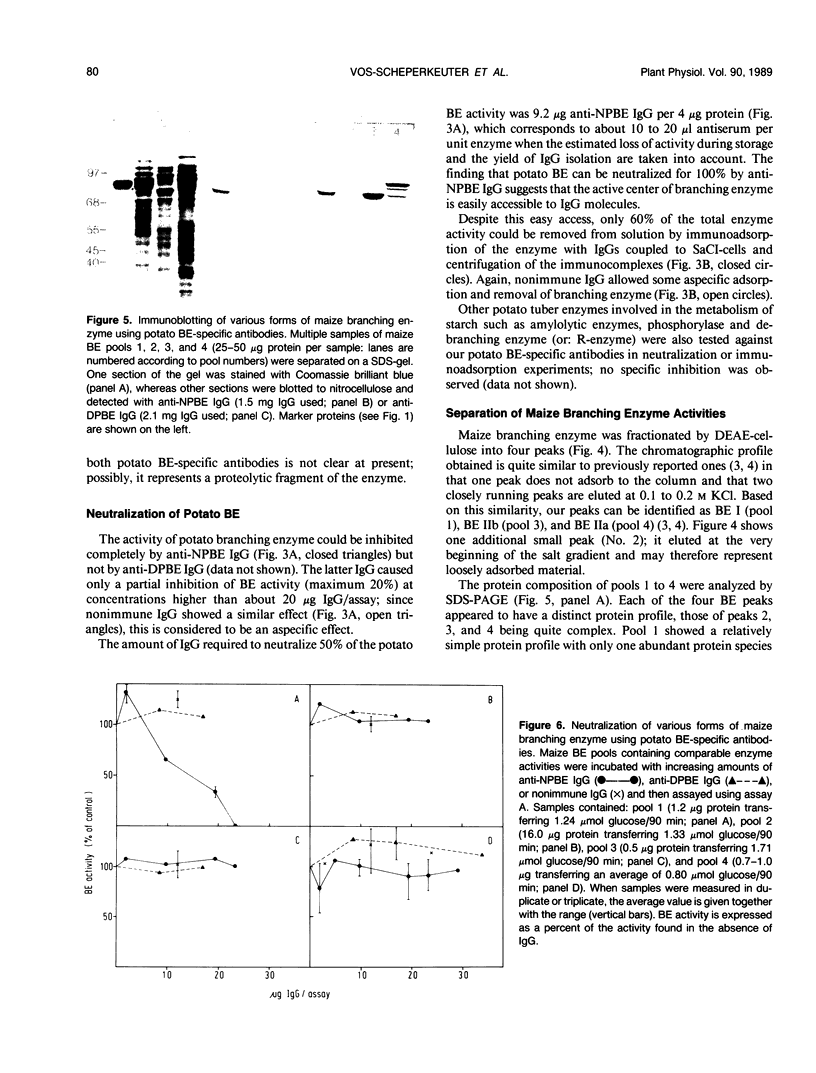
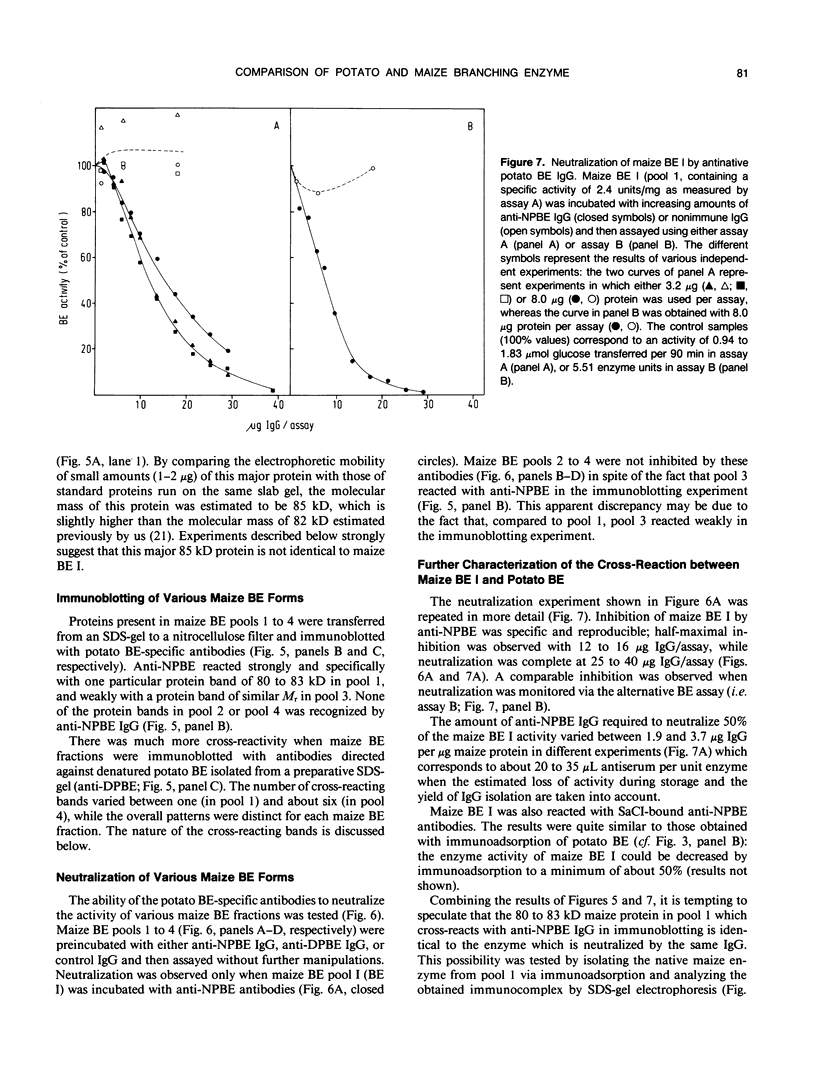
Starch branching enzyme was purified from potato (Solanum tuberosum L.) tubers as a single species of 79 kilodaltons and specific antibodies were prepared against both the native enzyme and against the gel-purified, denatured enzyme. The activity of potato branching enzyme could only be neutralized by antinative potato branching enzyme, whereas both types of antibodies reacted with denatured potato branching enzyme. Starch branching enzymes were also isolated from maize (Zea mays L.) kernels. All of the denatured forms of the maize enzyme reacted with antidenatured potato branching enzyme, whereas recognition by antinative potato branching enzyme was limited to maize branching enzymes I and IIb. Antibodies directed against the denatured potato enzyme were unable to neutralize the activity of any of the maize branching enzymes. Antinative potato branching enzyme fully inhibited the activity of maize branching enzyme I; the neutralized maize enzyme was identified as a 82 kilodalton protein. It is concluded that potato branching enzyme (Mr = 79,000) shares a high degree of similarity with maize branching enzyme I (Mr = 82,000), in the native as well as the denatured form. Cross-reactivity between potato branching enzyme and the other forms of maize branching enzyme was observed only after denaturation, which suggests mutual sequence similarities between these species.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borovsky D., Smith E. E., Whelan W. J. Purification and properties of potato 1,4-alpha-D-glucan:1,4-alpha-D-glucan 6-alpha-(1,4-alpha-glucano)-transferase. Evidence against a dual catalytic function in amylose-branching enzyme. Eur J Biochem. 1975 Nov 15;59(2):615–625. doi: 10.1111/j.1432-1033.1975.tb02490.x. [DOI] [PubMed] [Google Scholar]
- Boyer C. D., Preiss J. Evidence for independent genetic control of the multiple forms of maize endosperm branching enzymes and starch synthases. Plant Physiol. 1981 Jun;67(6):1141–1145. doi: 10.1104/pp.67.6.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond G. S., Smith E. E., Whelan W. J. Purification and properties of potato -1,4-glucan. -1,4-Glucan 6-glycosyltransferase (Q-enzyme). Eur J Biochem. 1972 Mar 27;26(2):168–176. doi: 10.1111/j.1432-1033.1972.tb01753.x. [DOI] [PubMed] [Google Scholar]
