Abstract
Background:
Epilepsy is one of the most common chronic brain diseases. Almost one-third of patients have drug-resistant epilepsy (DRE). Cannabidiol is being considered as a potential novel drug for treating DRE.
Objectives:
To investigate long-term efficacy and safety of cannabidiol in treatment of DRE and the differences in cannabidiol treatment among patients with different characteristics.
Design:
Systematic review and meta-analysis.
Data sources and methods:
Medline, Embase, and CENTRAL were searched for literature. RevMan5.4 was used for meta-analysis. The Intention-to-treat set and the random effect were used as the main analysis. Subgroup analyses were performed according to age, dose, concomitant antiseizure medications (ASMs), epilepsy syndromes, and study designs.
Results:
Fifty studies were included in this systematic review. A total of 4791 participants were collected. The responder rates (seizure frequency reduced at least 50%) at 12-, 24-, 48-, 72-, 96-, and 144-week were 0.40 [0.36, 0.45], 0.39 [0.34, 0.44], 0.37 [0.30, 0.44], 0.27 [0.17, 0.37], 0.22 [0.14, 0.30], and 0.38 [0.23, 0.53]. Seizure-free rates were 0.04 [0.03, 0.06], 0.04 [0.03, 0.05], 0.03 [0.02, 0.05], 0.03 [0.02, 0.03], 0.02 [0.01, 0.03], and 0.04 [0.01, 0.06]. Proportion of adverse events were 0.72 [0.61, 0.83], 0.62 [0.42, 0.81], 0.60 [0.41, 0.79], 0.35 [0.14, 0.56], 0.83 [0.75, 0.90], and 0.96 [0.94, 0.99]. The pooled 12-, 24-, 48-, 96-, and 144-week proportion of serious adverse events were 0.15 [0.09, 0.21], 0.23 [0.14, 0.31], 0.10 [0.06, 0.15], 0.31 [0.24, 0.38], and 0.40 [0.35, 0.45]. Subgroup analyses showed that there was no significant difference on efficacy and safety among age subgroups and epilepsy syndromes subgroups. For most periods, there were no significant difference on efficacy among subgroups of dose and concomitant ASMs. However, higher doses and more concomitant ASMs were associated with higher proportion of adverse events.
Conclusion:
Cannabidiol treatment of DRE has stable efficacy and fewer adverse events in early period. Long-term use may have decreased efficacy and increased adverse events. Dose escalation may not increase efficacy, but may increase adverse events. Furthermore, cannabidiol use may reduce dosage of other ASMs without reducing efficacy, thereby reducing adverse effects. Cannabidiol may have similar effects in various epilepsy syndromes.
Trial registration:
PROSPERO (CRD42022351250).
Keywords: antiseizure medications, drug combination, intractable epilepsy, marijuana
Introduction
Epilepsy is one of the most common chronic brain diseases, affecting nearly every age group. Epilepsy is the third most common neurological disorder in the global burden of disease, affecting an estimated 65 million people worldwide with a prevalence of approximately 6 cases per 1000 people and an annual incidence of approximately 68 cases per 100,000 people. 1 Currently, the treatment of epilepsy is primarily based on pharmacotherapy, and most people have a good prognosis. Antiseizure medications (ASMs) have been used successfully to treat about 65% of patients.1,2 Despite the discovery of many new ASMs in recent years, almost a third of patients still show resistance to multiple anticonvulsant therapies, which are referred to as drug-resistant epilepsy (DRE).1,3,4 Based on the consensus proposed by the International League Against Epilepsy, DRE is defined as ‘the failure of two tolerable and appropriately selected and used tolerable ASMs regimens, either as monotherapy or in combination therapy, to achieve sustained seizures freedom’. 5 DRE is associated with an increased risk of injury and death, increased medication burden and adverse events, increased psychiatric and neurocognitive comorbidities, socioeconomic disadvantage, and a reduction in the quality of life.1,3,4 This is despite the growing number of treatments available for DRE, including neuromodulation, surgery, and dietary interventions.1,3 Epilepsy surgery in particular can provide seizure-free outcomes in up to 80% of patients with DRE. 4 Surgical treatment, however, depends on the nature and location of the epileptogenic zone and is not appropriate for every patient. Other alternatives include ketogenic diets, neuromodulation, or biofeedback, but these regimens are not superior to further ASMs treatment.1,4 As a result, the search for novel ASMs remains one of the research hotspots in the treatment of DRE.
Recently, there has been strong interest in the use of cannabis for DRE. Cannabidiol (CBD) is being considered as a potential novel drug for treating DRE. The effectiveness of CBD derivatives in the treatment of epilepsy has been documented since antiquity, and the major active ingredients in the treatment of epilepsy are tetrahydrocannabinol (THC) and CBD. 6 Although the mechanism of the anticonvulsant action of CBD is not completely understood, in animal models of epilepsy, CBD has been shown to be capable of inhibiting seizures and has anti-inflammatory, neuroprotective, and antioxidant properties.6 –8 CBD is unlike THC in that it is not psychoactive.6 –8 Epidiolex is the first CBD medication approved by the US Food and Drug Administration (FDA) for the treatment of Dravet syndrome (DS) and Lennox-Gastaut syndrome (LGS). 6 Overall, there are multiple randomized controlled trials (RCTs) and prospective open-label trials providing data on the efficacy and safety of CBD in DRE. However, unanswered questions remain, such as whether CBD monotherapy might be effective and whether doses lower than 10 mg/kg daily might induce similar reductions in seizures with even fewer adverse events. 9 The broader controversy over the use of cannabis-based therapies in epilepsy also remains to be resolved. And there is a lack of high-level evidence to support the long-term efficacy and safety of CBD in the treatment of DRE.10 –12
The aim of this systematic review and meta-analysis will be to assess the efficacy and safety of long-term use of CBD in the treatment of DRE, including exploration of whether differences exist in different age groups, different doses, different combinations of ASMs, epilepsy syndromes, and study designs.
The systematic review and meta-analysis has been registered with PROSPERO on 19 October 2022 (CRD42022351250). 13
Methods
Eligibility criteria
Studies will be included and excluded according to the following criteria.
Study designs
We will include all intervention studies including RCTs, non-RCTs, cohort studies, and single-arm trials. We will exclude cross-sectional studies and case reports.
Participants
Studies examining patients with DRE will be included. There is no demographic restriction such as age, sex, or race. And there is no restriction on etiology and seizure type.
Interventions
We will include studies on CBD, whether or not in combination with other ASMs. There is no restriction on administration route and dose. Both Epidiolex and other types of CBD were included in the study. There were no restrictions on concomitant ASMs or other concomitant treatments.
Comparators
There is no restriction on the comparators whether or not the control was set.
Outcomes
Efficacy indicators include seizure-free rates and responder rates (responder: seizure frequency reduced at least 50% from baseline). Adverse effects indicators include proportion of adverse events and proportion of serious adverse events. Proportion of adverse events are the number of patients experiencing adverse events during the observation period divided by the number of observations. Proportion of serious adverse events are the number of patients experiencing serious adverse events during the observation period divided by the number of observations. There was no restriction on the definition of serious adverse events, and data were extracted as reported in individual studies.
Duration of follow-up
The duration of treatment in most of RCTs on CBD was about 3 months. To explore the long-term effect of CBD, that is, the efficacy of CBD use for more than 1 or even 2 years. Based on the duration of treatment in the original studies, the date was concomitant by the week closest to 12, 24, 48, 72, 96, and 144 weeks. We equate 1 month to 4 weeks.
Search strategy
We conducted a literature search combining subject headings and free-text words. We have searched Medline (via PubMed), Embase (via Ovid), and Cochrane Central Register of Controlled Trials (via Cochran Library). Searches were completed on 14 January 2023. The search formula is given in Supplemental Appendix 1. Only original articles published in English were included in this study.
Studies screening
After determining the retrieval scheme, two researchers screened the records separately according to the titles and abstracts. And the union of the two was used as the initial screening result. After removing duplicate records, the full texts of those that appeared eligible were examined independently by two researchers. Discrepancies or disagreements were resolved through discussion with the research team.
Quality and risk of bias in individual studies
Single-group rates were the outcomes of interest. Hence, we regarded all studies as single-arm trials and used the Methodological Index for Non-randomized Studies (MINORS) 14 to assess the quality. In our study, we selected only the non-control portion of MINORS with no consideration of whether to estimate sample size and thus the total score was 14. Consistent with our aim of the systematic review, we have conducted an assessment of high risk, uncertain risk, and low risk from five aspects of selection bias, intervention bias, measurement bias, follow-up bias, and reporting bias. Studies were evaluated independently by two researchers. When disagreements arose, we resolved them through discussion and expert consultation.
Data extraction
Data were extracted independently by two researchers, and a comparison was ultimately performed. Data extraction included basic information about studies. When RevMan5.4 (The Cochrane Collaboration, 2020) merges rates across studies, rates of 0% or 100% will be invalid. Hence, if a rate is 0, the number of events has been added 0.5 and if a rate is 100%, the number of events has been minus 0.5.
According to the follow-up time of studies, the date was concomitant by the week closest to 12, 24, 48, 72, 96, and 144 weeks. We equate 1 month to 4 weeks. Studies labeled as OLE were open-label expanding trials after the completion of RCTs. Because there were 12 weeks of the RCTs data before the onset of OLE, OLE data were extracted beginning at week 24.
Data synthesis and analysis
Main analysis and sensitivity analysis
Meta-analysis was performed using RevMan5.4. 15 The inverse variance was chosen as the statistical method. The random effect (RE) model was selected for pooling to make the results more robust. As a sensitivity analysis, we also used the fixed effect (FE) model. The intention-to-treat (ITT) set was used as the main analysis set, and the per-protocol (PP) set was used as the sensitivity analysis set. The PP set was not set for adverse effects, because the analyses of adverse effects in most studies were ITT sets. The main analysis included all types of study designs; however, after excluding non-prospective studies, the results were synthesized as a sensitivity analysis. In addition, the main analysis included all types of CBD; however, only studies using Epidiolex were included for a sensitivity analysis. We calculated the I 2 statistic to assess heterogeneity among studies. Funnel plots were used to assess publication bias. A test level of α = 0.05 was set. All tests in this study were two-sided.
Subgroup analysis and sensitivity analysis
We performed subgroup analyses. Subgroup variables included age, CBD dose, concomitant ASMs, epilepsy syndromes, and study designs. All studies that met the inclusion and exclusion criteria were included in the main analysis. However, studies that lacked a basis for subgroup analysis (e.g. a study that did not report the constituent ratio of epilepsy syndromes) were excluded from the subgroup analysis.
