Abstract
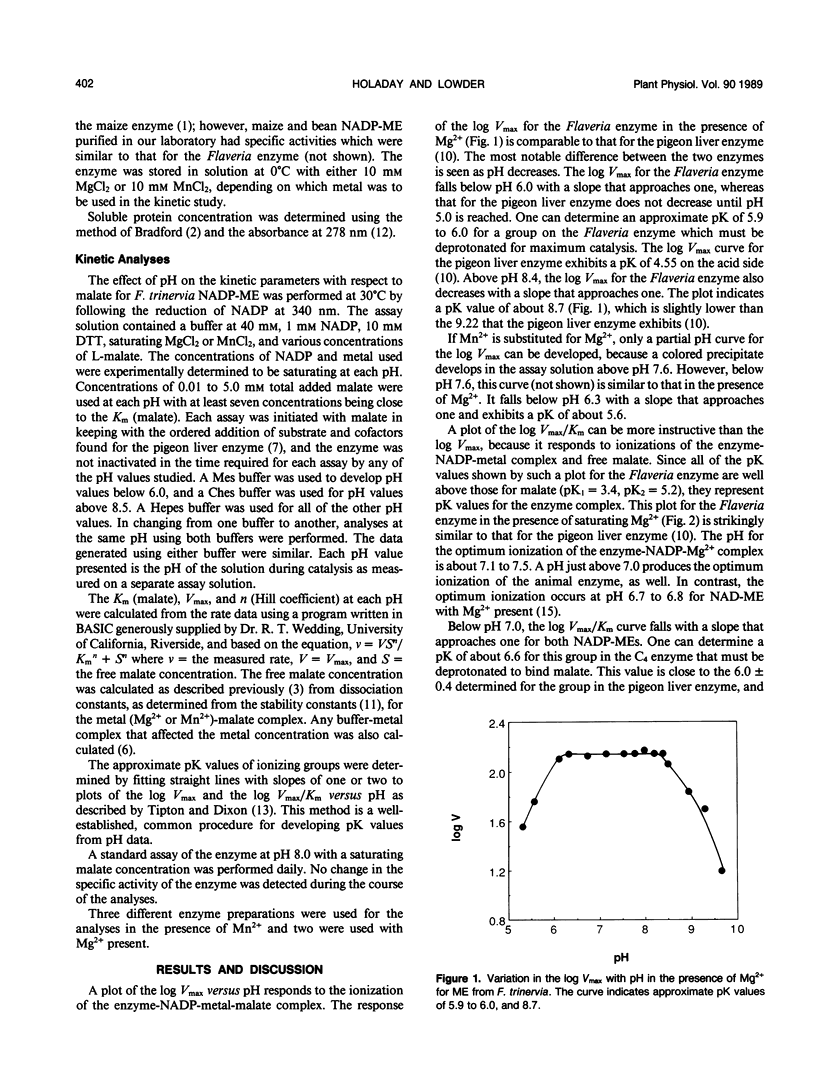
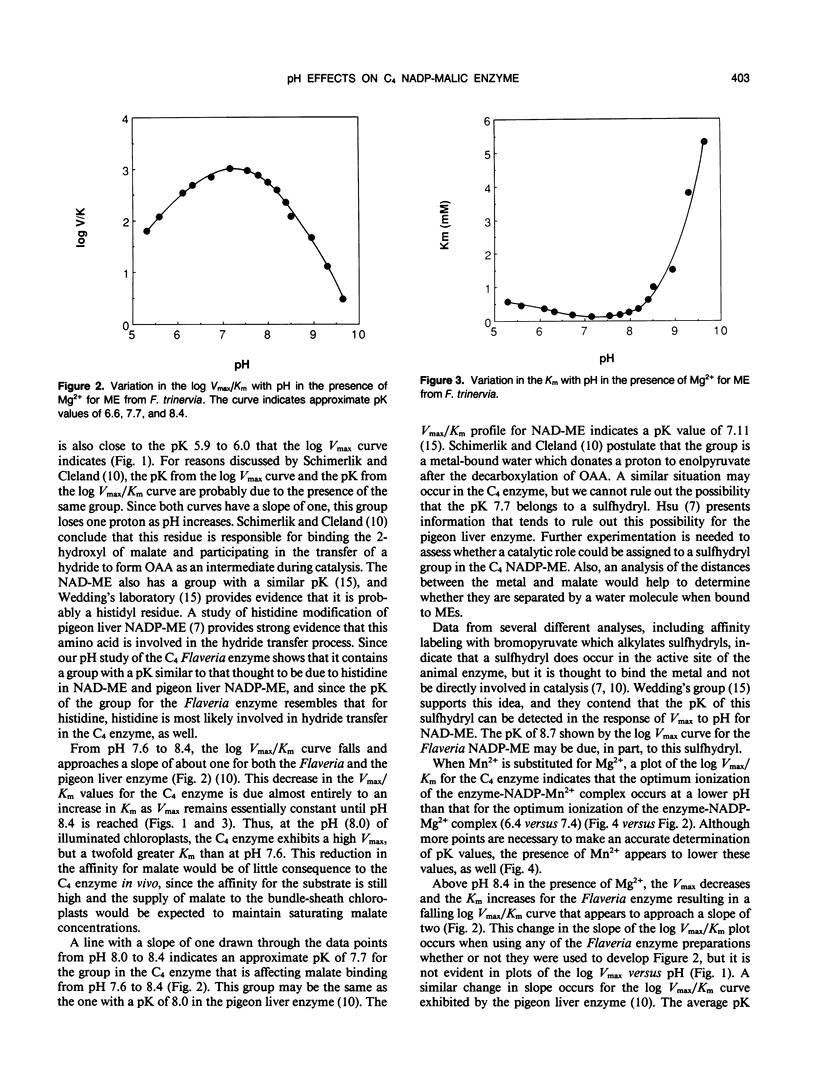
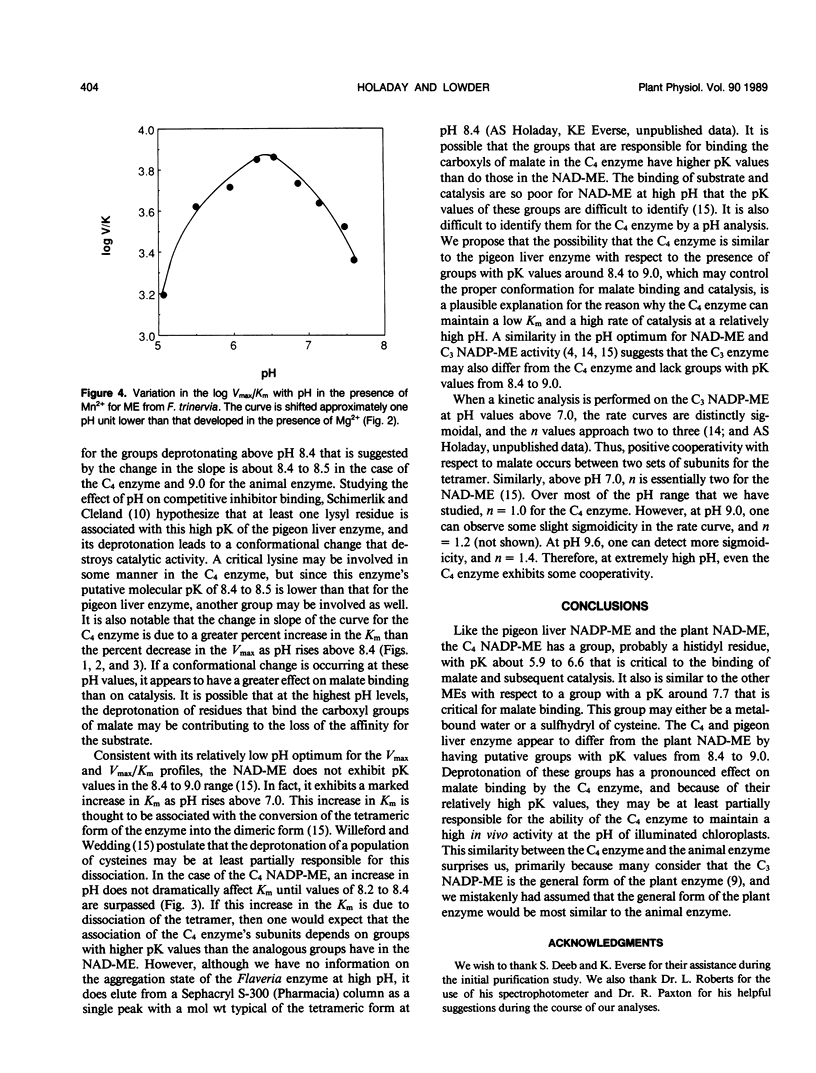
We have used the pH variation in the kinetic parameters with respect to malate of NADP-malic enzyme purified from the C4 species, Flaveria trinervia, to compare the pK values of its functional groups with those for the pigeon liver NADP-malic enzyme (MI Schimerlik, WW Cleland [1977] Biochemistry 16: 576-583) and the plant NAD-malic enzyme (KO Willeford, RT Wedding [1987] Plant Physiol 84: 1084-1087). Like the other enzymes, the C4 enzyme has a group with a pK of about 6.0 (6.6 for the C4 enzyme), as indicated from plots of the log Vmax/Km (Vmax = maximum rate of catalysis) versus pH, which must lose a proton for malate binding and subsequent catalysis. The optimum ionization for the C4 enzyme-NADP-Mg2+ complex occurs at pH 7.1 to 7.5. From pH 7.5 to 8.4, the Km increases, but Vmax remains constant. The log Vmax/Km plot in this pH range indicates a group with a pK of about 7.7. The other malic enzymes exhibit a similar pK. Above pH 8.4, deprotonation leads to a marked increase in Km and a decrease in Vmax for the C4 enzyme. As in the case of the animal enzyme, the log Vmax/Km plot for the C4 enzyme appears to approach a slope of two. The curve suggests an average pK of 8.4 for the groups involved, while the animal enzyme exhibits an average pK of 9.0. The NAD-malic enzyme does not exhibit any pK values at these high pK values. We hypothesize that the putative groups with the high pK values may be at least partially responsible for the ability of the C4 NADP-malic enzyme to maintain high activity at pH 8.0 in illuminated chloroplasts.
