Abstract
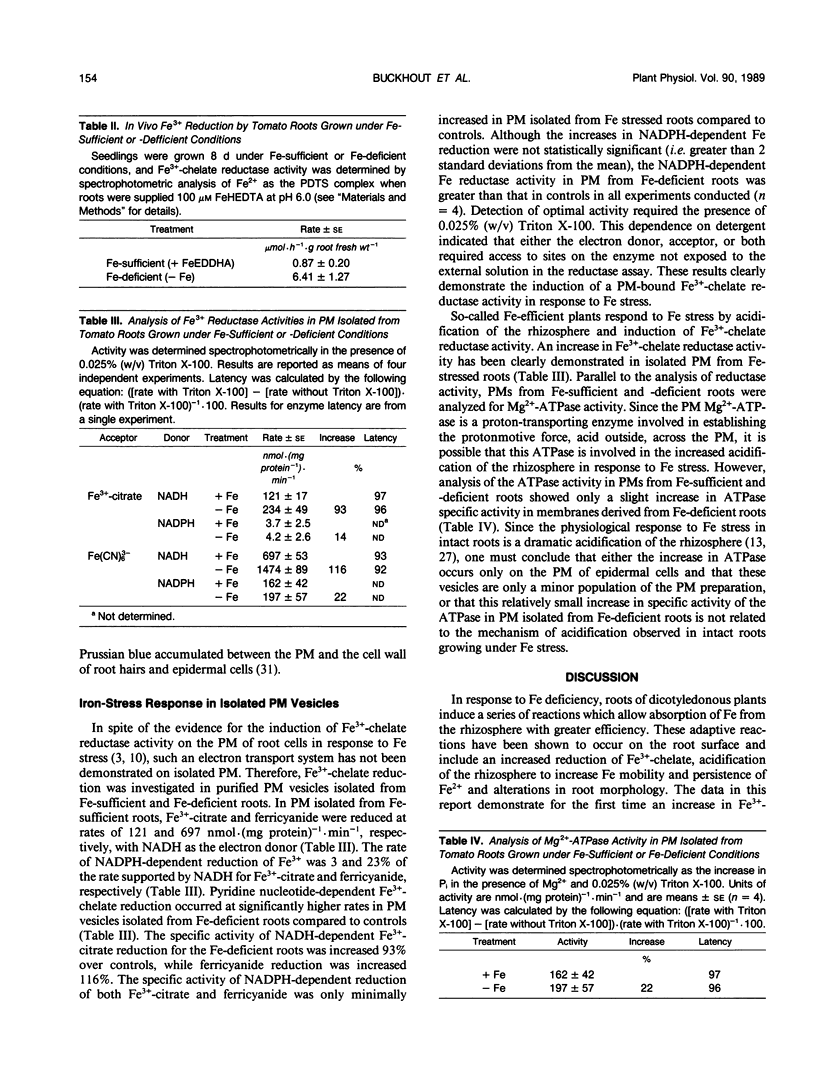
Tomato plants (Lycopersicum esculentum Mill.) were grown for 21-days in a complete hydroponic nutrient solution including Fe3+-ethylenediamine-di(o-hydroxyphenylacetate) and subsequently switched to nutrient solution withholding Fe for 8 days to induce Fe stress. The roots of Fe-stressed plants reduced chelated Fe at rates sevenfold higher than roots of plants grown under Fe-sufficient conditions. The response in intact Fe-deficient roots was localized to root hairs, which developed on secondary roots during the period of Fe stress. Plasma membranes (PM) isolated by aqueous two-phase partitioning from tomato roots grown under Fe stress exhibited a 94% increase in rates of NADH-dependent Fe3+-citrate reduction compared to PM isolated from roots of Fe-sufficient plants. Optimal detection of the reductase activity required the presence of detergent indicating structural latency. In contrast, NADPH-dependent Fe3+-citrate reduction was not significantly different in root PM isolated from Fe-deficient versus Fe-sufficient plants and proceeded at substantially lower rates than NADH-dependent reduction. Mg2+-ATPase activity was increased 22% in PM from roots of Fe-deficient plants compared to PM isolated from roots of Fe-sufficient plants. The results localized the increase in Fe reductase activity in roots grown under Fe stress to the PM.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett-Lennard E. G., Marschner H., Römheld V. Mechanism of Short Term Fe Reduction by Roots : Evidence against the Role of Secreted Reductants. Plant Physiol. 1983 Dec;73(4):893–898. doi: 10.1104/pp.73.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienfait H. F. Regulated redox processes at the plasmalemma of plant root cells and their function in iron uptake. J Bioenerg Biomembr. 1985 Apr;17(2):73–83. doi: 10.1007/BF00744199. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cakmak I., van de Wetering D. A., Marschner H., Bienfait H. F. Involvement of superoxide radical in extracellular ferric reduction by iron-deficient bean roots. Plant Physiol. 1987 Sep;85(1):310–314. doi: 10.1104/pp.85.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaney R. L., Brown J. C., Tiffin L. O. Obligatory reduction of ferric chelates in iron uptake by soybeans. Plant Physiol. 1972 Aug;50(2):208–213. doi: 10.1104/pp.50.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane F. L., Sun I. L., Clark M. G., Grebing C., Löw H. Transplasma-membrane redox systems in growth and development. Biochim Biophys Acta. 1985 Aug 1;811(3):233–264. doi: 10.1016/0304-4173(85)90013-8. [DOI] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Landsberg E. C. Function of Rhizodermal Transfer Cells in the Fe Stress Response Mechanism of Capsicum annuum L. Plant Physiol. 1986 Oct;82(2):511–517. doi: 10.1104/pp.82.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter R., van Zwieten R., Weening R. S., Hamers M. N., Roos D. Cytochrome b, flavins, and ubiquinone-50 in enucleated human neutrophils (polymorphonuclear leukocyte cytoplasts). J Biol Chem. 1984 Aug 10;259(15):9603–9606. [PubMed] [Google Scholar]
- Römheld V., Marschner H. Mechanism of iron uptake by peanut plants : I. Fe reduction, chelate splitting, and release of phenolics. Plant Physiol. 1983 Apr;71(4):949–954. doi: 10.1104/pp.71.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römheld V., Müller C., Marschner H. Localization and capacity of proton pumps in roots of intact sunflower plants. Plant Physiol. 1984 Nov;76(3):603–606. doi: 10.1104/pp.76.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijmons P. C., Lanfermeijer F. C., de Boer A. H., Prins H. B., Bienfait H. F. Depolarization of Cell Membrane Potential during Trans-Plasma Membrane Electron Transfer to Extracellular Electron Acceptors in Iron-Deficient Roots of Phaseolus vulgaris L. Plant Physiol. 1984 Dec;76(4):943–946. doi: 10.1104/pp.76.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijmons P. C., van den Briel W., Bienfait H. F. Cytosolic NADPH is the electron donor for extracellular fe reduction in iron-deficient bean roots. Plant Physiol. 1984 May;75(1):219–221. doi: 10.1104/pp.75.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipton C. L., Thowsen J. Fe reduction in cell walls of soybean roots. Plant Physiol. 1985 Oct;79(2):432–435. doi: 10.1104/pp.79.2.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos C. R., Lubberding H. J., Bienfait H. F. Rhizosphere acidification as a response to iron deficiency in bean plants. Plant Physiol. 1986 Jul;81(3):842–846. doi: 10.1104/pp.81.3.842. [DOI] [PMC free article] [PubMed] [Google Scholar]


