Abstract
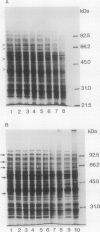
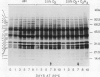
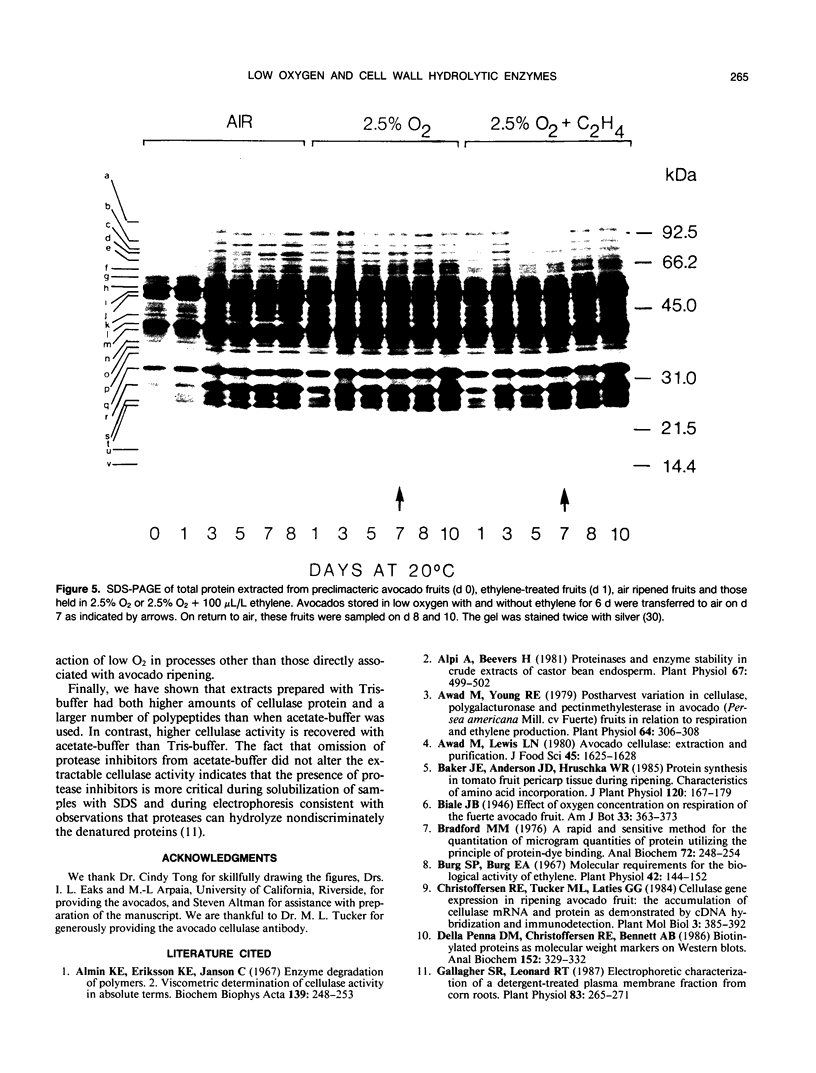
The effect of 2.5% O2 atmosphere with and without ethylene on the activities of hydrolytic enzymes associated with cell walls, and total protein profile during ripening of avocado fruits (Persea americana Mill., cv Hass) were investigated. The low 2.5% O2 atmosphere prevented the rise in the activities of cellulase, polygalacturonase, and acid phosphatase in avocado fruits whose ripening was initiated with ethylene. Addition of 100 microliters per liter ethylene to low O2 atmosphere did not alter these suppressive effects of 2.5% O2. Furthermore, 2.5% O2 atmosphere delayed the development of a number of polypeptides that appear during ripening of avocado fruits while at the same time new polypeptides accumulated. The composition of the extraction buffer and its pH greatly affected the recovery of cellulase activity and its total immunoreactive protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almin K. E., Eriksson K. E., Jansson C. Enzymic degradation of polymers. II. Viscometric determination of cellulase activity in absolute terms. Biochim Biophys Acta. 1967 Jul 11;139(2):248–253. doi: 10.1016/0005-2744(67)90029-0. [DOI] [PubMed] [Google Scholar]
- Alpi A., Beevers H. Proteinases and enzyme stability in crude extracts of castor bean endosperm. Plant Physiol. 1981 Mar;67(3):499–502. doi: 10.1104/pp.67.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad M., Young R. E. Postharvest Variation in Cellulase, Polygalacturonase, and Pectinmethylesterase in Avocado (Persea americana Mill, cv. Fuerte) Fruits in Relation to Respiration and Ethylene Production. Plant Physiol. 1979 Aug;64(2):306–308. doi: 10.1104/pp.64.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. Molecular requirements for the biological activity of ethylene. Plant Physiol. 1967 Jan;42(1):144–152. doi: 10.1104/pp.42.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Penna D., Christoffersen R. E., Bennett A. B. Biotinylated proteins as molecular weight standards on Western blots. Anal Biochem. 1986 Feb 1;152(2):329–332. doi: 10.1016/0003-2697(86)90417-3. [DOI] [PubMed] [Google Scholar]
- Gallagher S. R., Leonard R. T. Electrophoretic characterization of a detergent-treated plasma membrane fraction from corn roots. Plant Physiol. 1987 Feb;83(2):265–271. doi: 10.1104/pp.83.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin C. Y., Key J. L. Dissocation and reassembly of polyribosomes in relation to protein synthesis in the soybean root. J Mol Biol. 1967 Jun 14;26(2):237–247. doi: 10.1016/0022-2836(67)90294-x. [DOI] [PubMed] [Google Scholar]
- Mattoo A. K., Pick U., Hoffman-Falk H., Edelman M. The rapidly metabolized 32,000-dalton polypeptide of the chloroplast is the "proteinaceous shield" regulating photosystem II electron transport and mediating diuron herbicide sensitivity. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1572–1576. doi: 10.1073/pnas.78.3.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesis E., Fuchs Y., Zauberman G. Cellulase activity and fruit softening in avocado. Plant Physiol. 1978 Mar;61(3):416–419. doi: 10.1104/pp.61.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J. A. Acid Phosphatase Development during Ripening of Avocado. Plant Physiol. 1975 Feb;55(2):382–385. doi: 10.1104/pp.55.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs M. M., Freeling M., Okimoto R. The anaerobic proteins of maize. Cell. 1980 Jul;20(3):761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Solomos T., Laties G. G. Similarities between the Actions of Ethylene and Cyanide in Initiating the Climacteric and Ripening of Avocados. Plant Physiol. 1974 Oct;54(4):506–511. doi: 10.1104/pp.54.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker M. L., Laties G. G. Interrelationship of Gene Expression, Polysome Prevalence, and Respiration during Ripening of Ethylene and/or Cyanide-Treated Avocado Fruit. Plant Physiol. 1984 Feb;74(2):307–315. doi: 10.1104/pp.74.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]