Abstract
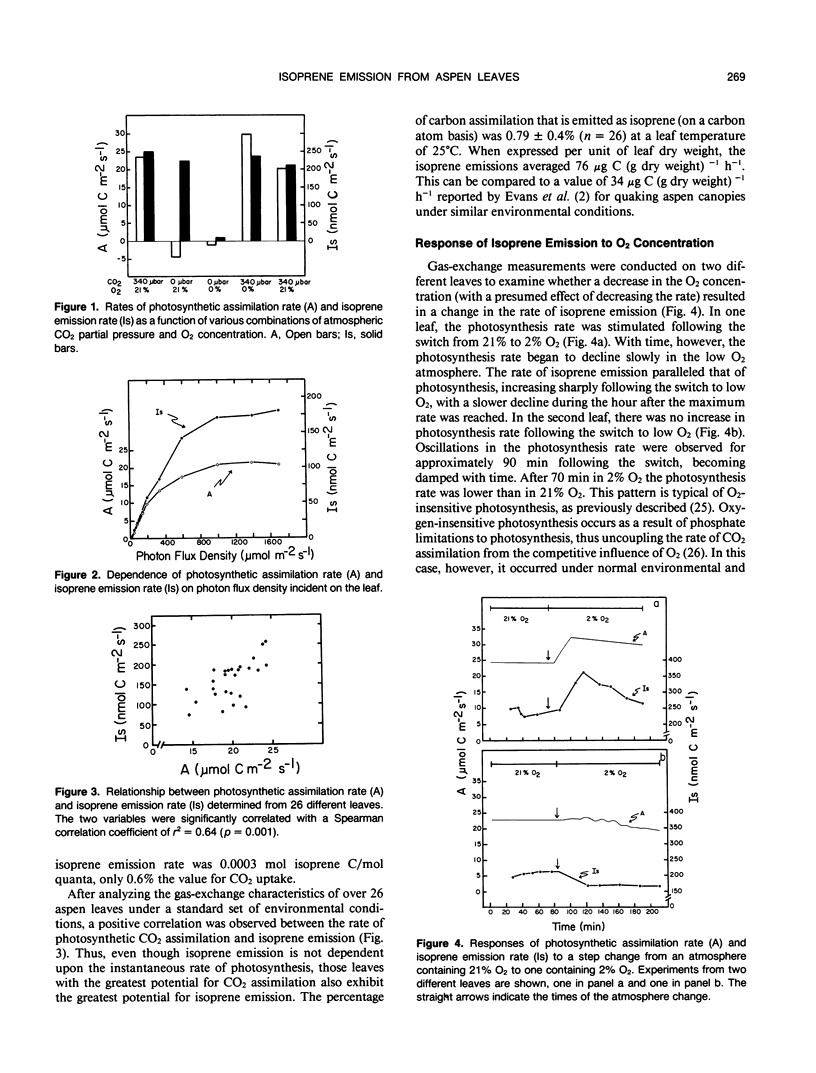
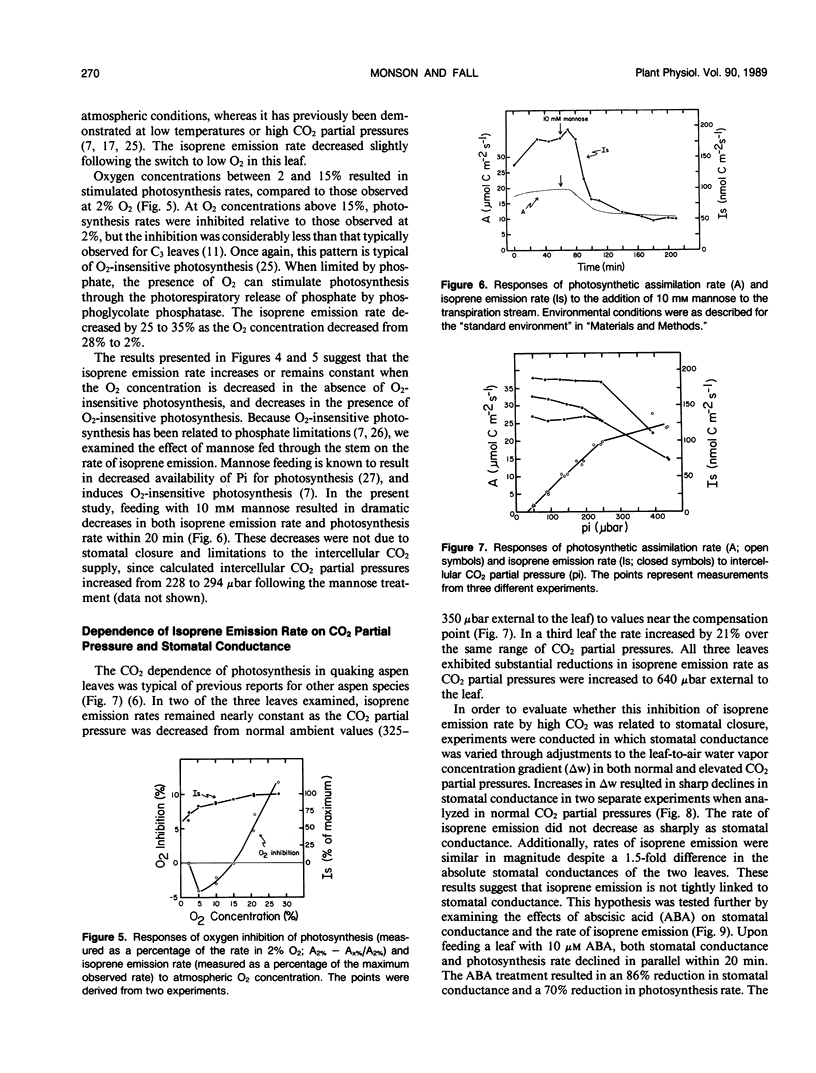
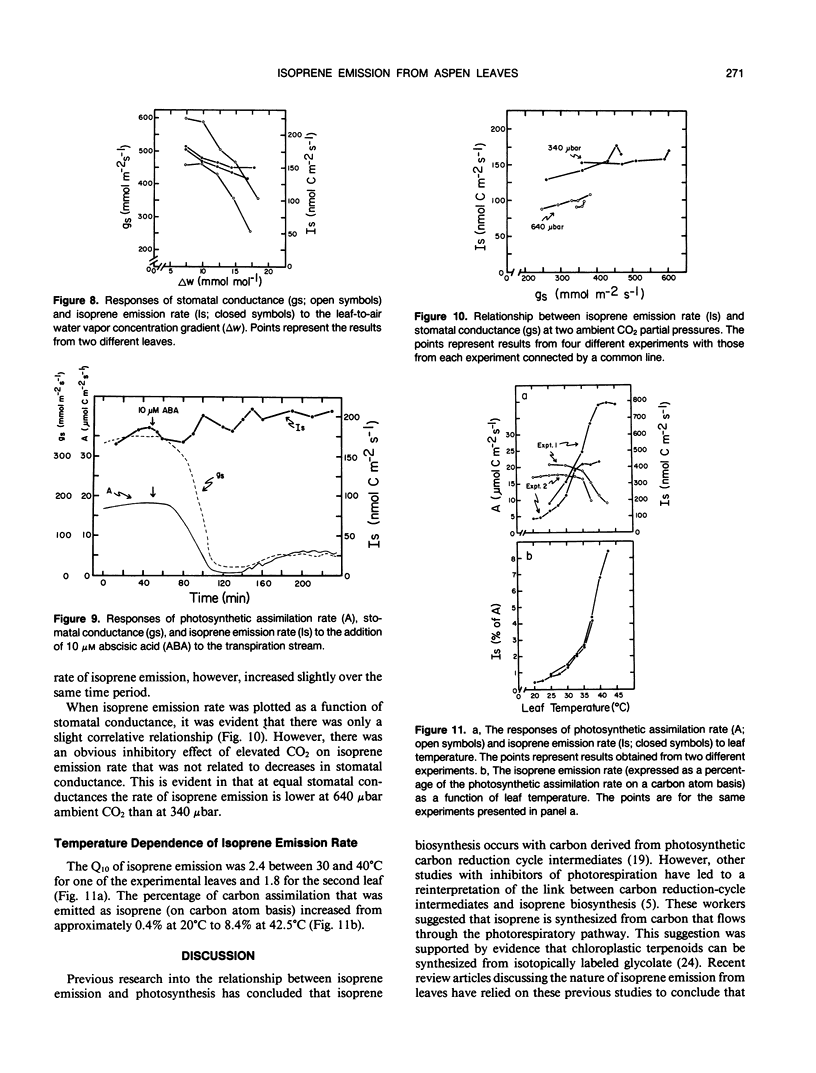
Isoprene emission rates from quaking aspen (Populus tremuloides Michx.) leaves were measured simultaneously with photosynthesis rate, stomatal conductance, and intercellular CO2 partial pressure. Isoprene emission required the presence of CO2 or O2, but not both. The light response of isoprene emission rate paralleled that of photosynthesis. Isoprene emission was inhibited by decreasing ambient O2 from 21% to 2%, only when there was oxygen insensitive photosynthesis. Mannose (10 millimolar) fed through cut stems resulted in strong inhibition of isoprene emission rate and is interpreted as evidence that isoprene biosynthesis requires either the export of triose phosphates from the chloroplast, or the continued synthesis of ATP. Light response experiments suggest that photosynthetically generated reductant or ATP is required for isoprene biosynthesis. Isoprene biosynthesis and emission are not directly linked to glycolate production through photorespiration, contrary to previous reports. Isoprene emission rate was inhibited by above-ambient CO2 partial pressures (640 microbar outside and 425 microbar inside the leaf). The inhibition was not due to stomatal closure. This was established by varying ambient humidity at normal and elevated CO2 partial pressures to measure isoprene emission rates over a range of stomatal conductances. Isoprene emission rates were inhibited at elevated CO2 despite no change in stomatal conductance. Addition of abscisic acid to the transpiration stream dramatically inhibited stomatal conductance and photosynthesis