Abstract
Ureidopeptidic natural products possess a wide variety of favorable pharmacological properties. In addition, they have been shown to mediate core physiological functions in producer bacteria. Here, we report that similar ureidopeptidic natural products with conserved biosynthetic gene clusters are produced by different bacterial genera that coinhabit marine invertebrate microbiomes. We demonstrate that a Microbulbifer strain isolated from a marine sponge can produce two different classes of ureidopeptide natural products encoded by two different biosynthetic gene clusters that are positioned on the bacterial chromosome and on a plasmid. The plasmid encoded ureidopeptide natural products, which we term the pseudobulbiferamides (5–8), resemble the ureidopeptide natural products produced by Pseudovibrio, a different marine bacterial genus that is likewise present in marine sponge commensal microbiomes. Using imaging mass spectrometry, we find that the two classes of Microbulbifer-derived ureidopeptides occupy different physical spaces relative to the bacterial colony, perhaps implying different roles for these two compound classes in Microbulbifer physiology and environmental interactions.
Natural products that play important roles in the physiology of the producer organisms and in mediating their interactions with the environment are widely conserved. Plants produce cyanogenic glycosides that deter herbivory.1 Soil-dwelling bacteria produce odoriferous sesquiterpenes to attract anthropods for spore dispersal,2 and natural product-mediated mutualistic and antagonistic interactions in entomology are likewise well-established.3,4 Gram-negative proteobacteria produce N-acyl homoserine lactones to sense and respond to quorum.5 In the marine realm, numerous marine algae biosynthesize halomethanes to ward off surface colonization by epiphytic bacteria.6 Marine bacteria produce chemical cues that induce settlement of coral larvae.7 The genetic potential to produce these natural products is likewise widespread. While the pharmacological activity of natural products often motivates their discovery, the true function of natural products is to fulfill these organismal and ecological roles.
Recently, Berlinck and Eustáquio reported the discovery of ureidopeptidic pseudovibriamide natural products from Pseudovibrio bacteria.8Pseudovibrio are marine Proteobacteria isolated from microbiomes of marine invertebrates such as sponges, tunicates, and corals.9 The pseudovibriamide natural products mediate swarming in Pseudovibrio, that is, the multicellular ensemble flagellar motion across solid surfaces.10 The biosynthetic gene cluster (BGC) responsible for production of pseudovibriamides was found to be positioned on a plasmid. Supporting the primal role of pseudovibriamides in Pseudovibrio physiology, the plasmid-encoded pseudovibriamide BGC was found to be widely distributed among Pseudovibrio genomes. In this study, we demonstrate that pseudovibriamide-like ureidopeptidic natural products, and highly similar plasmid-encoded BGCs, are shared among not only Pseudovibrio spp., but with other bacterial genera in marine invertebrate microbiomes.
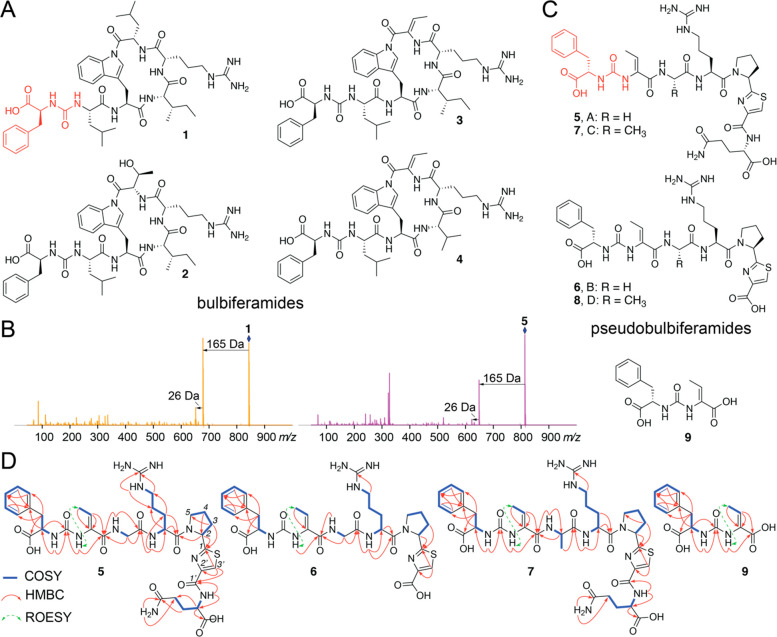
The Pseudovibrio and Microbulbifer genera coinhabit the commensal microbiomes of marine sponges.11,12 We, among others, have previously reported the discovery of bulbiferamides 1–4 from marine Microbulbifer spp. bacteria isolated from sponges Aplysina fulva and Aiolochroia crassa that were collected in the Florida Keys (Figure 1A).13,14 The mass spectrometric fragmentation spectra (MS/MS spectra) for 1–4 demonstrated 165 and 26 Da neutral losses characteristic of the Phe residue connected via the ureidopeptide bond. In extracts of the bacterium Microbulbifer sp. MKSA007 that was isolated from the sponge Smenospongia aurea also collected in the Florida Keys,11 we detected metabolites demonstrating similar features in their fragmentation spectra (Figure 1B). S. aurea, A. fulva, and A. crassa are high microbial abundance sponges that are ubiquitous on shallow reefs in the Florida Keys.15 However, structures of the Microbulbifer sp. MKSA007-derived metabolites could not be discerned based on progression of proteinogenic amino acids.13,16 These metabolites were then isolated from liquid culture extracts of Microbulbifer sp. MKSA007 and their structure elucidation pursued using spectroscopic and degradation experiments. Given their structural similarity to pseudovibriamides (vide infra), we have named these molecules pseudobulbiferamides (Figure 1C).
Figure 1.