Considering the differences in age demarcation between pediatric and adult departments in different countries, as well as the differences in the onset of adolescence among different individuals, we used two cut-off values separately. One was 14 years old, referring to the United Nations definition of youth 16 that is, 14 years old and below are children group, and over 14 years old are adolescent and adult group. In addition, a sensitivity analysis was performed with the cut-off value of 18 years. According to CBD dose, studies were divided into three subgroups: high dose (>20 mg/kg/day), medium dose (10–20 mg/kg/day), and low dose (⩽10 mg/kg/day). According to the proportion of patients who had used a certain concomitant ASM, studies were divided into three groups: <1/3, 1/3–2/3, and >2/3. In this systematic review, we selected the three most frequently used ASMs including clobazam (CLB), valproic acid (VPA), and levetiracetam (LEV) for subgroup analyses separately. According to the constituent ratio of epilepsy syndromes in the original studies, studies were divided into two groups: dominated by LGS and DS (LGS and DS accounted for more than 50%), and dominated by other syndromes (other syndromes accounted for more than 50%). In addition, subgroups were grouped according to the majority (95% or more) of LGS and DS and the majority (95% or more) of other syndromes as a sensitivity analysis. According to the designs of the original studies, studies were divided into three groups: OLE, RCT, and retrospective study. Because the duration of treatment in the RCTs was less than 3 months (12 weeks), subgroup analysis according to designs only pooled results at 12 weeks.
Confidence in cumulative evidence
We have used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) working group methodology 17 to assess the quality of the evidence for outcomes.
Results
Included studies
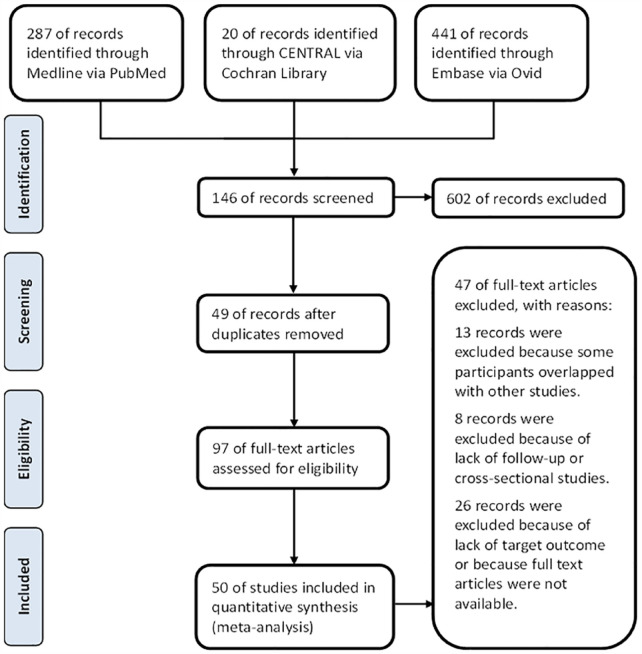
A total of 748 records were identified through the initial electronic database search strategy. Finally, 50 studies were included in this systematic review.18 –67 A total of 40 prospective studies (including 5 RCTS and 1 non-RCT) and 10 retrospective studies were included. Reasons for excluding full-text articles are presented in the PRISMA flow diagram (Figure 1). A total of 4791 participants (ITT set) were collected. The characteristics of each study are presented in Table 1.
Figure 1.
The PRISMA flow chart.
Table 1.
Studies’ characteristics.
| Study | Baseline sample size | Gender (male, %) | Age group | Epilepsy syndromes | Types of CBD/manufacturers | CBD dose | Concomitant ASMs | Follow-up time (weeks) | Outcomes | Study design | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LGS (%) | DS (%) | Others (%) | CLB | VPA | LEV | Responder | Seizure-free | Adverse events | Serious adverse events | ||||||||
| Anderson et al. 2021 18 | 33 | 48.5 | C | 0 | 0 | 100 | Epidiolex | H | 1/3–2/3 | 1/3–2/3 | <1/3 | 96 | √ | √ | √ | - | Prospective |
| Caraballo et al. 2020 19 | 50 | – | C | 76 | 6 | 18 | Rideau | M | 1/3–2/3 | 1/3–2/3 | 1/3–2/3 | 34 | √ | √ | – | – | Prospective |
| Caraballo and Valenzuela 2021 21 | 8 | 25.0 | C | 0 | 0 | 100 | Rideau | M | <1/3 | 1/3–2/3 | 1/3–2/3 | 38 | √ | √ | √ | √ | Retrospective |
| Caraballo et al. 2022 20 | 59 | – | C | 75 | 5 | 20 | Rideau | M | 1/3–2/3 | 1/3–2/3 | 1/3–2/3 | 40 | √ | √ | – | – | Prospective |
| Chen et al. 2018 22 | 40 | 55.0 | C | 20 | 15 | 65 | Epidiolex | M | 1/3–2/3 | 1/3–2/3 | <1/3 | 12 | – | – | √ | √ | Prospective |
| Cohen et al. 2022 23 | 31 | 45.2 | C | 42 | 6 | 52 | Epidiolex | H | <1/3 | <1/3 | <1/3 | 56 | √ | √ | √ | – | Retrospective |
| D’Onofrio et al. 2020 24 | 125 | 47.2 | C | 50 | 38 | 12 | Epidiolex | M | 1/3–2/3 | 1/3–2/3 | <1/3 | 24 | √ | √ | √ | - | Prospective |
| Devinsky et al. 2016 27 | 189 | 49.0 | C | 22 | 23 | 55 | Epidiolex | H | 1/3–2/3 | <1/3 | <1/3 | 12 | √ | √ | √ | √ | Prospective |
| Devinsky et al. 2017 25 | 61 | 57.4 | C | 0 | 100 | 0 | Epidiolex | M | 1/3–2/3 | 1/3–2/3 | <1/3 | 14 | √ | √ | √ | √ | RCT |
| Devinsky et al. 2018 28 | 73 | 54.8 | A | 100 | 0 | 0 | Epidiolex | L | 1/3–2/3 | 1/3–2/3 | <1/3 | 14 | – | – | √ | √ | RCT |
| Devinsky et al. 2018b 28 | 76 | 59.2 | A | 100 | 0 | 0 | Epidiolex | M | 1/3–2/3 | 1/3–2/3 | <1/3 | 14 | – | – | √ | √ | RCT |
| Devinsky et al. 2022 26 | 29 | 58.6 | A | 38 | 0 | 62 | Columbia Care | L | 1/3–2/3 | <1/3 | 1/3–2/3 | 24 | √ | √ | √ | √ | Prospective |
| Freeman 2018 29 | 20 | 25.0 | C | 60 | 0 | 40 | Epidiolex | M | 1/3–2/3 | 1/3–2/3 | <1/3 | 23 | √ | – | √ | √ | Prospective |
| Garza et al. 2017pp 30 | 38 | 60.5 | C | 100 | 0 | 0 | RSHO 5000 | L | – | – | – | 24 | √ | √ | – | – | Prospective |
| Gaston et al. 2021 31 | 80 | 45.0 | A | – | – | – | Epidiolex | H | – | – | – | 96 | √ | √ | – | – | Prospective |
| Gaston et al. 2021b 31 | 89 | 48.3 | C | – | – | – | Epidiolex | M, H | – | – | – | 96 | √ | √ | – | – | Prospective |
| Geffrey et al. 2015pp 32 | 13 | 53.8 | C | 0 | 23 | 77 | Epidiolex | H | >2/3 | <1/3 | <1/3 | 8 | √ | – | √ | – | Prospective |
| Hausman-Kedem et al. 2018 33 | 69 | – | C | – | – | – | Better and Tikun Olam | M | <1/3 | <1/3 | <1/3 | 62 | √ | √ | √ | – | Prospective |
| Herlopian et al. 2020 34 | 9 | 44.4 | C | 0 | 0 | 100 | Epidiolex | H | >2/3 | <1/3 | 1/3v2/3 | 48 | √ | √ | √ | – | Prospective |
| Iannone et al. 2021 35 | 93 | 52.7 | A | 68 | 32 | 0 | Epidiolex | M | 1/3–2/3 | 1/3–2/3 | <1/3 | 48 | √ | √ | √ | √ | Prospective |
| Kaplan et al. 2017 36 | 5 | 20.0 | C | 0 | 0 | 100 | Epidiolex | M | – | – | – | 14 | √ | √ | √ | – | Prospective |
| Klotz et al. 2019 37 | 35 | 54.3 | A | 17 | 14 | 69 | THC Pharm GmbH | M, H | <1/3 | 1/3–2/3 | <1/3 | 48 | √ | √ | √ | √ | Prospective |
| Klotz et al. 2021 38 | 37 | 48.6 | C | 11 | 14 | 76 | THC Pharm GmbH (77%) and Epidiolex (23%) | H | – | – | – | 12 | √ | √ | – | – | Prospective |
| Koo et al. 2020 39 | 44 | 63.6 | C | 77 | 23 | 0 | Epidiolex | L | 1/3–2/3 | >2/3 | <1/3 | 24 | √ | √ | √ | – | Prospective |
| Laux et al. 2019 40 | 152 | 61.2 | C | 62 | 38 | 0 | Epidiolex | H | >2/3 | 1/3–2/3 | 1/3–2/3 | 144 | √ | √ | √ | √ | Prospective |
| Laux et al. 2019b 40 | 455 | 48.4 | C | 0 | 0 | 100 | Epidiolex | H | – | – | – | 144 | √ | √ | – | – | Prospective |
| Marchese et al. 2022 41 | 37 | 54.1 | A | – | – | – | Enecta | L | <1/3 | 1/3–2/3 | <1/3 | 68 | √ | √ | √ | – | Retrospective |
| McCoy et al. 2018 42 | 20 | 50.0 | C | 0 | 100 | 0 | Tilray | M | >2/3 | 1/3–2/3 | 1/3–2/3 | 20 | √ | √ | √ | – | Prospective |
| Mitelpunkt et al. 2019 43 | 16 | 31.3 | C | – | – | – | AiFame-AiLab GmbH | M | – | – | – | 12 | √ | √ | √ | √ | Prospective |
| Navarro 2022 44 | 61 | 75.0 | A | – | – | – | Khiron Life Science Corp | L | <1/3 | <1/3 | 1/3–2/3 | 24 | √ | √ | √ | – | Prospective |
| Neubauer et al. 2018 45 | 70 | 55.7 | C | 3 | 0 | 97 | Bionorica | L | <1/3 | 1/3–2/3 | <1/3 | 14 | √ | √ | √ | √ | Retrospective |
| O’Brien et al. 2022 46 | 63 | 50.8 | A | 0 | 0 | 100 | Zynerba Pharmaceuticals | L | – | – | – | 12 | √ | √ | √ | √ | RCT |
