Abstract
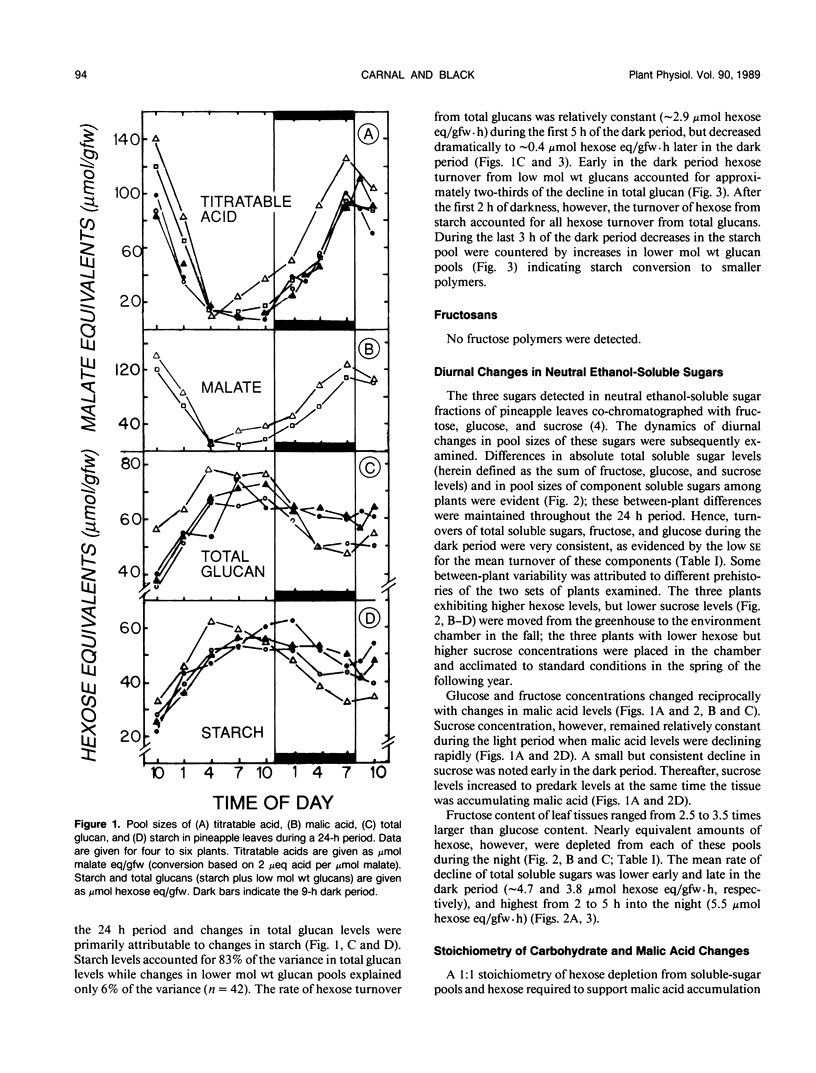
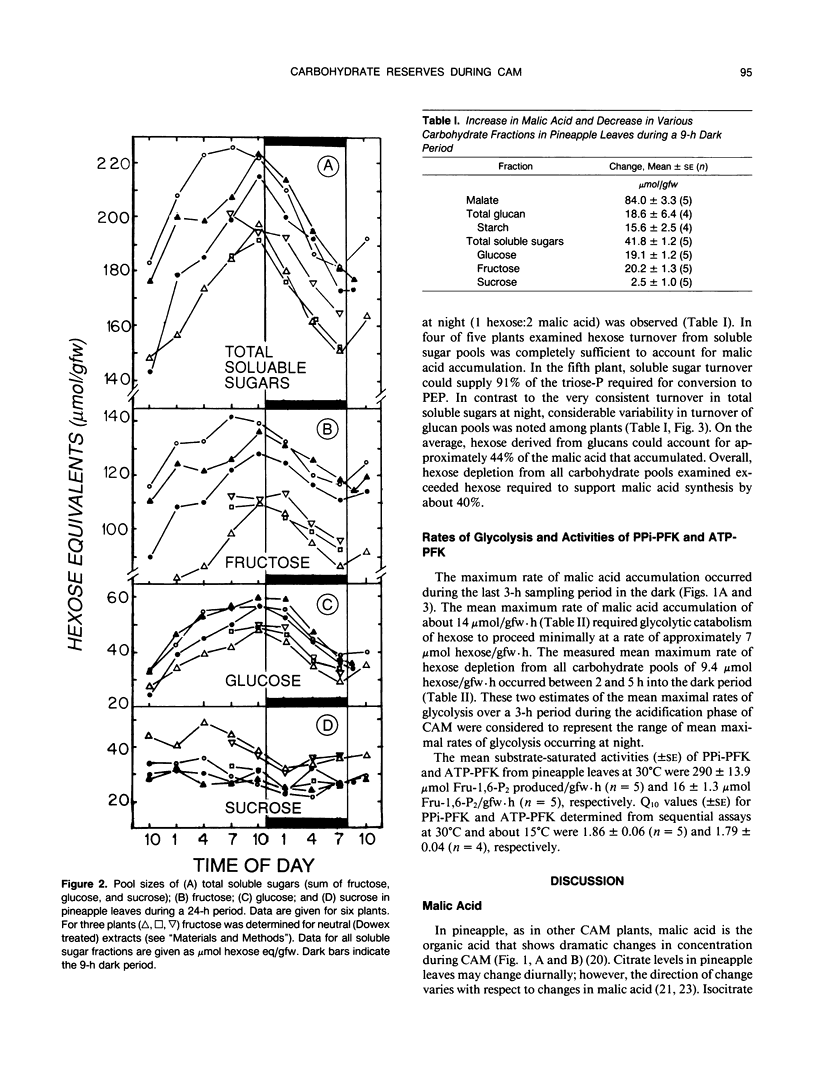
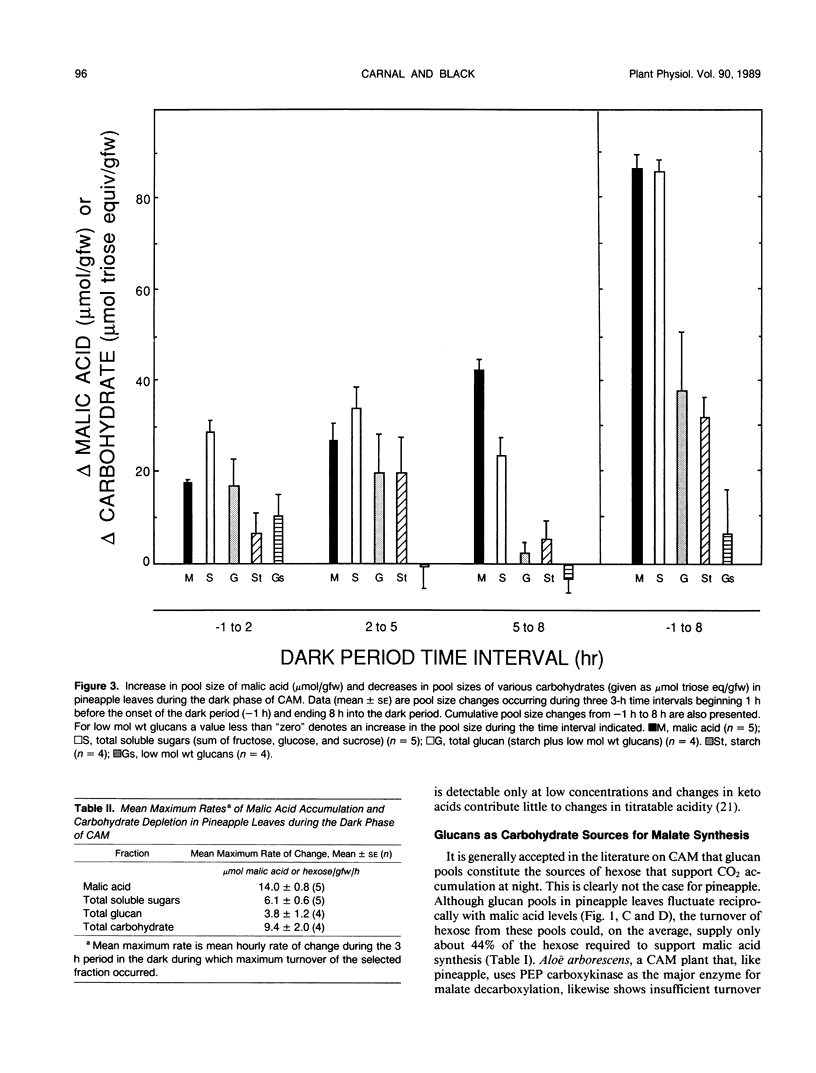
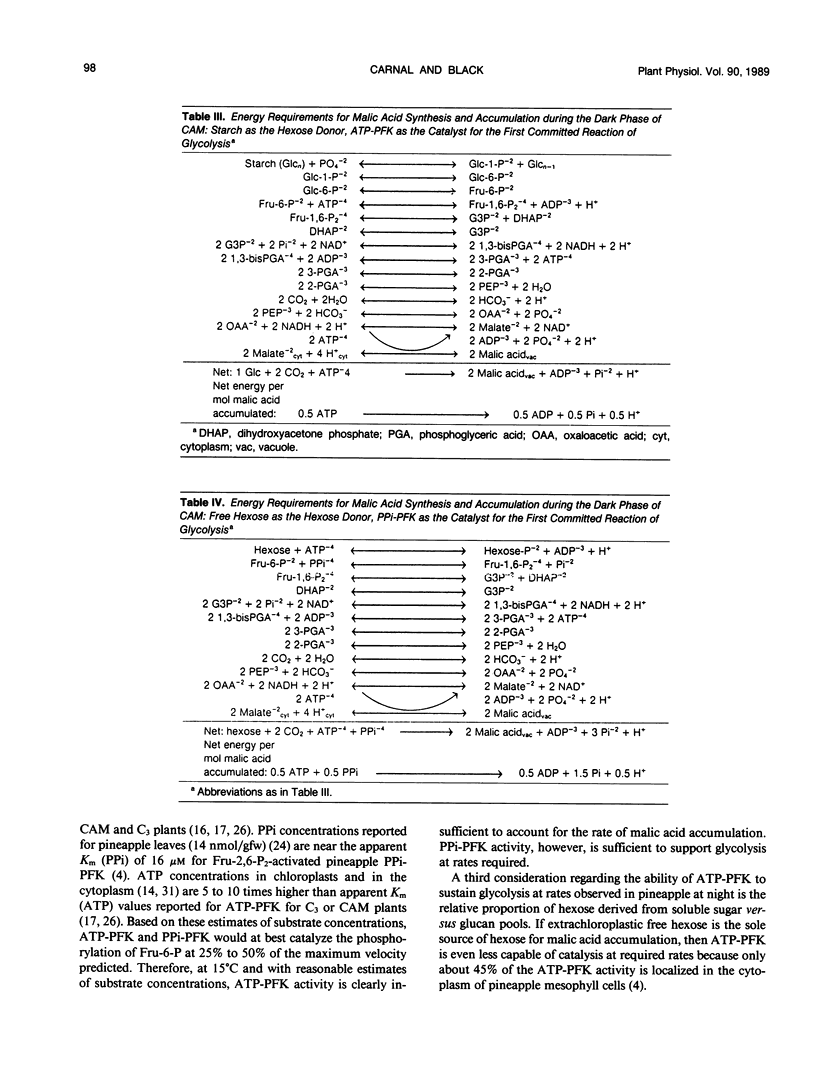
Neutral ethanol-soluble sugar pools serve as carbohydrate reserves for Crassulacean acid metabolism (CAM) in pineapple (Ananas comosus (L.) Merr.) leaves. Levels of neutral soluble sugars and glucans fluctuated reciprocally with concentrations of malic acid. Hexose loss from neutral soluble-sugar pools was sufficient to account for malic acid accumulation with about 95% of the required hexose accounted for by turnover of fructose and glucose pools. Hexose loss from starch or starch plus lower molecular weight glucan pools was insufficient to account for nocturnal accumulation of malic acid. The apparent maximum catalytic capacity of pyrophosphate:6-phosphofructokinase (PPi-PFK) at 15°C was about 16 times higher than the mean maximum rate of glycolysis that occurred to support malic acid accumulation in pineapple leaves at night and 12 times higher than the mean maximum rate of hexose turnover from all carbohydrate pools. The apparent maximum catalytic capacity of ATP-PFK at 15°C was about 70% of the activity required to account for the mean maximal rate of hexose turnover from all carbohydrate pools if turnover were completely via glycolysis, and marginally sufficient to account for mean maximal rates of acidification. Therefore, at low night temperatures conducive to CAM and under subsaturating