- Griffin H. L., Wu Y. V. Corn and potato -1,4-glucan: -1,4-glucan 6-glycosyltransferase: evidence for separate hydrolytic and branching components. Biochemistry. 1971 Nov;10(23):4330–4335. doi: 10.1021/bi00799a027. [DOI] [PubMed] [Google Scholar]
- Hawker J. S., Ozbun J. L., Ozaki H., Greenberg E., Preiss J. Interaction of spinach leaf adenosine diphosphate glucose alpha-1,4-glucan alpha-4-glucosyl transferase and alpha-1,4-glucan, alpha-1,4-glucan-6-glycosyl transferase in synthesis of branched alpha-glucan. Arch Biochem Biophys. 1974 Feb;160(2):530–551. doi: 10.1016/0003-9861(74)90430-5. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Singh B. K., Preiss J. Starch Branching Enzymes from Maize : Immunological Characterization using Polyclonal and Monoclonal Antibodies. Plant Physiol. 1985 Sep;79(1):34–40. doi: 10.1104/pp.79.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos-Scheperkeuter G. H., Witholt B. Assembly pathway of newly synthesized LamB protein an outer membrane protein of Escherichia coli K-12. J Mol Biol. 1984 Jun 5;175(4):511–528. doi: 10.1016/0022-2836(84)90182-7. [DOI] [PubMed] [Google Scholar]
- Vos-Scheperkeuter G. H., de Boer W., Visser R. G., Feenstra W. J., Witholt B. Identification of granule-bound starch synthase in potato tubers. Plant Physiol. 1986 Oct;82(2):411–416. doi: 10.1104/pp.82.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensink J., Witholt B. Identification of different forms of the murein-bound lipoprotein found in isolated outer membranes of Escherichia coli. Eur J Biochem. 1981 Jan;113(2):349–357. doi: 10.1111/j.1432-1033.1981.tb05073.x. [DOI] [PubMed] [Google Scholar]
- Zakin M. M., Garel J. R., Dautry-Varsat A., Cohen G. N., Boulot G. Detection of the homology among proteins by immunochemical cross-reactivity between denatured antigens. Application to the threonine and methionine regulated aspartokinases-homoserine dehydrogenases from Escherichia coli K 12. Biochemistry. 1978 Oct 3;17(20):4318–4323. doi: 10.1021/bi00613a032. [DOI] [PubMed] [Google Scholar]