| O’Brien et al. 2022b 46 | 62 | 41.9 | A | 0 | 0 | 100 | Zynerba Pharmaceuticals | L | – | – | – | 12 | √ | √ | √ | √ | RCT |
| OLE (Patel et al. 2021) 48 | 366 | 54.1 | A | 100 | 0 | 0 | Epidiolex | H | 1/3–2/3 | 1/3–2/3 | 1/3–2/3 | 156 | √ | √ | √ | √ | Prospective |
| OLE (Scheffer et al. 2021) 55 | 315 | 49.5 | C | 0 | 100 | 0 | Epidiolex | H | >2/3 | >2/3 | <1/3 | 144 | √ | √ | √ | √ | Prospective |
| OLE (Thiele et al. 2022) 60 | 199 | 59.3 | C | 0 | 0 | 100 | Epidiolex | H | <1/3 | 1/3–2/3 | <1/3 | 48 | √ | √ | √ | √ | Prospective |
| Park et al. 2020 47 | 50 | 57.4 | C | – | – | – | Epidiolex | H | 1/3–2/3 | <1/3 | 1/3–2/3 | 144 | √ | – | √ | √ | Retrospective |
| Patel et al. 2021 49 | 54 | 57.4 | C | 0 | 9 | 91 | Epidiolex | H | – | – | – | 182 | √ | √ | – | – | Prospective |
| Pesántez Ríos et al. 2022pp 50 | 34 | 55.9 | C | 35 | 3 | 62 | – | L | – | – | – | 48 | √ | √ | – | – | Retrospective |
| Pietrafusa et al. 2019 51 | 29 | 41.4 | C | – | – | – | BotexPharma | M | – | – | – | 45 | √ | √ | √ | – | Prospective |
| Press et al. 2015pp 52 | 75 | 45.3 | C | 12 | 17 | 71 | – | – | – | – | – | 22 | √ | √ | √ | – | Retrospective |
| Sands et al. 2019 53 | 26 | 50.0 | C | 15 | 23 | 62 | Epidiolex | H | – | – | – | 96 | √ | √ | √ | √ | Prospective |
| Savage et al. 2020 54 | 32 | 62.5 | A | 0 | 16 | 84 | Epidiolex | H | >2/3 | <1/3 | <1/3 | 8 | √ | √ | – | – | Prospective |
| Savage et al. 2020b 54 | 15 | 53.3 | A | 0 | 13 | 87 | Epidiolex | H | <1/3 | <1/3 | 1/3–2/3 | 8 | √ | √ | – | – | Prospective |
| Scheffer et al. 2021 56 | 48 | 54.2 | C | 10 | 17 | 73 | Zynerba Pharmaceuticals | H | 1/3–2/3 | >2/3 | 1/3–2/3 | 26 | – | – | √ | √ | Non-RCT |
| Sianati et al. 2019 57 | 22 | 30.0 | A | – | – | – | – | – | – | – | – | 12 | √ | – | – | – | Prospective |
| Szaflarski et al. 2018 58 | 607 | – | C | 15 | 10 | 75 | Epidiolex | H | 1/3–2/3 | <1/3 | 1/3–2/3 | 96 | √ | √ | √ | √ | Prospective |
| Thiele et al. 2018 61 | 86 | 52.3 | A | 100 | 0 | 0 | Epidiolex | M | 1/3–2/3 | 1/3–2/3 | <1/3 | 14 | – | – | √ | √ | RCT |
| Thiele et al. 2021 59 | 75 | 57.3 | C | 0 | 0 | 100 | Epidiolex | H | <1/3 | 1/3–2/3 | <1/3 | 16 | √ | √ | √ | √ | RCT |
| Thiele et al. 2021b 59 | 73 | 58.9 | C | 0 | 0 | 100 | Epidiolex | H | <1/3 | 1/3–2/3 | <1/3 | 16 | √ | √ | √ | √ | RCT |
| Tzadok et al. 2016pp 63 | 74 | – | C | – | – | – | Better and Tikun Olam | L | – | – | – | 22 | √ | – | √ | – | Retrospective |
| Tzadok et al. 2022 62 | 114 | 53.5 | C | – | – | – | Better and Tikun Olam | L | – | – | – | 48 | √ | – | √ | – | Retrospective |
| Uliel-Sibony et al. 2021 64 | 92 | 64.1 | C | – | – | – | Better and Tikun Olam | M | <1/3 | – | – | 12 | √ | √ | √ | – | Prospective |
| Vezyroglou et al. 2017 65 | 24 | – | C | – | – | – | Epidiolex | M | – | – | – | 12 | √ | – | – | – | Prospective |
| Wheless et al. 2019pp 66 | 61 | 54.1 | C | – | – | – | INSYS Manufacturing LLC | H | – | – | – | 2 | – | – | √ | – | Prospective |
| Zilmer and Olofsson 2021 67 | 78 | 48.7 | C | 19 | 81 | Glostrup Pharmacy | M | – | – | – | 96 | √ | – | √ | – | Retrospective | |
–, not reported or unknown; b, subgroup of the same study; pp, only the PP set was available for the study; A, adolescents and adults (>14 years); C, children (⩽14 years); L, low dose (⩽10 mg/kg/day); M, moderate dose (10, 20 mg/kg/day); H, high dose (>20 mg/kg/day); 1/3, 1/3–2/3, >2/3: proportion of ASMs used in study.
ASM, antiseizure medication; CBD, cannabidiol; CLB, clobazam; DS, Dravet syndrome; LEV, levetiracetam; LGS, Lennox-Gastaut syndrome; OLE, open-label expand; RCT, randomized controlled trial; VPA, valproic acid.
The MINORS scores and risk of bias are presented in Supplemental eFigure 1. The evaluation of the evidence can be found in Supplemental eTable 1. Overall, about half of the studies had no selection bias, and about half of the studies had uncertain selection bias. Most of the studies had no intervention bias, measurement bias, and reporting bias. Most studies were subject to follow-up bias. Despite high follow-up bias with responder rates and seizure-free rates, because an ITT analysis was used, the effect of bias may have been an underestimation of the results (negative bias). Efficacy outcomes may therefore be more conservative.
Efficacy
Responder rates
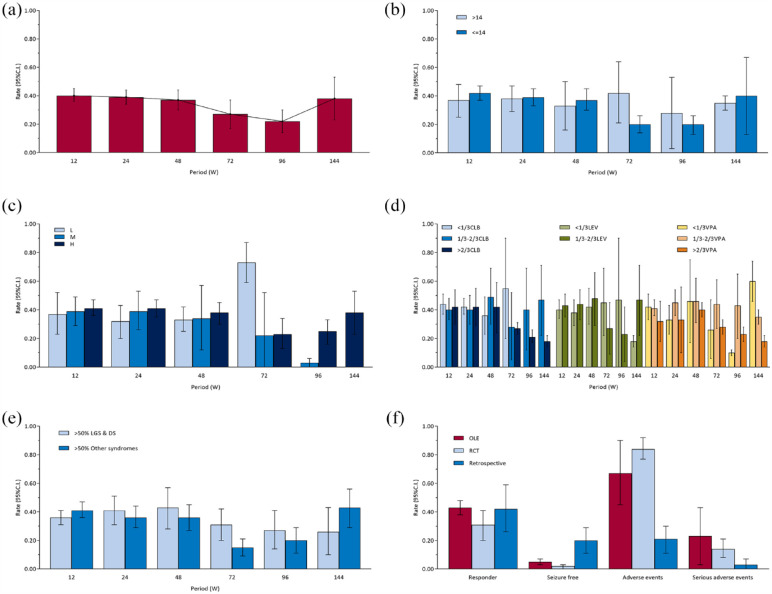
Overall, the pooled responder rates at 12-, 24-, 48-, 72-, 96-, and 144-week (ITT and RE) were 0.40 [0.36, 0.45], 0.39 [0.34, 0.44], 0.37 [0.30, 0.44], 0.27 [0.17, 0.37], 0.22 [0.14, 0.30], and 0.38 [0.23, 0.53] respectively [Figure 2(a) and Supplemental eFigure 2]. There were differences among periods (χ2 = 19.77, ν = 5, p = 0.001). For pairwise comparisons, we used the maximum responder rate (i.e. 12-week responder rate) as the reference (adjusted α value after multiple testing was 0.010). Responder rates at 24-week (χ2 = 0.27, ν = 1, p = 0.60), 48-week (χ2 = 0.77, ν = 1, p = 0.38), and 144-week (χ2 = 0.10, ν = 1, p = 0.75) were not different from 12-week, while 72-week (χ2 = 5.91, ν = 1, p = 0.02) and 96-week (χ2 = 15.37, ν = 1, p < 0.001) were different from 12-week. Overall, the responder rates seem to have stabilized at around 40% until 48 weeks and then have tended to decline. The results of sensitivity analyses of the main analysis are shown in Supplemental eFigure 3A. There was a tendency to increase with time in PP set, which was contrary to the main analysis. Responder rates of FE model, excluding retrospective studies set, and Epidiolex set were broadly consistent with the main analysis.
Figure 2.
(a–e) Responder rates. (a) Overall, (b) age subgroups, (c) CBD dose subgroups, (d) concomitant ASMs subgroups, (e) epilepsy syndromes subgroups, and (f) pooled outcomes at 12 weeks across study designs.
ASM, antiseizure medication; CBD, cannabidiol.
In addition, subgroup analyses according to age, dose, concomitant ASMs, epilepsy syndromes, and study designs are shown in Figure 2(b) to (f).
There was no difference between two subgroups of age [Figure 2(b)] at 12-week (χ2 = 0.56, ν = 1, p = 0.45), 24-week (χ2 = 0.02, ν = 1, p = 0.89), 48-week (χ2 = 0.26, ν = 1, p = 0.61), 72-week (χ2 = 3.74, ν = 1, p > 0.05), 96-week (χ2 = 0.41, ν = 1, p = 0.52), and 144-week (χ2 = 0.12, ν = 1, p = 0.73). The responder rates were not statistically different between the two subgroups at all periods. But overall, it appears that the responder rates of the adult and adolescent group (>14 years) were stable longer than the child group (⩽14 years), with little tendency to decrease with duration of CBD usage. However, the results of sensitivity analysis (Supplemental eFigure 4A) appear to be somewhat different. After adjusting the cut-off value from 14 to 18 years, there were no statistically significant differences in responder rates between the children group (⩽18 years) and adults (>18 years) group except for 48 weeks. But it appears that responder rates of the adult group declined faster and earlier with duration of CBD usage, which is contrary to the main analysis.