substrate concentrations, PPi-PFK activity, but not ATP-PFK activity, would be sufficient to support the rate of glycolytic carbohydrate processing required for acid accumulation. These data for pineapple establish that there are at least two types of CAM plants with respect to the nature of the carbohydrate reserve utilized to support nighttime CO2 accumulation. The data further indicate that the glycolytic carbohydrate processing that supports acidification proceeds in different subcellular compartments in plants utilizing different carbohydrate reserves.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carnal N. W., Black C. C. Phosphofructokinase activities in photosynthetic organisms : the occurrence of pyrophosphate-dependent 6-phosphofructokinase in plants and algae. Plant Physiol. 1983 Jan;71(1):150–155. doi: 10.1104/pp.71.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn W., McAulay A. Changes in Metabolite Levels in Kalanchoë daigremontiana and the Regulation of Malic Acid Accumulation in Crassulacean Acid Metabolism. Plant Physiol. 1977 Mar;59(3):455–458. doi: 10.1104/pp.59.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cséke C., Weeden N. F., Buchanan B. B., Uyeda K. A special fructose bisphosphate functions as a cytoplasmic regulatory metabolite in green leaves. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4322–4326. doi: 10.1073/pnas.79.14.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrendorf T., Holtum J. A., Mukherjee U., Latzko E. Fructose 2,6-bisphosphate, carbohydrate partitioning, and crassulacean Acid metabolism. Plant Physiol. 1987 May;84(1):182–187. doi: 10.1104/pp.84.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland W. J., Dennis D. T. Plastid and cytosolic phosphofructokinase from the developing endosperm of Ricinus communis. I. Separation, purification, and initial characterization of the isozymes. Arch Biochem Biophys. 1980 Oct 1;204(1):302–309. doi: 10.1016/0003-9861(80)90037-5. [DOI] [PubMed] [Google Scholar]
- Graham D., Smydzuk J. Use of anthrone in the quantitative determination of hexose phosphates. Anal Biochem. 1965 May;11(2):246–255. doi: 10.1016/0003-2697(65)90012-6. [DOI] [PubMed] [Google Scholar]
- Heuer B., Hansen M. J., Anderson L. E. Light modulation of phosphofructokinase in pea leaf chloroplasts. Plant Physiol. 1982 Jun;69(6):1404–1406. doi: 10.1104/pp.69.6.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly G. J., Latzko E. Chloroplast Phosphofructokinase: II. Partial Purification, Kinetic and Regulatory Properties. Plant Physiol. 1977 Aug;60(2):295–299. doi: 10.1104/pp.60.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly G. J., Latzko E. Chloroplast phosphofructokinase: I. Proof of phosphofructokinase activity in chloroplasts. Plant Physiol. 1977 Aug;60(2):290–294. doi: 10.1104/pp.60.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon W. H., Severson R. F., Black C. C. Maintenance carbon cycle in crassulacean Acid metabolism plant leaves : source and compartmentation of carbon for nocturnal malate synthesis. Plant Physiol. 