(A) Bulbiferamides isolated from marine Microbulbifer sp. bacteria. The Phe residue and the ureido linkage are highlighted in red. (B) MS/MS fragmentation spectra for bulbiferamide A (1, left) and pseudobulbiferamide A (5, right), demonstrating the conserved 165 and 26 Da neutral losses. Parent ions are marked by the blue diamonds. (C) Structures of pseudobulbiferamides A–D (5–8) and the shunt metabolite 9 described in this study. (D) COSY, HMBC, and selected ROESY correlations for 5–7, 9.
Pseudobulbiferamide A (5) was isolated as a yellow oil with the molecular formula C35H47N11O10S, indicating 18 degrees of unsaturation. The 1H NMR spectrum revealed amide proton signals (δH 6.54–8.44 Table 1) and amino acid α-proton signals (δH 3.63–5.33), which were suggestive of its peptidic nature. This inference was supported by the observation of amide carbonyl carbon signals (δC 160.5–173.6) and amino acid Cα carbon signals (δC 42.3–58.3) in the 13C NMR spectra. Analysis of the 2D NMR spectra, including HSQC, HMBC, and COSY allowed the identification of five proteinogenic amino acid residues Phe, Gly, Arg, Pro, and Gln (Figures 1D and S1–S9). Two additional modified residues were identified. First, a dehydrobutyrine (Dhb) residue was determined to be present by the COSY correlation between methyl group protons (δH 1.62, d, J = 7.0 Hz) and a vinyl proton (δH 5.89, q, J = 7.0 Hz) and HMBC correlations from the above-mentioned vinyl proton and an amide proton (δH 7.93, s) to a Cα carbon atom (δC 132.1) and an amino acid main chain carbonyl carbon (δC 165.6). The configuration of the Cα-Cβ double bond in the Dhb residue was deduced to be Z by the ROESY correlation between its amide proton and methyl group.17 Second, a thiazole heterocycle (Thz) derived from a Cys residue was elucidated based on HMBC correlations from the Thz Hβ (δH 8.17, s) to Thz carbonyl carbon (δC 160.5), Cα-carbon (δC 149.1), and Pro carbonyl carbon (δC 173.6). A hexapeptide partial structure Dhb2-Gly3-Arg4-Pro5-Thz6-Gln7 could then be established based on HMBC correlations between the amino acid amide protons or α-protons and the neighboring carbonyl carbon atoms. A ureido bond was deduced to be linking Phe1 and Dhb2 based on HMBC correlations from both amide protons of Phe1 and Dhb2 to a deshielded carbon signal at δC 155.3, which is a characteristic chemical shift of ureido carbonyl carbons.18,19 The planar structure of 5 was thus deduced as a linear heptapeptide Phe1-Dhb2-Gly3-Arg4-Pro5-Thz6-Gln7 with a ureido linkage between the Phe1 and Dhb2 residues. The absolute configurations of all amino acids were determined to be L by Marfey’s analysis (Figures S10–S13). Progressing from 5, using MS/MS fragmentation spectra, we could discern the structure of pseudobulbiferamide B (6), wherein the terminal Gln residue was omitted (Figure S14). Structural assignment of 6 was supported by examination of NMR spectra (Figures S15–S21, Table S1).
Table 1. 13C (176 MHz) and 1H (700 MHz) NMR Chemical Shifts of Compounds 5 and 7 in DMSO-d6 (J in Hz, δ in ppm).
| 5 |
7 |
||||
|---|---|---|---|---|---|
| residue | no. | δC, typea | δH, (J, Hz)b | δC, typea | δH, (J, Hz)b |
| Phe1 | 1 | 173.1, C | 173.2, C | ||
| 2 | 53.8, CH | 4.43, dt (7.4, 6.0) | 53.8, CH | 4.39, overlap | |
| 3 | 37.5, CH2 | 3.05, dd (13.7, 5.3) | 37.5, CH2 | 3.03, dd (13.7, 5.3) | |
| 2.95, dd (13.7, 7.4) | 2.95, dd (13.7, 6.9) | ||||
| 4 | 137.0, C | 137.1, C | |||
| 5/9 | 129.5, CH | 7.17, d (7.1) | 129.4, CH | 7.20, d (7.0) | |
| 6/8 | 128.2, CH | 7.28, t (7.3) | 128.2, CH | 7.29, t (7.6) | |
| 7 | 126.5, CH | 7.22, t (7.3) | 126.6, CH | 7.35, t (7.3) | |
| NH | 6.54, d (7.6) | 6.47, d (7.9) | |||
| Ureido | CO | 155.3, C | 155.1, C | ||
| Dhb2 | 1 | 165.6, C | 164.9, C | ||