rate, with a slight increase in isoprene emission rate. Thus, isoprene emission is independent of stomatal conductance, and may occur through the cuticle. Temperature had an influence on isoprene emission rate, with the Q10 being 1.8 to 2.4 between 35 and 45°C. At these high temperatures the amount of carbon lost through isoprene emission was between 2.5 and 8% of that assimilated through photosynthesis. This represents a significant carbon cost that should be taken into account in determining midsummer carbon budgets for plants that are isoprene emitters.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Harris G. C., Cheesbrough J. K., Walker D. A. Measurement of CO(2) and H(2)O Vapor Exchange in Spinach Leaf Discs : Effects of Orthophosphate. Plant Physiol. 1983 Jan;71(1):102–107. doi: 10.1104/pp.71.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. A., Rasmussen R. A. Production of isoprene by leaf tissue. Plant Physiol. 1975 Jun;55(6):982–987. doi: 10.1104/pp.55.6.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurik T. W., Weber J. A., Gates D. M. Short-Term Effects of CO(2) on Gas Exchange of Leaves of Bigtooth Aspen (Populus grandidentata) in the Field. Plant Physiol. 1984 Aug;75(4):1022–1026. doi: 10.1104/pp.75.4.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütke-Brinkhaus F., Liedvogel B., Kleinig H. On the biosynthesis of ubiquinones in plant mitochondria. Eur J Biochem. 1984 Jun 15;141(3):537–541. doi: 10.1111/j.1432-1033.1984.tb08226.x. [DOI] [PubMed] [Google Scholar]