There was no difference among different doses [Figure 2(c)] at 12-week (χ2 = 0.32, ν = 2, p = 0.85), 24-week (χ2 = 2.08, ν = 2, p = 0.35), and 48-week (χ2 = 0.53, ν = 2, p = 0.77), but there were differences at 72-week (χ2 = 31.09, ν = 2, p < 0.001) and 96-week (χ2 = 22.41, ν = 1, p < 0.001). There was only one group at 144-week.
There was no difference among different proportions of CLB [Figure 2(d)] at 12-week (χ2 = 0.60, ν = 2, p = 0.74), 24-week (χ2 = 0.22, ν = 2, p = 0.89), 48-week (χ2 = 0.22, ν = 2, p = 0.89), 72-week (χ2 = 0.22, ν = 2, p = 0.89), and 96-week (χ2 = 1.68, ν = 1, p = 0.19), but there was a difference at 144-week (χ2 = 5.02, ν = 1, p = 0.03). There was no difference among different proportions of VPA [Figure 2(d)] at 12-week (χ2 = 1.74, ν = 2, p = 0.42), 24-week (χ2 = 3.04, ν = 2, p = 0.22), 48-week (χ2 = 0.71, ν = 2, p = 0.70), and 72-week (χ2 = 3.36, ν = 2, p = 0.19), but there were differences at 96-week (χ2 = 30.54, ν = 2, p < 0.001) and 144-week (χ2 = 40.79, ν = 2, p < 0.001). There was no difference among different proportions of LEV [Figure 2(d)] at 12-week (χ2 = 0.20, ν = 1, p = 0.66), 24-week (χ2 = 0.64, ν = 1, p = 0.42), 48-week (χ2 = 0.21, ν = 1, p = 0.64), 72-week (χ2 = 1.47, ν = 1, p = 0.23), and 96-week (χ2 = 0.83, ν = 1, p = 0.36), but there was a difference at 144-week (χ2 = 5.02, ν = 1, p = 0.03).
There was no difference between two subgroups of epilepsy syndromes [Figure 2(e)] at 12-week (χ2 = 1.74, ν = 1, p = 0.19), 24-week (χ2 = 0.57, ν = 1, p = 0.45), 48-week (χ2 = 0.63, ν = 1, p = 0.43), 96-week (χ2 = 0.84, ν = 1, p = 0.36), and 144-week (χ2 = 2.28, ν = 1, p = 0.13), but there was a difference at 72-week (χ2 = 6.39, ν = 1, p = 0.01). Consistent with the main subgroup analysis, the results of the sensitivity analysis of the subgroup analysis of epilepsy syndromes (Supplemental eFigure 5A) show that the LGS and DS group was statistically different from the other syndromes group only at 72 weeks. Overall, responder rates were not statistically different between the two groups for most periods.
There was no difference among three subgroups of study designs [Figure 2(f)] at 12-week (χ2 = 4.03, ν = 2, p = 0.13).
The funnel plot is shown in Supplemental eFigure 6A. There may be little publication bias at 12-week and 24-week. Publication bias may exist at 48-, 72-, and 96-week. It is possible that studies with large sample sizes and high responder rates are more likely to be published. There are too few studies to assess publication bias at 144-week.
Seizure-free rates
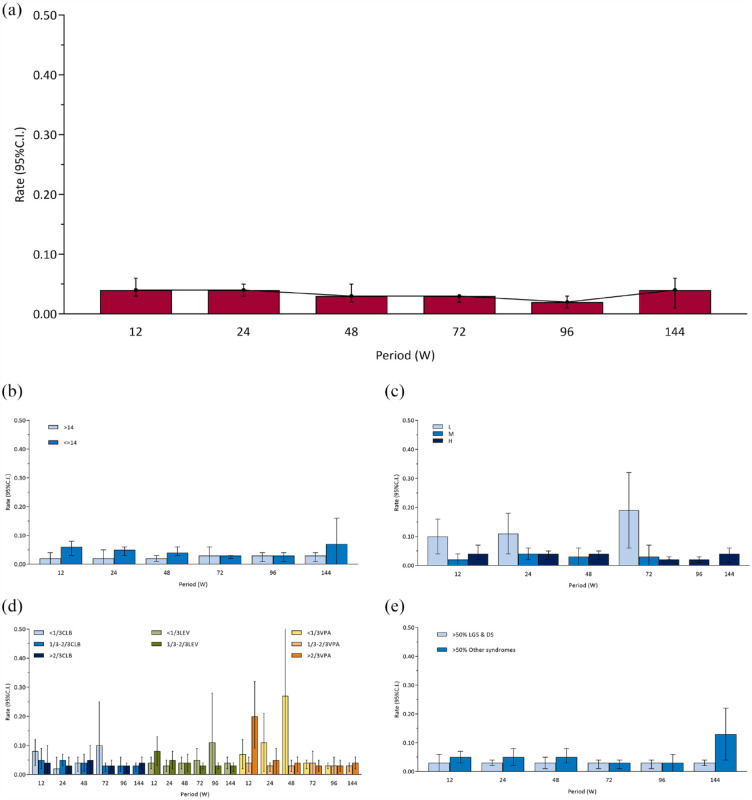
The overall pooled 12-, 24-, 48-, 72-, 96-, and 144-week seizure-free rates (ITT and RE) were 0.04 [0.03, 0.06], 0.04 [0.03, 0.05], 0.03 [0.02, 0.05], 0.03 [0.02, 0.03], 0.02 [0.01, 0.03], and 0.04 [0.01, 0.06], respectively [Figure 3(a) and Supplemental eFigure 7]. There was no difference among periods (χ2 = 7.90, ν = 5, p = 0.16). In general, the seizure-free rates remained stable at about 4% in all periods. The results of sensitivity analyses are shown in Supplemental eFigure 3B. Similar to responder rates, there was a tendency to increase with time in PP set, which was contrary to the main analysis. Seizure-free rates of FE model, excluding retrospective studies set, and Epidiolex set were generally consistent with the main analysis.
Figure 3.
Seizure-free rates: (a) overall, (b) age subgroups, (c) CBD dose subgroups, (d). concomitant ASMs subgroups, and (e) epilepsy syndromes subgroups.
ASM, antiseizure medication; CBD, cannabidiol.
In addition, subgroup analyses according to age, dose, the concomitant ASMs, epilepsy syndromes, and study designs are shown in Figures 2(f) and 3(b) to (e).
There was no difference between two subgroups of age [Figure 3(b)] at 24-week (χ2 = 2.50, ν = 1, p = 0.11), 72-week (χ2 < 0.01, ν = 1, p = 0.98), 96-week (χ2 < 0.01, ν = 1, p = 0.95), and 144-week (χ2 = 0.91, ν = 1, p = 0.34), but there were differences at 12-week (χ2 = 5.72, ν = 1, p = 0.02) and 48-week (χ2 = 5.41, ν = 1, p = 0.02). Similar to the main subgroup analysis, sensitivity analysis (Supplemental eFigure 4B) found no statistically significant differences in seizure-free rates between the adult group and the child group except at 12 weeks. Overall, the seizure-free rates did not differ significantly between adults and children.
There was no difference among different doses [Figure 3(c)] at 12-week (χ2 = 5.78, ν = 2, p = 0.06), 24-week (χ2 = 3.60, ν = 2, p = 0.16), and 48-week (χ2 = 0.21, ν = 1, p = 0.65), but there was a difference at 72-week (χ2 = 6.55, ν = 2, p = 0.04). There was only one group at 96-week and 144-week.
There was no difference among different proportions of CLB [Figure 3(d)] at 12-week (χ2 = 0.89, ν = 2, p = 0.64), 24-week (χ2 = 2.20, ν = 2, p = 0.33), 48-week (χ2 = 0.09, ν = 2, p = 0.96), 72-week (χ2 = 0.85, ν = 2, p = 0.66), 96-week (χ2 = 0.33, ν = 1, p = 0.57), and 144-week (χ2 = 0.35, ν = 1, p = 0.55). There was no difference among different proportions of VPA [Figure 3(d)] at 24-week (χ2 = 2.90, ν = 2, p = 0.23), 48-week (χ2 = 1.15, ν = 2, p = 0.56), 72-week (χ2 = 0.18, ν = 2, p = 0.91), 96-week (χ2 = 0.01, ν = 2, p = 0.99), and 144-week (χ2 = 0.35, ν = 1, p = 0.55), but there was a difference at 12-week (χ2 = 8.17, ν = 2, p = 0.02). There was no difference among different proportions of LEV [Figure 3(d)] at 12-week (χ2 = 2.92, ν = 1, p = 0.09), 24-week (χ2 = 1.22, ν = 1, p = 0.27), 48-week (χ2 = 0.08, ν = 1, p = 0.78), 72-week (χ2 = 0.56, ν = 1, p = 0.45), 96-week (χ2 = 0.80, ν = 1, p = 0.37), and 144-week (χ2 = 0.35, ν = 1, p = 0.55).
There was no difference between two subgroups of epilepsy syndromes [Figure 3(e)] at 12-week (χ2 = 1.24, ν = 1, p = 0.27), 24-week (χ2 = 1.69, ν = 1, p = 0.19), 48-week (χ2 = 1.87, ν = 1, p = 0.17), 96-week (χ2 = 0.14, ν = 1, p = 0.71), and 72-week (χ2 = 0.19, ν = 1, p = 0.66), but there was a difference at 144-week (χ2 = 4.58, ν = 1, p = 0.03). Consistent with the main subgroup analysis, sensitivity analysis (Supplemental eFigure 5B) found no statistically significant differences in the seizure-free rates between the LGS and DS group and the other syndromes group from 12 to 96 weeks. Overall, the seizure-free rates did not differ significantly between subgroups of epilepsy syndromes.
There was a difference among three subgroups of study designs [Figure 2(f)] at 12-week (χ2 = 17.98, ν = 2, p < 0.001). The pairwise comparison (adjusted α value after multiple testing was 0.017) found that the seizure-free rates of the retrospective study group differ from both the RCT group (χ2 = 14.14, ν = 1, p < 0.001) and the OLE group (χ2 = 9.68, ν = 1, p = 0.002).
The funnel plot is shown in Supplemental eFigure 6B. There may be little publication bias at 72-week. Publication bias may exist at 12-, 24-, 48-, and 96-week. It is possible that studies with large sample sizes and high seizure-free rates are more likely to be published. There are too few studies to assess publication bias at 144-week.