| 2 | 132.1, C | 131.7, C | |||
| 3 | 120.6, CH | 5.89, q (7.0) | 121.9, CH | 5.96, q (7.0) | |
| 4 | 12.3, CH3 | 1.62, d (7.0) | 12.7, CH3 | 1.59, d (7.0) | |
| NH | 7.93, s | 7.76, s | |||
| Gly3 | 1 | 169.2, C | |||
| 2 | 42.3, CH2 | 3.79, overlap | |||
| 3.63, dd (16.7, 5.8) | |||||
| NH | 8.08, t (5.9) | ||||
| Ala3 | 1 | 172.3, C | |||
| 2 | 48.3, CH | 4.30, dq (7.2, 6.9) | |||
| 3 | 18.0, CH3 | 1.23, d (7.1) | |||
| NH | 7.71, d (7.5) | ||||
| Arg4 | 1 | 170.7, C | 172.4, C | ||
| 2 | 50.2, CH | 4.55, m | 48.3, CH | 4.52, m | |
| 3 | 28.0, CH2 | 1.77, m | 28.2, CH2 | 1.76, m | |
| 1.67, m | 1.61, overlap | ||||
| 4 | 24.9, CH2 | 1.56, m | 24.9, CH2 | 1.55, m | |
| 1.52, m | 1.52, m | ||||
| 5 | 40.4, CH2 | 3.09, m | 40.1, CH2 | 3.10, m | |
| 6 | 156.7, C | 156.6, C | |||
| αNH | 8.12, d (7.5) | 8.15, d (7.5) | |||
| δNH | 7.59, t (5.0) | 7.48, br s | |||
| εNH | nd | nd | |||
| Pro5 -Thz6 | 1 | 173.6, C | 173.6, C | ||
| 2 | 58.3, CH | 5.33, dd, (6.4, 4.9) | 58.4, CH | 5.32, dd, (8.0, 2.8) | |
| 3 | 31.4, CH2 | 2.23, m | 31.4, CH2 | 2.21, m | |
| 4 | 24.0, CH2 | 1.99, overlap | 24.1, CH2 | 1.97, m | |
| 2.03, m | 1.99, m | ||||
| 5 | 46.8, CH2 | 3.77, overlap | 46.7, CH2 | 3.73, m | |
| 1′ | 160.5, C | 160.4, C | |||
| 2′ | 149.1, C | 149.1, C | |||
| 3′ | 123.9, CH | 8.17, s | 124.0, CH | 8.18, s | |
| Gln7 | 1 | 173.2, C | 173.3, C | ||
| 2 | 51.8, CH | 4.39, m | 51.8, CH | 4.39, m | |
| 3 | 26.4, CH2 | 2.11, m 1.97, overlap | 26.3, CH2 | 2.11, m 1.97, overlap | |
| 4 | 31.4, CH2 | 2.15, t, (6.4) | 31.4, CH2 | 2.15, t, (6.4) | |
| 5 | 173.6, C | 173.5, C | |||
| αNH | 8.44, d (7.9) | 8.43, d (7.8) | |||
| δNH | 7.32, s | 7.32, s | |||
| 6.78, s | 6.78, s | ||||
Recorded at 176 MHz.
Recorded at 700 MHz. nd: not detected.
Pseudobulbiferamide C (7) was isolated as a yellow oil with the molecular formula C36H49N11O10S. The 1H and 13C NMR data of 7 were highly similar to those of 5 (Table 1). Analysis of MS/MS fragmentation and 2D NMR spectra indicated that the Gly residue in 5 was replaced by an Ala residue in 7, as discerned by the COSY correlation between the Ala methyl protons (δH 1.23, d, J = 7.1 Hz) and the Ala α-proton (δH 4.30, dq, J = 7.2, 6.9 Hz), as well as HMBC correlations from the Ala methyl protons to the Ala amide carbonyl carbon (δC 172.3) (Figures S22–S31). As before, the configuration of Dhb residue was deduced to be Z by a ROESY correlation between its amide proton and methyl group. Thus, the planar structure of 7 was determined as Phe1-Dhb2-Ala3-Arg4-Pro5-Thz6-Gln7 with a ureido bond connecting the Phe1 and the Dhb2 residues (Figure 1D). Marfey’s analysis of 7 allowed assignments of the amino acids as L (Figures S32–S36). Akin to 6, MS/MS data demonstrated the presence of pseudobulbiferamide D (8) wherein the terminal Gln residue was omitted (Figure S37). Molecule 8 was produced in drastically reduced amounts which precluded isolation and NMR spectra acquisition. We also detected the presence of the shunt metabolite 9 comprising of just the Phe and Dhb residues connected via the ureido linkage. Examination of the NMR spectra allowed assignment of the Dhb Cα-Cβ double bond in 9 to be in the Z configuration (Figures S38–S44, Table S1). Marfey’s analysis allowed assignment of the Phe residue in 9 as l-Phe (Figure S45).
The structures of the pseudobulbiferamides are similar to the pseudovibriamides, hybrid nonribosomal peptide synthetase/polyketide synthase (NRPS/PKS) derived natural products isolated from extracts of a marine Pseudovibrio bacterium, which, akin to Microbulbifer sp. MKSA007, was isolated from a marine sponge. Crucially, the Phe1 residue in pseudobulbiferamides is replaced with Tyr in pseudovibriamides which distinguishes the two compound families.