1985 Jan;77(1):183–189. doi: 10.1104/pp.77.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucher G. W., Vickery H. B., Abrahams M. D., Leavenworth C. S. STUDIES IN THE METABOLISM OF CRASSULACEAN PLANTS: DIURNAL VARIATION OF ORGANIC ACIDS AND STARCH IN EXCISED LEAVES OF BRYOPHYLLUM CALYCINUM. Plant Physiol. 1949 Oct;24(4):610–620. doi: 10.1104/pp.24.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sideris C. P., Young H. Y., Chun H. H. DIURNAL CHANGES AND GROWTH RATES AS ASSOCIATED WITH ASCORBIC ACID, TITRATABLE ACIDITY, CARBOHYDRATE AND NITROGENOUS FRACTIONS IN THE LEAVES OF ANANAS COMOSUS (L.) MERR. Plant Physiol. 1948 Jan;23(1):38–69. doi: 10.1104/pp.23.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth D. A., Black C. C. Measurement of the pyrophosphate content of plant tissues. Plant Physiol. 1984 Jul;75(3):862–864. doi: 10.1104/pp.75.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickery H. B. The Effect of Abnormally Prolonged Alternating Periods of Light and Darkness upon the Composition of Bryophyllum calycinum Leaves. Plant Physiol. 1954 Nov;29(6):520–526. doi: 10.1104/pp.29.6.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz W., Stitt M., Heldt H. W. Enzymic determination of metabolites in the subcellular compartments of spinach protoplasts. Plant Physiol. 1980 Jul;66(1):187–193. doi: 10.1104/pp.66.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]