Adverse effects
Proportion of adverse events
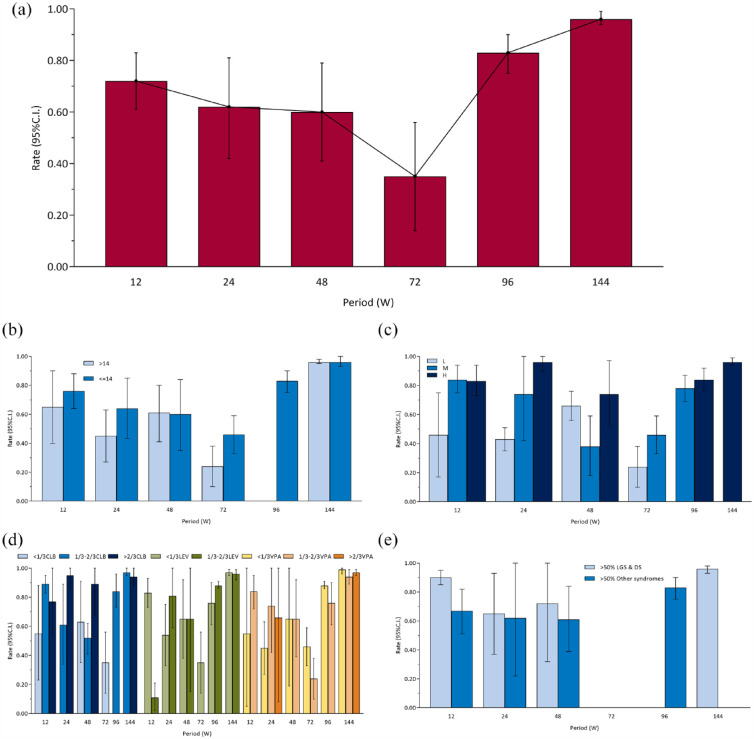
The overall pooled 12-, 24-, 48-, 72-, 96-, and 144-week proportion of adverse events (RE) were 0.72 [0.61, 0.83], 0.62 [0.42, 0.81], 0.60 [0.41, 0.79], 0.35 [0.14, 0.56], 0.83 [0.75, 0.90], and 0.96 [0.94, 0.99], respectively [Figure 4(a) and Supplemental eFigure 8]. There were differences among periods (χ2 = 82.65, ν = 5, p < 0.001). The maximum adverse events rate (i.e. 144-week adverse events rate) was used as reference for pairwise comparisons (adjusted α value after multiple testing was 0.010). Twelve-week (χ2 = 18.33, ν = 1, p < 0.001), 24-week (χ2 = 12.05, ν = 1, p < 0.001), 48-week (χ2 = 13.53, ν = 1, p < 0.001), 72-week (χ2 = 32.82, ν = 1, p < 0.001), and 96-week (χ2 = 13.04, ν = 1, p < 0.001) were different from 144-week. The results of sensitivity analyses are provided in Supplemental eFigure 3C. Proportion of adverse events of FE model, excluding retrospective studies set, and Epidiolex set were generally consistent with the main analysis.
Figure 4.
Proportion of adverse events: (a) overall, (b) age subgroups, (c) CBD dose subgroups, (d) concomitant ASMs subgroups, and (e) epilepsy syndromes subgroups.
ASM, antiseizure medication; CBD, cannabidiol.
In addition, subgroup analyses according to age, dose, the concomitant ASMs, epilepsy syndromes, and study designs are shown in Figures 2(f) and 4(b) to (e).
There was no difference between two subgroups of age [Figure 4(b)] at 12-week (χ2 = 0.62, ν = 1, p = 0.43), 24-week (χ2 = 1.81, ν = 1, p = 0.18), 48-week (χ2 = 0.01, ν = 1, p = 0.94), and 144-week (χ2 = 0.01, ν = 1, p = 0.91), but there was a difference at 72-week (χ2 = 4.86, ν = 1, p = 0.03). There was only one group at 96-week. Sensitivity analysis was similar to the main subgroup analysis (Supplemental eFigure 4C).
There was no difference among different doses [Figure 4(c)] at 96-week (χ2 = 1.05, ν = 1, p = 0.31), but there were differences at 12-week (χ2 = 6.39, ν = 2, p = 0.04), 24-week (χ2 = 113.56, ν = 2, p < 0.001), 48-week (χ2 = 6.74, ν = 2, p = 0.03), and 72-week (χ2 = 4.86, ν = 1, p = 0.03). There was only one group at 144-week.
There was no difference among different proportions of CLB [Figure 4(d)] at 12-week (χ2 = 4.84, ν = 2, p = 0.09) and 144-week (χ2 = 0.83, ν = 1, p = 0.36), but there were differences at 24-week (χ2 = 5.15, ν = 1, p = 0.02) and 48-week (χ2 = 10.26, ν = 2, p = 0.006). There was only one group at 72-week and 96-week. There was no difference among different proportions of VPA [Figure 4(d)] at 12-week (χ2 = 1.13, ν = 1, p = 0.29), 24-week (χ2 = 2.70, ν = 2, p = 0.26), 48-week (χ2 < 0.01, ν = 1, p = 0.99), 96-week (χ2 = 2.72, ν = 1, p = 0.10), and 144-week (χ2 = 2.61, ν = 2, p = 0.27), but there was a difference at 72-week (χ2 = 4.86, ν = 1, p = 0.03). There was no difference among different proportions of LEV [Figure 4(d)] at 24-week (χ2 = 2.96, ν = 1, p = 0.09), 48-week (χ2 < 0.01, ν = 1, p > 0.99), 96-week (χ2 = 2.72, ν = 1, p = 0.10), and 144-week (χ2 = 0.37, ν = 1, p = 0.54), but there was a difference at 12-week (χ2 = 103.98, ν = 1, p < 0.001). There was only one group at 72-week.
There was no difference between two subgroups of epilepsy syndromes [Figure 4(e)] at 24-week (χ2 = 0.01, ν = 1, p = 0.90) and 48-week (χ2 = 0.22, ν = 1, p = 0.64), but there was a difference at 12-week (χ2 = 7.60, ν = 1, p < 0.001). There was only one group at 96-week and 144-week. Sensitivity analysis was similar to the main subgroup analysis (Supplemental eFigure 5C).
There was a difference among three subgroups of study designs [Figure 2(f)] at 12-week (χ2 = 106.81, ν = 2, p < 0.001). The pairwise comparison (adjusted α value after multiple testing was 0.017) found that the proportion of adverse events of the retrospective study group differ from both the RCT group (χ2 = 14.18, ν = 1, p < 0.001) and the OLE group (χ2 = 106.51, ν = 1, p < 0.001).
The funnel plot is shown in Supplemental eFigure 6C. There may be little publication bias at 12-, 24-, and 48-week. It is possible that studies with large sample sizes are more likely to be published. There are too few studies to assess publication bias at 72-, 96-, and 144-week.
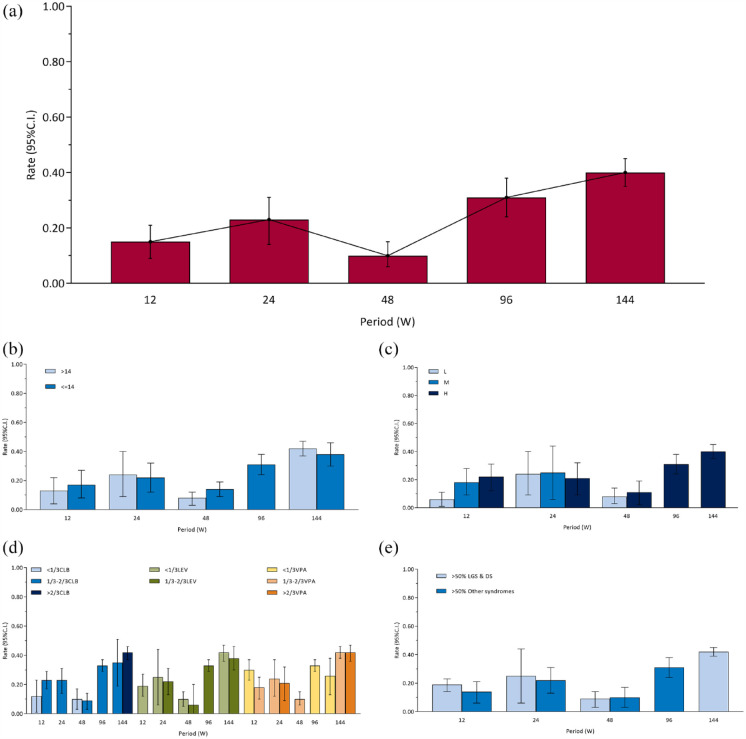
Proportion of serious adverse events
The overall pooled 12-, 24-, 48-, 96-, and 144-week proportion of serious adverse events (RE) were 0.15 [0.09, 0.21], 0.23 [0.14, 0.31], 0.10 [0.06, 0.15], 0.31 [0.24, 0.38], and 0.40 [0.35, 0.45], respectively [Figure 5(a) and Supplemental eFigure 9]. There were differences among periods (χ2 = 85.72, ν = 4, p < 0.001). The maximum serious adverse events rate (i.e. 144-week serious adverse events rate) was used as reference for pairwise comparisons (adjusted α value after multiple testing was 0.013). Proportion of serious adverse events at 12-week (χ2 = 36.94, ν = 1, p < 0.001), 24-week (χ2 = 12.10, ν = 1, p < 0.001), and 48-week (χ2 = 73.91, ν = 1, p < 0.001) were different from 144-week. But 96-week (χ2 = 3.81, ν = 1, p = 0.05) was not different from 144-week. The results of sensitivity analyses are provided in Supplemental eFigure 3D. Proportion of serious adverse events of FE model, excluding retrospective studies set, and Epidiolex set were generally consistent with the main analysis.
Figure 5.
Proportion of serious adverse events: (a) overall, (b) age subgroups, (c) CBD dose subgroups, (d) concomitant ASMs subgroups, and (e) epilepsy syndromes subgroups.
ASM, antiseizure medication; CBD, cannabidiol.
In addition, subgroup analyses according to age, dose, the concomitant ASMs, epilepsy syndromes, and study designs are shown in Figures 2(f) and 5(b) to (e).
There was no difference between two subgroups of age [Figure 5(b)] at 12-week (χ2 = 0.39, ν = 1, p = 0.53), 24-week (χ2 = 0.05, ν = 1, p = 0.82), 48-week (χ2 = 3.62, ν = 1, p = 0.06), and 144-week (χ2 = 0.87, ν = 1, p = 0.35). There was only one group at 96-week. Sensitivity analysis was similar to the main subgroup analysis (Supplemental eFigure 4D).