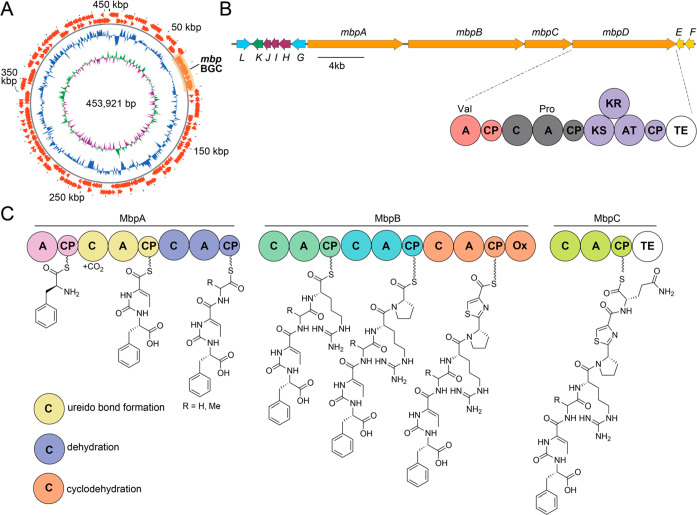
The biosynthesis of pseudovibriamides was mapped to the plasmid-encoded ppp BGC.8 The ppp BGC is widespread in marine Pseudovibrio strains. Sequencing and assembly of a draft genome of Microbulbifer sp. MKSA007 identified a highly similar BGC, which we term the Microbulbifer-derived ppp-like BGC (mbp BGC). Just like the ppp BGC, the mbp BGC is plasmid encoded (Figure 2A). The presence of the ppp and the mbp BGCs on plasmids could have functional relevance to the distribution of these BGCs and the corresponding ureidopeptidic natural products in marine invertebrate microbiomes. The organization of the mbp BGC wherein genes mbpA–C encode a heptamodular NRPS assembly line leads to a biosynthetic proposal for the production of pseudobulbiferamides (Figure 2B). Ureido bond formation, dehydration of the Thr side chain to Dhb and the Cys cyclodehydration to thiazoline is proposed to be catalyzed by the corresponding NRPS condensation domains.20−23 An oxidase domain at the C-terminus of the NRPS MbpB is proposed to catalyze the thiazoline to thiazole transformation. Of note is the presence of the mbpD gene which encodes two additional NRPS modules followed by a PKS module. The corresponding gene in the ppp BGC, pppD, extends the pseudovibriamide ureidoheptapeptides with an additional acetate unit and a prolyl dipeptide.8 The correspondingly extended congeners were not detected for the pseudobulbiferamides. Genes mbpE and mbpF encode a phosphopantetheinyl transferase and a thioesterase, respectively. Additional thioesterases are encoded at the C-termini of the MbpC and MbpD polypeptides. Putative transporters are encoded by mbpG and mbpL. The catalytic roles if any, of the unannotated open reading frames MbpH–J, and the α-ketoglutarate-dependent oxidase MbpK in the biosynthesis of pseudobulbiferamides are not apparent.
Figure 2.
(A) Map of the 454 kbp Microbulbifer sp. MKSA007 plasmid bearing the mbp BGC. From outside in predicted open reading frames (ORFs) on the leading and lagging DNA strands with the position of the mbp BGC highlighted are the normalized plot of GC content (blue) and normalized plot of GC skew (purple/green). (B) mbp BGC. (Abbreviations: A, adenylation; CP, carrier protein; C, condensation; Ox, oxidase; TE, thioesterase; KS, ketosynthase; KR, ketoreductase; and AT, acyl transferase). Hybrid NRPS-PKS MbpD is rationalized to contain two NRPS modules with predicted A-domain specificities for Val and Pro and a single PKS module. (C) Inferred assembly line biosynthesis of pseudobulbiferamides mediated by NRPSs MbpA–C. Three C domains, color-coded, conceivably catalyze ureido bond formation, dehydration of a Thr side chain, and Cys to thiazoline oxidation.
Mining the draft genome of the Microbulbifer sp. MKSA007 bacterium led to the identification of a second BGC encoding six NRPS modules. Unlike the mbp BGC, this second NRPS BGC was present on the bacterial chromosome and not on a plasmid. The organization of this BGC was highly similar to the bulbiferamide-producing bulb BGCs that we had previously described from two other marine Microbulbifer strains that were likewise cultured from marine sponge microbiomes, Microbulbifer sp. MLAF003 and Microbulbifer sp. VAAF005 (Figure 3A).13 Both, Microbulbifer sp. MLAF003 and Microbulbifer sp. VAAF005 constitutively produce bulbiferamides in liquid media, while neither strain produces pseudobulbiferamides. Despite the detection of the bulb BGC, no bulbiferamides were detected in liquid culture extracts of Microbulbifer sp. MKSA007 from which the pseudobulbiferamides were isolated.
Figure 3.
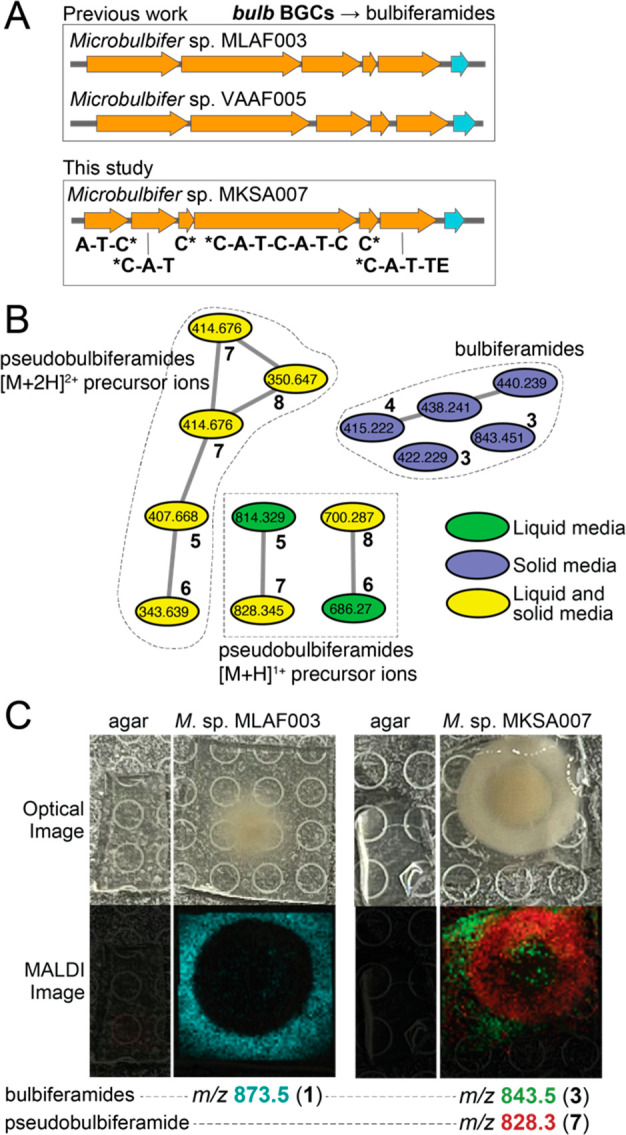
(A) bulb BGCs detected in Microbulbifer strains, Microbulbifer sp. MLAF003, Microbulbifer sp. VAAF005,13 and Microbulbifer sp. MKSA007. The domain organization of the Microbulbifer sp. MKSA007 bulb NRPSs is denoted. The gene colored cyan encodes a putative transporter. C domains marked by * are split between two polypeptides. (B) Molecular network with nodes denoting bulbiferamides and pseudobulbiferamides detected in Microbulbifer sp. MKSA007 culture extracts. Nodes colored green, blue, and yellow are detected in liquid, solid, and both liquid and solid media culture extracts, respectively. The precursor ion m/z are labeled for each node. Nodes corresponding to different ionization states for both classes of molecules do not cluster. (C) Imaging mass spectrometry denoting differential localization of bulbiferamides and pseudobulbiferamides for bulbiferamide-only producing Microbulbifer sp. MLAF003 (left) and bulbiferamide and pseudobulbiferamides producing Microbulbifer sp. MKSA007 (right). Different bulbiferamide congeners detected in the two bacterial strains.