- Monson R. K., Stidham M. A., Williams G. J., Edwards G. E., Uribe E. G. Temperature Dependence of Photosynthesis in Agropyron smithii Rydb. : I. FACTORS AFFECTING NET CO(2) UPTAKE IN INTACT LEAVES AND CONTRIBUTION FROM RIBULOSE-1,5-BISPHOSPHATE CARBOXYLASE MEASURED IN VIVO AND IN VITRO. Plant Physiol. 1982 Apr;69(4):921–928. doi: 10.1104/pp.69.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney H. A., Vitousek P. M., Matson P. A. Exchange of materials between terrestrial ecosystems and the atmosphere. Science. 1987 Nov 13;238(4829):926–932. doi: 10.1126/science.238.4829.926. [DOI] [PubMed] [Google Scholar]
- Rasmussen R. A. What do the hydrocarbons from trees contribute to air pollution? J Air Pollut Control Assoc. 1972 Jul;22(7):537–543. doi: 10.1080/00022470.1972.10469676. [DOI] [PubMed] [Google Scholar]
- Sage R. F., Sharkey T. D. The Effect of Temperature on the Occurrence of O(2) and CO(2) Insensitive Photosynthesis in Field Grown Plants. Plant Physiol. 1987 Jul;84(3):658–664. doi: 10.1104/pp.84.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S. P., Rogers L. J. Compartmentation of terpenoid biosynthesis in green plants. A proposed route of acetyl-coenzyme A synthesis in maize chloroplasts. Biochem J. 1969 Sep;114(2):395–405. doi: 10.1042/bj1140395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey T. D. O(2)-insensitive photosynthesis in c(3) plants : its occurrence and a possible explanation. Plant Physiol. 1985 May;78(1):71–75. doi: 10.1104/pp.78.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey T. D., Stitt M., Heineke D., Gerhardt R., Raschke K., Heldt H. W. Limitation of Photosynthesis by Carbon Metabolism : II. O(2)-Insensitive CO(2) Uptake Results from Limitation Of Triose Phosphate Utilization. Plant Physiol. 1986 Aug;81(4):1123–1129. doi: 10.1104/pp.81.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]