There was no difference among different doses [Figure 5(c)] at 24-week (χ2 = 0.19, ν = 2, p = 0.91) and 48-week (χ2 = 0.20, ν = 1, p = 0.66), but there was a difference at 12-week (χ2 = 10.18, ν = 2, p = 0.006). There was only one group at 96-week and 144-week.
There was no difference among different proportions of CLB [Figure 5(d)] at 12-week (χ2 = 2.75, ν = 1, p = 0.10), 48-week (χ2 = 0.13, ν = 1, p = 0.72), and 144-week (χ2 = 0.60, ν = 1, p = 0.44). There was only one group at 24-week and 96-week. There was no difference among different proportions of VPA [Figure 5(d)] at 24-week (χ2 = 0.19, ν = 1, p = 0.67), but there were differences at 12-week (χ2 = 5.27, ν = 1, p = 0.02) and 144-week (χ2 = 6.21, ν = 2, p = 0.04). There was only one group at 48-week and 96-week. There was no difference among different proportions of LEV [Figure 5(d)] at 24-week (χ2 = 0.08, ν = 1, p = 0.78), 48-week (χ2 = 0.19, ν = 1, p = 0.66), 96-week (χ2 = 2.72, ν = 1, p = 0.10), and 144-week (χ2 = 0.62, ν = 1, p = 0.43). There was only one group at 12-week and 72-week.
There was no difference between two subgroups of epilepsy syndromes [Figure 5(e)] at 12-week (χ2 = 1.26, ν = 1, p = 0.26), 24-week (χ2 = 0.08, ν = 1, p = 0.78), and 48-week (χ2 = 0.13, ν = 1, p = 0.72). There was only one group at 96-week and 144-week. In contrast to the main subgroup analysis, a sensitivity analysis (Supplemental eFigure 5D) found a statistically significant difference in the serious adverse events rate between the two groups at 12 weeks.
There was a difference among three subgroups of study designs [Figure 2(f)] at 12-week (χ2 = 11.18, ν = 2, p = 0.004). The pairwise comparison (adjusted α value after multiple testing was 0.017) found that the proportion of serious adverse events of the retrospective study group differ from the RCT group (χ2 = 8.63, ν = 1, p = 0.003).
The funnel plot is shown in Supplemental eFigure 6D. There may be little publication bias at 12-week. There are too few studies to assess publication bias at 24-, 48-, 96-, and 144-week.
Discussion
DRE has a tremendous impact on the cognitive and behavioral function and quality of life of patients, and its treatment is both a challenge and a rare opportunity. CBD is the main non-psychotropic compound in cannabis, and CBD has received considerable attention over the past decade. Basic research provides strong evidence for the safety and anticonvulsant properties of CBD. A growing number of clinical studies have also emerged. However, the major limitation of most clinical studies is that they are open-label, observational studies. The follow-up period was short and there was no control group. Many questions remain, not least the long-term effects of CBD on the brain and the extent of benefit after full consideration of drug interactions. Although CBD does not have psychotropic properties and appears to be safe for short-term use, safety data on long-term use are scarce. This systematic review is the first meta-analysis of CBD for DRE with rate as the outcome measure rather than ratio and with subgroup analyses according to duration of use, age, dose, concomitant ASMs, epilepsy syndromes, and study designs. The results may provide evidence on the long-term efficacy of CBD in the treatment of DRE and provide ideas for future studies.
Period
In summary, the meta-analysis found that responder rates remained stable up to 48-week, but trended downward after 48-week. In contrast, responder rates of PP set trended upward. The results of some long-term open-label studies also show a growing trend with time.18,48,49,55,58 This finding also differs from that of another study in which efficacy decreased rapidly over the first 6 months. 53 Seizure-free rates remained at approximately 4% across all periods. Similar to responder rates, PP set and some long-term open-label studies showed an increasing trend over time. Patient retention decreased with the duration of CBD usage, particularly as the loss to follow-up was due to poor efficacy. Hence, there may be selection bias in PP set, that is, patients with more CBD sensitivity than in ITT set. Therefore, the results of meta-analysis using ITT set may be more robust and conservative. Proportion of adverse events and proportion of serious adverse events tended to increase with increasing duration of CBD usage, while the meta-analysis showed that the adverse effect rate at 72-week appeared to be the lowest. However, the result at 72-week was pooled from only two studies, and the sample size was small, which may exhibit publication bias.
The pooled results of the main analysis were obtained from all the original studies that met the inclusion and exclusion criteria, that is, the study population was not limited by epilepsy syndrome type, age, etc. That is, the results of this study may be more generalizable. Therefore, this conservative pooled result may more confirm the potential of CBD in the adjuvant treatment of DRE.
Age
A longitudinal observational study found that CBD may be more effective at younger ages (<10 years). 33 In contrast, a long-term open-label study found no difference in efficacy between adults and children. 31 The results of meta-analysis showed that there was no difference in either responder rates or seizure-free rates between the two age subgroups. It is possible that the results of these studies differ due to differences in cut-off values for age groups, or because children are more likely to be lost to follow-up, and efficacy seems to be better with the use of PP set analysis for children. In addition, a sensitivity analysis with a cut-off value of 18 years compared with a main subgroup analysis with a cut-off value of 14 years, the responder rates appeared to be higher and more stable in the adult group than in the child group after the cut-off value was raised. This suggests that adolescents aged 14–18 years may have a higher responder rate. In addition, studies on the treatment of elderly DRE patients with CBD are currently lacking.
Dosage
A longitudinal observational study found that higher doses were associated with better efficacy. 33 In contrast, several other studies have found no relationship between dose and effectiveness.23,35 Similarly, a RCT also found no difference in efficacy between the 25 and 50 mg dose groups. 59 The meta-analysis found that responder rates were similar and not statistically different across three groups of dosage. It has even been shown that lower doses may have higher free seizure rates. This suggests that treatment of DRE may be more effective with lower doses of CBD. However, the greater effect of the lower dose could be due to potential survivor bias, especially at later stages. Patients who are more responsive to CBD treatment may be more likely to reduce the dose, whereas patients who respond poorly to CBD treatment may try to increase the dose.
During the initial period of CBD use (before 24 or 48 weeks), adverse effects increased as doses were increased. But after approximately 24 or 48 weeks, there was no difference between dosages. It is possible that the adverse effects caused by long-term CBD use masked the adverse effects caused by dose increase. Or the patient is gradually tolerating CBD after long-term use.
Previous studies have found that the common adverse reactions of CBD in the treatment of epilepsy include rash, diarrhea, nausea, decreased appetite, insomnia, and elevated transaminase. Most adverse effects were dose-dependent and resolved spontaneously or with dose reduction or discontinuation.11,12 A systematic review and meta-analysis of the adverse effects of CBD in the treatment of epilepsy found that CBD resulted in two times as many adverse events as placebo. Compared with placebo, CBD was more likely to cause diarrhea, insomnia, rash, decreased appetite, and elevated aminotransferase levels (p < 0.05). 68
Concomitant ASMs
A retrospective study found that the combination of CLB and CBD may have superior efficacy. 67 In contrast, another retrospective study and some prospective studies did not find a difference in efficacy with or without the combination of CLB.24,32,35,54 Some studies also found that CBD use can reduce the dose of concomitant ASMs.41,49 A study of CBD interactions with other ASMs found no difference in seizure severity or frequency between patients taking any of the potentially interacting ASMs compared with those not taking them. 69 However, the results of the meta-analysis found that regardless of the proportion of the population treated with concomitant ASMs (CLB, VPA, or LEV), the responder rates and seizure-free rates were not significantly different across most periods. Thus, the efficacy of CBD may be independent of other ASMs. This is consistent with the results of a previous study exploring whether the efficacy of CBD is affected by concomitant ASM drugs. 69
Two prospective studies found that adverse effects increased as the dose of concomitant CLB increased.24,32 The results of the meta-analysis found that proportion of adverse events increased with the proportion of patients using ASMs increasing. However, there was no difference in proportion of serious adverse events. Most prospective studies of drug combination are CBD concomitant with CLB, and studies of other ASMs like LEV and VPA are lacking. The results of the meta-analysis were based on the proportion of patients treated with ASMs, and the specific doses of ASMs were not available, so the extrapolation of findings is limited.
CBD metabolism occurs primarily through the cytochrome (CY) P450 enzyme, which is known to be a substrate for CYP3A4 and CYP2C19. A previous study found that the increase in plasma N-desmethylclobazam (N-CLB) in patients using CBD was significantly higher than that in the placebo group. 70 N-CLB is the active metabolite of CLB, presumably because of CYP2C19 inhibition, which inactivates N-CLB. The dose of CLB is reduced in DRE patients after using CBD, which may be related to the increase of active components of CLB caused by the interaction between CLB and CBD, so that smaller doses can have the same efficacy. At the same time, this may also lead to an increase in adverse effects such as sleepiness. 11 Previous studies have found that the interaction between VPA and CBD may induce the elevation of transaminase levels. Somnolence and transaminase elevation were the only two clinically significant adverse effects possibly caused by drug interactions reported in all pivotal clinical trials, in combination with CLB and VPA, respectively.11,25 The results of this systematic review and meta-analysis also support these findings.
Epilepsy syndromes
Although the FDA currently approved CBD for patients ⩾2 years of age with LGS or DS, off-label use of CBD is emerging, increasingly for DRE that do not meet the diagnostic criteria for LGS or DS, including genetic epilepsy syndromes (i.e. CDKL5 deficiency, Aicardi syndrome, Dup15q syndrome, and Doose syndrome), and febrile infection-related epilepsy syndrome.71,72 Moreover, the efficacy and safety of CBD in the treatment of these four syndromes (CDKL5 deficiency, Aicardi syndrome, Dup15q syndrome, and Doose syndrome) are similar. 71 This systematic review and meta-analysis found that the efficacy of LGS and DS group was basically similar to those of other syndromes group in the main subgroup analyses and sensitivity analyses in most periods. This may suggest that CBD has similar effects on all types of epilepsy syndromes. It is possible to expand the range of indications for CBD in the future.
Study designs
In general, the results of both efficacy and adverse effects are not exactly the same among the three study designs, especially the retrospective study group was different from the other two. Retrospective studies appear to have better efficacy and lower adverse effects than the other two. This may be related to the higher selectivity bias of retrospective studies, that is, retrospective studies may include fewer patients who discontinued the drug because of poor efficacy and/or large adverse effects. However, the results of sensitivity analyses that excluded retrospective studies were generally consistent with those of the main analyses, which suggests that the results of this systematic review and meta-analysis were less influenced by retrospective studies.