To test whether changes in culture conditions might induce the production of bulb BGC-encoded bulbiferamides, using molecular networking,24 we queried the metabolomes of Microbulbifer sp. MKSA007 bacterium when cultured in liquid and in solid media. In both culture conditions, we detected the constitutive production of pseudobulbiferamides. In line with the presence of the bulb BGC in the chromosomal DNA, the production of bulbiferamides was indeed detected but only when Microbulbifer sp. MKSA007 was cultured on solid media; bulbiferamide production was not detected in liquid media for this strain (Figure 3B). These data establish that Microbulbifer sp. MKSA007 can produce two distinct families of ureidopeptide natural products: the hexapeptidic bulbiferamides that are derived from the chromosome encoded bulb BGC and the heptapeptidic pseudobulbiferamides that are derived from the plasmid encoded mbp BGC. Both families of molecules are produced in solid media, while only the pseudobulbiferamides are produced in liquid media.
The functional relevance, if any, of the coproduction of bulbiferamides and pseudobulbiferamides by the same bacterial strain, and how these two families of ureidopeptidic natural products influence each other’s production is presently not clear. Using imaging mass spectrometry, we detect that bulbiferamides produced by both bulbiferamide-only producing Microbulbifer sp. MLAF003 and bulbiferamide and pseudobulbiferamides producing Microbulbifer sp. MKSA007 are excreted out of the bacterial colony into the surrounding media. In contrast, pseudobulbiferamides are largely retained within the bacterial colony (Figure 3C). Hence, the two families of NRPS-derived ureidopeptidic natural products are fated to occupy different physical regions in Microbulbifer colonies. We know that pseudovibriamides mediate swarming behavior in marine Pseudovibrio bacteria;8 it is thus conceivable that bulbiferamides and/or pseudobulbiferamides also play fundamentally important roles in Microbulbifer organismal biology. The yet to be developed tools for the genetic manipulation of Microbulbifer bacteria will be instrumental in answering this lingering question and in the assignment of the physiological role(s) for these two classes of molecules in the Microbulbifer genus. With no detectable activity for either class of molecules against Gram-positive or Gram-negative bacteria, it is unlikely that these molecules are mediating competitive interactions in marine sponge microbiomes.
Experimental Section
General Experimental Procedures
Optical rotation, circular dichroism, and UV spectra were measured on a JASCO J-815 spectropolarimeter (JASCO). One-dimensional (1D) and two-dimensional (2D) NMR spectra were recorded on a Bruker Avance IIIHD 700 MHz NMR. The 1H and 13C NMR chemical shifts were referenced to the solvent peaks for DMSO-d6 at δH 2.50 and δC 39.52. High-resolution electrospray ionization mass data were recorded using a 1290 Infinity II ultraperformance liquid chromatography (UPLC, Agilent Technologies) coupled to a ImpactII ultrahigh-resolution Q-ToF mass spectrometer equipped with an electron spray ionization (ESI) source (Bruker Daltonics). A Kinetex C18 reversed phase UPLC column (50 × 2.1 mm, 1.7 μm) was used for chromatographic separation. Data were acquired in the positive ionization mode with m/z 50–2000 Da. Low-resolution electrospray ionization mass data were recorded on a 1260 Infinity high performance liquid chromatography (HPLC, Agilent Technologies) coupled to a amaZon mass spectrometer equipped with an electron spray ionization (ESI) source (Bruker Daltonics). A Poroshell 120 EC-C18 reverse phase HPLC column (100 × 4.6 mm, 5.0 μm) was used for chromatographic separation. Data were acquired in negative ionization mode with m/z 50–2000 Da. Analytical thin-layer chromatography (TLC) was carried out on silica gel 60 F254 aluminum-backed TLC plates (Merck), with compounds visualized using short-wave UV (254 nm) and by spraying with phosphomolybdic acid reagent followed by heating. Open column chromatography (CC) was performed over silica gel (60–200 mesh, SiliaFlash) and ODS (50 μm, Merck). Semipreparative HPLC was performed on an Agilent 1260 Infinity II HPLC system equipped with a VWD detector, using a Luna C18 reverse phase column (250 × 10 mm, 5 μm). All solvents used in CC and HPLC were analytical grade (VWR) and HPLC grade (Fisher and Sigma-Aldrich), respectively.
Liquid Fermentation, Extraction, and Compound Purification
The isolation of Microbulbifer sp. MKSA007 used in this study from a marine sponge has been previously described.11 Liquid fermentation and extraction were the same as previously described.13 The extract (3.1 g) was subjected to a silica gel column chromatography using stepwise gradient elution with CH2Cl2/MeOH (v/v, 20:1, 10:1, 5:1, 1:1, 0:1, each 600 mL) to yield 10 fractions (1–10). Fractions 7–10 (210 mg) were combined and further fractionated using semipreparative HPLC [C18 column, 250 × 10 mm, 5 μm, 10–100% MeCN/H2O (+0.1% v/v TFA) gradient across 25 min at 2 mL/min flow rate] to yield 5 (7.3 mg), 6 (0.9 mg), 7 (1.2 mg), and 9 (2.5 mg).
Pseudobulbiferamide A (5)
Yellow oil; [α]25D= −159.5 (c 0.2, MeOH); UV (MeOH) λmax (log ε) 200 (4.91, end absorption), 209 (4.80); ECD (MeOH) λmax (Δε) 219 (3.43), 235 (−3.04); 1H and 13C NMR data, Table 1; HRESIMS m/z 814.3294 [M + H]+ (calcd for C35H48N11O10S, 814.3301).
Pseudobulbiferamide B (6)
Yellow oil; [α]25D= −96.9 (c 0.1, MeOH); UV (MeOH); λmax (log ε) 200 (4.84, end absorption), 207 (4.76); ECD (MeOH) λmax (Δε) 220 (7.92), 241 (−5.83); 1H and 13C NMR data, Table S1; HRESIMS m/z 686.2714 [M + H]+ (calcd for C30H40N9O8S, 686.2715).
Pseudobulbiferamide C (7)
Yellow oil; [α]25D= −181.1 (c 0.2, MeOH); UV (MeOH) λmax (log ε) 200 (4.66, end absorption), 205 (4.60); ECD (MeOH) λmax (Δε) 210 (−12.69), 230 (−11.96); 1H and 13C NMR data, Table 1; HRESIMS m/z 828.3445 [M + H]+ (calcd for C36H50N11O10S, 828.3457).
Pseudobulbiferamide D (8)
Positive HRESIMS m/z 700.2872 [M + H]+ (calcd for C31H42N9O8S, 700.2872).
Compound 9
Yellow oil; [α]25D= −48.3 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 200 (4.42, end absorption), 207 (4.33); ECD (MeOH) λmax (Δε) 203 (16.09), 220 (10.11); 1H and 13C NMR data, Table S1; HRESIMS m/z 293.1122 [M + H]+ (calcd for C14H17N2O5, 293.1132).
Solid Cultivation and Extraction
Microbulbifer sp. MKSA007 was routinely cultured on three MB1/2 agar plates each containing 20 mL MB1/2 with 0.5% agar for 5 days at 30 °C. The agar media and cells were combined and frozen at −80 °C, followed by lyophilization. The dried media and cells were extracted with 40 mL MeOH and acetone by sonication for 15 min, separately. The MeOH and acetone extracts were recovered by filtration, combined, and dried in vacuo to yield an organic extract. The MB1/2 agar blank extract was prepared the same way.
Marfey’s Analysis
Compounds 5, 7, and 9, as well as amino acid standards were derivatized by Marfey’s reaction as previously described.13
MALDI-ToF Imaging Mass Spectrometry
A 1 μL inoculum of each isolate Microbulbifer sp. MLAF003 and Microbulbifer sp. MKSA007 (OD600 of 0.05 and 0.01) was spotted on MB1/2 agar (10 mL agar poured in a standard 10 cm Petri dish) and incubated at 30 °C for 4 and 3 days, respectively. The agar containing the colony and the uninoculated section of the agar were then excised from the Petri dish and gently placed on a Bruker Daltonics ground steel MALDI 96 anchor target plate. The recrystallized and finely powdered matrix of 1:1 2,5-dihydroxybenzoic acid:α-cyano-4-hydroxycinnaimic acid was applied to the sample using the sieve method and dried for ∼8 h at 30 °C and ∼1 h in a desiccator. For calibration of the instrument, peptide calibration standard (Bruker Daltonics Inc., 8206195) was mixed with matrix and spotted on the target plate next to the dried agar slice. All samples were imaged using rapifleX MALDI-TOF at a scan range of 25 × 25 μm resulting in a field size of 104 × 104 μm in reflectron positive mode in the mass range from 200 to 3520 m/z. The laser intensity and detector gain were optimized for individual experiments, and spectra were acquired using a M5 defocus as laser setting. Data were analyzed using Bruker Daltonics FlexImaging 5.0.
Acknowledgments
Genome sequencing and assembly was performed at the Georgia Genomics and Bioinformatics Core facility at the University of Georgia. The authors are grateful to the National Science Foundation (NSF) for grant support (IOS-2047235 to N.G. and CHE-2238650 to V.A.).
Data Availability Statement
The Microbulbifer sp. MKSA007 genome has been deposited in GenBank with the bioproject accession number PRJNA941849. Mass spectrometry fragmentation spectra for pseudobulbiferamides have been deposited to spectral databases as outlined in Table S2. NMR spectra for molecules 5–7 and 9 have been deposited to the NP-MRD database (NP0331779, NP0331780, NP0331778, and NP0331781, respectively).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jnatprod.3c00595.