Precise treatment of CBD in DRE
It is possible that the benefit for the population at the initial (before 48 weeks) use of CBD for DRE is broad and stable, while the responder rate appears to gradually decrease with increasing duration of CBD use. However, after 2 years (96 weeks), more than 20% of patients still responded to CBD treatment. In addition, the seizure-free rate was stable at around 4% in the long term. This seems to suggest that there may be a subset of ‘special’ DRE patients who are sensitive to and respond long-term to CBD treatment. Identifying such ‘special’ patients may lead to greater benefits of CBD use. A pharmacogenetic study of CBD treatment of DRE found an influence of genetic variants on seizure reduction and susceptibility to adverse effects in DRE patients treated with CBD. The metabolic process of CBD is complex, and there may be genetic heterogeneity in tolerance and response to CBD in different patients. 73 The involvement of steroid hormone-related pathways found in the study suggests that CBD responses may be gender- and age-dependent. 73 The subgroup analysis and sensitivity analysis of this systematic review and meta-analysis suggest that adolescents aged 14–18 years may have a higher response rate, which may be due to the hormonal changes in adolescents affecting the pathway related to CBD metabolism, or because some genes of the adolescent-associated epilepsy syndromes are associated with the efficacy of CBD. In addition, these pathways identified by the pharmacogenetic study are also related to other neurodegenerative diseases, and the pathways involved (such as cholesterol metabolism and glutathione binding) suggest a possible interaction between CBD and commonly used drugs (such as statins, acetaminophen, etc.). 73 This suggests that comorbidity of epilepsy and its medication may also affect the efficacy of CBD in the treatment of DRE. Future studies may explore the influence of comorbidity of epilepsy and its medications on the treatment of DRE with CBD.
Summary
In conclusion, CBD treatment of DRE has stable efficacy and fewer adverse events in the early period (before 48 weeks). Long-term use may have decreased efficacy and increased adverse events. The best therapeutic dose may be a low dose (less than 10 mg/kg/day). Dose escalation may not only not increase efficacy, but may also increase the adverse events. Furthermore, CBD use may reduce the dosage of other ASMs without reducing efficacy, thereby reducing adverse effects. In addition, CBD seems to have similar efficacy for epilepsy syndromes other than LGS and DS approved by the FDA, and it is possible to expand the range of CBD indications in the future.
However, because the studies included in this systematic review are mainly single-arm studies, the greatest limitation is the lack of control, which makes direct comparison with placebo or other ASMs impossible. There was also substantial follow-up bias, although the results may have been more biased toward the null by using ITT set. In addition, this study did not investigate the effect of seizure subtype and comorbidity of epilepsy on the treatment of DRE with CBD. In the future, more real-world studies or RCTs may be needed to explore the long-term effects of CBD, especially its interactions with other ASMs and to explore the characteristics of the population with good efficacy of CBD, especially the 4% of long-term stable seizure-free population, including related genes and comorbidities, so as to provide reference for precise treatment.
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_17562864231207755 for Long-term efficacy and adverse effects of cannabidiol in adjuvant treatment of drug-resistant epilepsy: a systematic review and meta-analysis by Shengyi Liu, Zihua He and Jinmei Li in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-docx-2-tan-10.1177_17562864231207755 for Long-term efficacy and adverse effects of cannabidiol in adjuvant treatment of drug-resistant epilepsy: a systematic review and meta-analysis by Shengyi Liu, Zihua He and Jinmei Li in Therapeutic Advances in Neurological Disorders
Acknowledgments
We thank Yinping Li for her help and support for this study.
Footnotes
ORCID iDs: Shengyi Liu  https://orcid.org/0000-0002-1912-9774
https://orcid.org/0000-0002-1912-9774
Jinmei Li  https://orcid.org/0000-0002-7411-8269
https://orcid.org/0000-0002-7411-8269
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Shengyi Liu, Department of Neurology, West China Hospital, Sichuan University, Chengdu, China.
Zihua He, Department of Neurology, West China Hospital, Sichuan University, Chengdu, China.
Jinmei Li, Department of Neurology, West China Hospital, Sichuan University, No. 37 Guoxue Alley, Wuhou District, Chengdu, Sichuan 610041, China.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Shengyi Liu: Conceptualization; Data curation; Formal analysis; Visualization; Writing – original draft; Writing – review & editing.
Zihua He: Conceptualization; Data curation; Formal analysis.
Jinmei Li: Conceptualization; Project administration; Supervision; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: All data generated during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
- 1. Devinsky O, Vezzani A, O’Brien TJ, et al. Epilepsy. Nat Rev Dis Primers 2018; 4: 18024–20180503. [DOI] [PubMed] [Google Scholar]
- 2. Chisholm D. and WHO-CHOICE. Cost-effectiveness of first-line antiepileptic drug treatments in the developing world: a population-level analysis. Epilepsia 2005; 46: 751–759. [DOI] [PubMed] [Google Scholar]
- 3. Hwang ST, Stevens SJ, Fu AX, et al. Intractable generalized epilepsy: therapeutic approaches. Curr Neurol Neurosci Rep 2019; 19: 16–20190226. [DOI] [PubMed] [Google Scholar]
- 4. Lerche H. Drug-resistant epilepsy – time to target mechanisms. Nat Rev Neurol 2020; 16: 595–596. [DOI] [PubMed] [Google Scholar]
- 5. Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2009; 51: 1069–1077. [DOI] [PubMed] [Google Scholar]
- 6. Billakota S, Devinsky O, Marsh E. Cannabinoid therapy in epilepsy. Curr Opin Neurol 2019; 32: 220–226. [DOI] [PubMed] [Google Scholar]
- 7. Boleti APA, Frihling BEF, E Silva PS, et al. Biochemical aspects and therapeutic mechanisms of cannabidiol in epilepsy. Neurosci Biobehav Rev 2022; 132: 1214–1228. [DOI] [PubMed] [Google Scholar]
- 8. Campos AC, Fogaça MV, Sonego AB, et al. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol Res 2016; 112: 119–127. [DOI] [PubMed] [Google Scholar]
- 9. Kaufman SK, Del Tredici K, Thomas TL, et al. Tau seeding activity begins in the transentorhinal/entorhinal regions and anticipates phospho-tau pathology in Alzheimer’s disease and PART. Acta Neuropathol 2018; 136: 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Detyniecki K, Hirsch LJ. Cannabidiol for epilepsy: trying to see through the haze. Lancet Neurol 2016; 15: 235–237. [DOI] [PubMed] [Google Scholar]
- 11. Abu-Sawwa R, Scutt B, Park Y. Emerging use of Epidiolex (cannabidiol) in epilepsy. J Pediatr Pharmacol Ther 2020; 25: 485–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abu-Sawwa R, Stehling C. Epidiolex (cannabidiol) primer: frequently asked questions for patients and caregivers. J Pediatr Pharmacol Ther 2020; 25: 75–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu S, He Z, Li J. Efficacy and adverse effects of cannabidiol in the treatment of drug-resistant epilepsy: a systematic review and meta-analysis. PROSPERO (CRD42022351250), https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022351250 (2022, accessed 22 March 2023). [DOI] [PMC free article] [PubMed]
- 14. Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg 2003; 73: 712–716. [DOI] [PubMed] [Google Scholar]
- 15. The Cochrane Collaboration. Review Manager (RevMan) [Computer program], version 5.4, 2020.
- 16. United Nations. Youth, https://www.un.org/en/global-issues/youth (2021, accessed 13 June 2023).
- 17. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anderson CL, Evans V, Gorham L, et al. Seizure frequency, quality of life, behavior, cognition, and sleep in pediatric patients enrolled in a prospective, open-label clinical study with cannabidiol. Epilepsy Behav 2021; 124: 108325. [DOI] [PubMed] [Google Scholar]
- 19. Caraballo R, Demirdjian G, Reyes G, et al. Effectiveness of cannabidiol in a prospective cohort of children with drug-resistant epileptic encephalopathy in Argentina. Seizure 2020; 80: 75–80. [DOI] [PubMed] [Google Scholar]
- 20. Caraballo R, Reyes G, Demirdjian G, et al. Long-term use of cannabidiol-enriched medical cannabis in a prospective cohort of children with drug-resistant developmental and epileptic encephalopathy. Seizure 2022; 95: 56–63. [DOI] [PubMed] [Google Scholar]
- 21. Caraballo R, Valenzuela GR. Cannabidiol-enriched medical cannabis as add-on therapy in children with treatment-resistant West syndrome: a study of eight patients. Seizure 2021; 92: 238–243. [DOI] [PubMed] [Google Scholar]
- 22. Chen KA, Farrar M, Cardamone M, et al. Cannabidiol for treating drug-resistant epilepsy in children: the New South Wales experience. Med J Aust 2018; 209: 217–221. [DOI] [PubMed] [Google Scholar]
- 23. Cohen NT, Bahar B, Conry JA, et al. Variability in serum concentrations and clinical response in artisanal versus pharmaceutical cannabidiol treatment of pediatric pharmacoresistant epilepsy. J Pediatr Pharmacol Ther 2022; 27: 558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. D’Onofrio G, Kuchenbuch M, Hachon-Le Camus C, et al. Slow titration of cannabidiol add-on in drug-resistant epilepsies can improve safety with maintained efficacy in an open-label study. Front Neurol 2020; 11: 829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. New Engl J Med 2017; 376: 2011–2020. [DOI] [PubMed] [Google Scholar]
- 26. Devinsky O, Marmanillo A, Hamlin T, et al. Observational study of medical marijuana as a treatment for treatment-resistant epilepsies. Ann Clin Transl Neurol 2022; 9: 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Devinsky O, Marsh E, Friedman D, et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol 2016; 15: 270–278. [DOI] [PubMed] [Google Scholar]
- 28. Devinsky O, Patel AD, Cross JH, et al. Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. New Engl J Med 2018; 378: 1888–1897. [DOI] [PubMed] [Google Scholar]
- 29. Freeman JL. Safety of cannabidiol prescribed for children with refractory epilepsy. Med J Aust 2018; 209: 228–229. [DOI] [PubMed] [Google Scholar]