High-resolution mass spectra for 5–9; 1H, 13C, DEPT, HSQC, HMBC, COSY, TOCSY, and ROESY NMR spectra for 5–7 and 9; and tables of NMR data for 6 and 9 (PDF)
Author Contributions
∥ N.M.A. and J.M.D. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Gleadow R. M.; Møller B. L. Cyanogenic glycosides: synthesis, physiology, and phenotypic plasticity. Annual Review of Plant Biology 2014, 65 (1), 155–185. 10.1146/annurev-arplant-050213-040027. [DOI] [PubMed] [Google Scholar]
- Becher P. G.; Verschut V.; Bibb M. J.; Bush M. J.; Molnár B. P.; Barane E.; Al-Bassam M. M.; Chandra G.; Song L.; Challis G. L.; Buttner M. J.; Flärdh K. Developmentally regulated volatiles geosmin and 2-methylisoborneol attract a soil arthropod to Streptomyces bacteria promoting spore dispersal. Nat. Microbiol. 2020, 5 (6), 821–829. 10.1038/s41564-020-0697-x. [DOI] [PubMed] [Google Scholar]
- Beemelmanns C.; Guo H.; Rischer M.; Poulsen M. Natural products from microbes associated with insects. Beilstein J. Org. Chem. 2016, 12, 314–327. 10.3762/bjoc.12.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y.-M.; Hirschmann M.; Shi Y.-N.; Ahmed S.; Abebew D.; Tobias N. J.; Grün P.; Crames J. J.; Pöschel L.; Kuttenlochner W.; Richter C.; Herrmann J.; Müller R.; Thanwisai A.; Pidot S. J.; Stinear T. P.; Groll M.; Kim Y.; Bode H. B. Global analysis of biosynthetic gene clusters reveals conserved and unique natural products in entomopathogenic nematode-symbiotic bacteria. Nat. Chem. 2022, 14 (6), 701–712. 10.1038/s41557-022-00923-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster M.; Joseph Sexton D.; Diggle S. P.; Peter Greenberg E. Acyl-homoserine lactone quorum sensing: from evolution to application. Annu. Rev. Microbiol. 2013, 67 (1), 43–63. 10.1146/annurev-micro-092412-155635. [DOI] [PubMed] [Google Scholar]
- Paul N. A.; de Nys R.; Steinberg P. D. Chemical defence against bacteria in the red alga Asparagopsis armata: linking structure with function. Mar. Ecol.: Prog. Ser. 2006, 306, 87–101. 10.3354/meps306087. [DOI] [Google Scholar]
- Puglisi M. P.; Sneed J. M.; Ritson-Williams R.; Young R. Marine chemical ecology in benthic environments. Nat. Prod. Rep. 2019, 36 (3), 410–429. 10.1039/C8NP00061A. [DOI] [PubMed] [Google Scholar]
- Ióca L. P.; Dai Y.; Kunakom S.; Diaz-Espinosa J.; Krunic A.; Crnkovic C. M.; Orjala J.; Sanchez L. M.; Ferreira A. G.; Berlinck R. G. S.; Eustáquio A. S. A family of nonribosomal peptides modulate collective behavior in Pseudovibrio bacteria isolated from marine sponges. Angew. Chem., Int. Ed. 2021, 60 (29), 15891–15898. 10.1002/anie.202017320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano S. Ecology and biotechnological potential of bacteria belonging to the genus Pseudovibrio. Appl. Environ. Microbiol. 2018, 84 (8), e02516. 10.1128/AEM.02516-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns D. B. A field guide to bacterial swarming motility. Nature Reviews Microbiology 2010, 8 (9), 634–644. 10.1038/nrmicro2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch J. M.; Green M. O.; Akavaram P.; Davis A. C.; Diskalkar S. S.; Du Plessis I. A.; Fallon H. A.; Grason E. M.; Kauf E. G.; Kim Z. M.; Miller J. R.; Neal A. L.; Riera T.; Stroeva S.-E.; Tran J.; Tran V.; Coronado A. V.; Coronado V. V.; Wall B. T.; Yang C. M.; Mohanty I.; Abrahamse N. H.; Freeman C. J.; Easson C. G.; Fiore C. L.; Onstine A. E.; Djeddar N.; Biliya S.; Bryksin A. V.; Garg N.; Agarwal V. Limited metabolomic overlap between commensal bacteria and marine sponge holobionts revealed by large scale culturing and mass spectrometry-based metabolomics: an undergraduate laboratory pedagogical effort at Georgia Tech. Marine Drugs 2023, 21 (1), 53. 10.3390/md21010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dat T. T. H.; Steinert G.; Cuc N. T. K.; Smidt H.; Sipkema D.. Bacteria cultivated from sponges and bacteria not yet cultivated from sponges—a review. Front. Microbiol. 2021, 12, 10.3389/fmicb.2021.737925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W.; Deutsch J. M.; Yi D.; Abrahamse N. H.; Mohanty I.; Moore S. G.; McShan A. C.; Garg N.; Agarwal V. Discovery and biosynthesis of ureidopeptide natural products macrocyclized via indole N-acylation in marine Microbulbifer spp. bacteria. ChemBioChem. 2023, 24 (12), e202300190 10.1002/cbic.202300190. [DOI] [PubMed] [Google Scholar]
- Lu S.; Zhang Z.; Sharma A. R.; Nakajima-Shimada J.; Harunari E.; Oku N.; Trianto A.; Igarashi Y. Bulbiferamide, an antitrypanosomal hexapeptide cyclized via an N-acylindole linkage from a marine obligate Microbulbifer. J. Nat. Prod. 2023, 86 (4), 1081–1086. 10.1021/acs.jnatprod.2c01083. [DOI] [PubMed] [Google Scholar]