- 30. Garza Morales SJ, Benavides Aguilar O, Bastida Mercado E. Use of cannabidiol (RSHO) in the treatment of refractory epilepsy (Lennox-Gastaut syndrome), experience of 38 cases. Epilepsia 2017; 58 (Suppl. 5): S53. (Conference Abstract). [Google Scholar]
- 31. Gaston TE, Ampah SB, Martina Bebin E, et al. Long-term safety and efficacy of highly purified cannabidiol for treatment refractory epilepsy. Epilepsy Behav 2021; 117: 107862. [DOI] [PubMed] [Google Scholar]
- 32. Geffrey AL, Pollack SF, Bruno PL, et al. Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia 2015; 56: 1246–1251. [DOI] [PubMed] [Google Scholar]
- 33. Hausman-Kedem M, Menascu S, Kramer U. Efficacy of CBD-enriched medical cannabis for treatment of refractory epilepsy in children and adolescents: an observational, longitudinal study. Brain Dev 2018; 40: 544–551. [DOI] [PubMed] [Google Scholar]
- 34. Herlopian A, Hess EJ, Barnett J, et al. Cannabidiol in treatment of refractory epileptic spasms: an open-label study. Epilepsy Behav 2020; 106: 106988. [DOI] [PubMed] [Google Scholar]
- 35. Iannone LF, Arena G, Battaglia D, et al. Results from an Italian expanded access program on cannabidiol treatment in highly refractory Dravet syndrome and Lennox–Gastaut syndrome. Front Neurol 2021; 12: 673135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kaplan EH, Offermann EA, Sievers JW, et al. Cannabidiol treatment for refractory seizures in Sturge-Weber syndrome. Pediatr Neurol 2017; 71: 18–23.e2. [DOI] [PubMed] [Google Scholar]
- 37. Klotz KA, Grob D, Hirsch M, et al. Efficacy and tolerance of synthetic cannabidiol for treatment of drug resistant epilepsy. Front Neurol 2019; 10: 1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Klotz KA, Grob D, Schönberger J, et al. Effect of cannabidiol on interictal epileptiform activity and sleep architecture in children with intractable epilepsy: a prospective open-label study. CNS Drugs 2021; 35: 1207–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koo CM, Kim SH, Lee JS, et al. Cannabidiol for treating Lennox-Gastaut syndrome and Dravet syndrome in Korea. J Korean Med Sci 2020; 35: e427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Laux LC, Bebin EM, Checketts D, et al. Long-term safety and efficacy of cannabidiol in children and adults with treatment resistant Lennox-Gastaut syndrome or Dravet syndrome: expanded access program results. Epilepsy Res 2019; 154: 13–20. [DOI] [PubMed] [Google Scholar]
- 41. Marchese F, Vari MS, Balagura G, et al. An open retrospective study of a standardized cannabidiol based-oil in treatment-resistant epilepsy. Cannabis Cannabinoid Res 2022; 7: 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McCoy B, Wang L, Zak M, et al. A prospective open-label trial of a CBD/THC cannabis oil in Dravet syndrome. Ann Clin Transl Neurol 2018; 5: 1077–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mitelpunkt A, Kramer U, Hausman Kedem M, et al. The safety, tolerability, and effectiveness of PTL-101, an oral cannabidiol formulation, in pediatric intractable epilepsy: a phase II, open-label, single-center study. Epilepsy Behav 2019; 98: 233–237. [DOI] [PubMed] [Google Scholar]
- 44. Navarro CE. Cannabis-based magistral formulation is highly effective as an adjuvant treatment in drug-resistant focal epilepsy in adult patients: an open-label prospective cohort study. Neurol Sci 2023; 44: 297–304. [DOI] [PubMed] [Google Scholar]
- 45. Neubauer D, Perković Benedik M, Osredkar D. Cannabidiol for treatment of refractory childhood epilepsies: experience from a single tertiary epilepsy center in Slovenia. Epilepsy Behav 2018; 81: 79–85. [DOI] [PubMed] [Google Scholar]
- 46. O’Brien TJ, Berkovic SF, French JA, et al. Adjunctive transdermal cannabidiol for adults with focal epilepsy: a randomized clinical trial. JAMA Netw Open 2022; 5: e2220189–20220701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Park YD, Linder DF, Pope J, et al. Long-term efficacy and safety of cannabidiol (CBD) in children with treatment-resistant epilepsy: results from a state-based expanded access program. Epilepsy Behav 2020; 112: 107474. [DOI] [PubMed] [Google Scholar]
- 48. Patel AD, Mazurkiewicz-Bełdzińska M, Chin RF, et al. Long-term safety and efficacy of add-on cannabidiol in patients with Lennox-Gastaut syndrome: results of a long-term open-label extension trial. Epilepsia 2021; 62: 2228–2239. [DOI] [PubMed] [Google Scholar]
- 49. Patel S, Grinspoon R, Fleming B, et al. The long-term efficacy of cannabidiol in the treatment of refractory epilepsy. Epilepsia 2021; 62: 1594–1603. [DOI] [PubMed] [Google Scholar]
- 50. Pesántez Ríos G, Armijos Acurio L, Jimbo Sotomayor R, et al. A pilot study on the use of low doses of CBD to control seizures in rare and severe forms of drug-resistant epilepsy. Life 2022; 12: 20221209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pietrafusa N, Ferretti A, Trivisano M, et al. Purified cannabidiol for treatment of refractory epilepsies in pediatric patients with developmental and epileptic encephalopathy. Paediatr Drugs 2019; 21: 283–290. [DOI] [PubMed] [Google Scholar]
- 52. Press CA, Knupp KG, Chapman KE. Parental reporting of response to oral cannabis extracts for treatment of refractory epilepsy. Epilepsy Behav 2015; 45: 49–52. [DOI] [PubMed] [Google Scholar]
- 53. Sands TT, Rahdari S, Oldham MS, et al. Long-term safety, tolerability, and efficacy of cannabidiol in children with refractory epilepsy: results from an expanded access program in the US. CNS Drugs 2019; 33: 47–60. [DOI] [PubMed] [Google Scholar]
- 54. Savage TE, Sourbron J, Bruno PL, et al. Efficacy of cannabidiol in subjects with refractory epilepsy relative to concomitant use of clobazam. Epilepsy Res 2020; 160: 106263. [DOI] [PubMed] [Google Scholar]
- 55. Scheffer IE, Halford JJ, Miller I, et al. Add-on cannabidiol in patients with Dravet syndrome: results of a long-term open-label extension trial. Epilepsia 2021; 62: 2505–2517. [DOI] [PubMed] [Google Scholar]
- 56. Scheffer IE, Hulihan J, Messenheimer J, et al. Safety and tolerability of transdermal cannabidiol gel in children with developmental and epileptic encephalopathies: a nonrandomized controlled trial. JAMA Netw Open 2021; 4: e2123930–20210901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sianati B, Schramke C, Valeriano J. Medical Marijuana and its use in treatment-refractory seizures: the Pittsburgh experience. In: Neurology conference: 71st annual meeting of the American Academy of Neurology, AAN 2019 meeting held in Philadelphia, p. 92. Conference Abstract. [Google Scholar]
- 58. Szaflarski JP, Bebin EM, Comi AM, et al. Long-term safety and treatment effects of cannabidiol in children and adults with treatment-resistant epilepsies: expanded access program results. Epilepsia 2018; 59: 1540–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Thiele EA, Marsh ED, French JA, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2018; 391: 1085–1096. [DOI] [PubMed] [Google Scholar]
- 60. Thiele EA, Bebin EM, Filloux F, et al. Long-term cannabidiol treatment for seizures in patients with tuberous sclerosis complex: an open-label extension trial. Epilepsia 2022; 63: 426–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Thiele EA, Bebin EM, Bhathal H, et al. Add-on cannabidiol treatment for drug-resistant seizures in tuberous sclerosis complex: a placebo-controlled randomized clinical trial. JAMA Neurol 2021; 78: 285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tzadok M, Uliel-Siboni S, Linder I, et al. CBD-enriched medical cannabis for intractable pediatric epilepsy: the current Israeli experience. Seizure 2016; 35: 41–44. [DOI] [PubMed] [Google Scholar]
- 63. Tzadok M, Hamed N, Heimer G, et al. The long-term effectiveness and safety of cannabidiol-enriched oil in children with drug-resistant epilepsy. Pediatr Neurol 2022; 136: 15–19. [DOI] [PubMed] [Google Scholar]
- 64. Uliel-Sibony S, Hausman-Kedem M, Fattal-Valevski A, et al. Cannabidiol-enriched oil in children and adults with treatment-resistant epilepsy-does tolerance exist? Brain Dev 2021; 43: 89–96. [DOI] [PubMed] [Google Scholar]
- 65. Vezyroglou K, Eltze C, Varadkar S, et al. Cannabidiol as add on therapy in children with complex epilepsy. Dev Med Child Neurol 2017; 59: 17. (Conference Abstract). [Google Scholar]
- 66. Wheless JW, Dlugos D, Miller I, et al. Pharmacokinetics and tolerability of multiple doses of pharmaceutical-grade synthetic cannabidiol in pediatric patients with treatment-resistant epilepsy. CNS Drugs 2019; 33: 593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zilmer M, Olofsson K. Cannabidiol treatment of severe refractory epilepsy in children and young adults. Dan Med J 2021; 68: 20210428. [PubMed] [Google Scholar]
- 68. Fazlollahi A, Zahmatyar M, ZareDini M, et al. Adverse events of cannabidiol use in patients with epilepsy: a systematic review and meta-analysis. JAMA Netw Open 2023; 6: e239126–20230403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gaston TE, Bebin EM, Cutter GR, et al. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia 2017; 58: 1586–1592. [DOI] [PubMed] [Google Scholar]
- 70. Devinsky O, Patel AD, Thiele EA, et al. Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology 2018; 90: e1204–e1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Devinsky O, Verducci C, Thiele EA, et al. Open-label use of highly purified CBD (Epidiolex®) in patients with CDKL5 deficiency disorder and Aicardi, Dup15q, and Doose syndromes. Epilepsy Behav 2018; 86: 131–137. [DOI] [PubMed] [Google Scholar]
- 72. Gofshteyn JS, Wilfong A, Devinsky O, et al. Cannabidiol as a potential treatment for febrile infection-related epilepsy syndrome (FIRES) in the acute and chronic phases. J Child Neurol 2017; 32: 35–40. [DOI] [PubMed] [Google Scholar]
- 73. Davis BH, Beasley TM, Amaral M, et al. Pharmacogenetic predictors of cannabidiol response and tolerability in treatment-resistant epilepsy. Clin Pharmacol Ther 2021; 110: 1368–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_17562864231207755 for Long-term efficacy and adverse effects of cannabidiol in adjuvant treatment of drug-resistant epilepsy: a systematic review and meta-analysis by Shengyi Liu, Zihua He and Jinmei Li in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-docx-2-tan-10.1177_17562864231207755 for Long-term efficacy and adverse effects of cannabidiol in adjuvant treatment of drug-resistant epilepsy: a systematic review and meta-analysis by Shengyi Liu, Zihua He and Jinmei Li in Therapeutic Advances in Neurological Disorders