- Mohanty I.; Tapadar S.; Moore S. G.; Biggs J. S.; Freeman C. J.; Gaul D. A.; Garg N.; Agarwal V. Presence of bromotyrosine alkaloids in marine sponges is independent of metabolomic and microbiome architectures. mSystems 2021, 6 (2), e01387 10.1128/mSystems.01387-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty I.; Nguyen N. A.; Moore S. G.; Biggs J. S.; Gaul D. A.; Garg N.; Agarwal V. Enzymatic synthesis assisted discovery of proline-rich macrocyclic peptides in marine sponges. Chembiochem 2021, 22 (16), 2614–2618. 10.1002/cbic.202100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao X.; Yu J.; Wang Y.; Connolly J. A.; Liu Y.; Zhang Y.; Yu L.; Cen S.; Goss R. J. M.; Gan M. Zelkovamycins B-E, cyclic octapeptides containing rare amino acid residues from an endophytic Kitasatospora sp. Org. Lett. 2020, 22 (23), 9346–9350. 10.1021/acs.orglett.0c03565. [DOI] [PubMed] [Google Scholar]
- Kanki D.; Nakamukai S.; Ogura Y.; Takikawa H.; Ise Y.; Morii Y.; Yamawaki N.; Takatani T.; Arakawa O.; Okada S.; Matsunaga S. Homophymamide A, heterodetic cyclic tetrapeptide from a Homophymia sp. marine sponge: a cautionary note on configurational assignment of peptides that contain a ureido linkage. J. Nat. Prod. 2021, 84 (6), 1848–1853. 10.1021/acs.jnatprod.1c00336. [DOI] [PubMed] [Google Scholar]
- Cheruku P.; Plaza A.; Lauro G.; Keffer J.; Lloyd J. R.; Bifulco G.; Bewley C. A. Discovery and synthesis of namalide reveals a new anabaenopeptin scaffold and peptidase inhibitor. J. Med. Chem. 2012, 55 (2), 735–742. 10.1021/jm201238p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imker H. J.; Walsh C. T.; Wuest W. M. SylC catalyzes ureido-bond formation during biosynthesis of the proteasome inhibitor syringolin A. J. Am. Chem. Soc. 2009, 131 (51), 18263–18265. 10.1021/ja909170u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patteson J. B.; Fortinez C. M.; Putz A. T.; Rodriguez-Rivas J.; Bryant L. H. III; Adhikari K.; Weigt M.; Schmeing T. M.; Li B. Structure and function of a dehydrating condensation domain in nonribosomal peptide biosynthesis. J. Am. Chem. Soc. 2022, 144 (31), 14057–14070. 10.1021/jacs.1c13404. [DOI] [PubMed] [Google Scholar]
- Duerfahrt T.; Eppelmann K.; Müller R.; Marahiel M. A. Rational design of a bimodular model system for the investigation of heterocyclization in nonribosomal peptide biosynthesis. Chem. Biol. 2004, 11 (2), 261–271. 10.1016/j.chembiol.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Bloudoff K.; Schmeing T. M. Structural and functional aspects of the nonribosomal peptide synthetase condensation domain superfamily: discovery, dissection and diversity. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics 2017, 1865 (11), 1587–1604. 10.1016/j.bbapap.2017.05.010. [DOI] [PubMed] [Google Scholar]
- Wang M.; Carver J. J; Phelan V. V; Sanchez L. M; Garg N.; Peng Y.; Nguyen D. D.; Watrous J.; Kapono C. A; Luzzatto-Knaan T.; Porto C.; Bouslimani A.; Melnik A. V; Meehan M. J; Liu W.-T.; Crusemann M.; Boudreau P. D; Esquenazi E.; Sandoval-Calderon M.; Kersten R. D; Pace L. A; Quinn R. A; Duncan K. R; Hsu C.-C.; Floros D. J; Gavilan R. G; Kleigrewe K.; Northen T.; Dutton R. J; Parrot D.; Carlson E. E; Aigle B.; Michelsen C. F; Jelsbak L.; Sohlenkamp C.; Pevzner P.; Edlund A.; McLean J.; Piel J.; Murphy B. T; Gerwick L.; Liaw C.-C.; Yang Y.-L.; Humpf H.-U.; Maansson M.; Keyzers R. A; Sims A. C; Johnson A. R; Sidebottom A. M; Sedio B. E; Klitgaard A.; Larson C. B; Boya P C. A; Torres-Mendoza D.; Gonzalez D. J; Silva D. B; Marques L. M; Demarque D. P; Pociute E.; O'Neill E. C; Briand E.; Helfrich E. J N; Granatosky E. A; Glukhov E.; Ryffel F.; Houson H.; Mohimani H.; Kharbush J. J; Zeng Y.; Vorholt J. A; Kurita K. L; Charusanti P.; McPhail K. L; Nielsen K. F.; Vuong L.; Elfeki M.; Traxler M. F; Engene N.; Koyama N.; Vining O. B; Baric R.; Silva R. R; Mascuch S. J; Tomasi S.; Jenkins S.; Macherla V.; Hoffman T.; Agarwal V.; Williams P. G; Dai J.; Neupane R.; Gurr J.; Rodriguez A. M C; Lamsa A.; Zhang C.; Dorrestein K.; Duggan B. M; Almaliti J.; Allard P.-M.; Phapale P.; Nothias L.-F.; Alexandrov T.; Litaudon M.; Wolfender J.-L.; Kyle J. E; Metz T. O; Peryea T.; Nguyen D.-T.; VanLeer D.; Shinn P.; Jadhav A.; Muller R.; Waters K. M; Shi W.; Liu X.; Zhang L.; Knight R.; Jensen P. R; Palsson B. Ø; Pogliano K.; Linington R. G; Gutierrez M.; Lopes N. P; Gerwick W. H; Moore B. S; Dorrestein P. C; Bandeira N. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34 (8), 828–837. 10.1038/nbt.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Microbulbifer sp. MKSA007 genome has been deposited in GenBank with the bioproject accession number PRJNA941849. Mass spectrometry fragmentation spectra for pseudobulbiferamides have been deposited to spectral databases as outlined in Table S2. NMR spectra for molecules 5–7 and 9 have been deposited to the NP-MRD database (NP0331779, NP0331780, NP0331778, and NP0331781, respectively).