Full text
PDF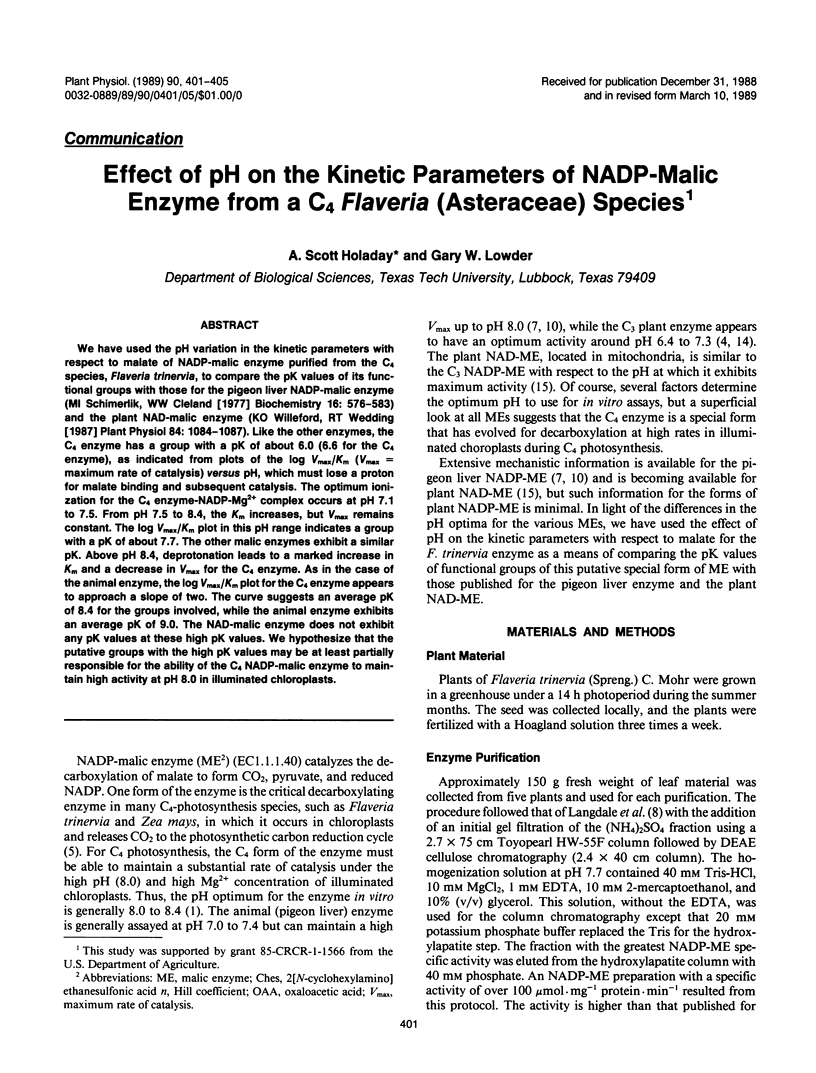

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asami S., Inoue K., Akazawa T. NADP-malic enzyme from maize leaf: regulatory properties. Arch Biochem Biophys. 1979 Sep;196(2):581–587. doi: 10.1016/0003-9861(79)90311-4. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Canellas P. F., Wedding R. T. Substrate and metal ion interactions in the NAD+ malic enzyme from cauliflower. Arch Biochem Biophys. 1980 Jan;199(1):259–264. doi: 10.1016/0003-9861(80)90279-9. [DOI] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Hsu R. Y. Pigeon liver malic enzyme. Mol Cell Biochem. 1982 Mar 5;43(1):3–26. doi: 10.1007/BF00229535. [DOI] [PubMed] [Google Scholar]
- Langdale J. A., Metzler M. C., Nelson T. The argentia mutation delays normal development of photosynthetic cell-types in Zea mays. Dev Biol. 1987 Jul;122(1):243–255. doi: 10.1016/0012-1606(87)90349-6. [DOI] [PubMed] [Google Scholar]
- Schimerlik M. I., Cleland W. W. pH variation of the kinetic parameters and the catalytic mechanism of malic enzyme. Biochemistry. 1977 Feb 22;16(4):576–583. doi: 10.1021/bi00623a003. [DOI] [PubMed] [Google Scholar]
- Thorniley M. S., Dalziel K. NADP-linked malic enzyme. Purification from maize leaves, Mr and subunit composition. Biochem J. 1988 Aug 15;254(1):229–233. doi: 10.1042/bj2540229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipton K. F., Dixon H. B. Effects of pH on enzymes. Methods Enzymol. 1979;63:183–234. doi: 10.1016/0076-6879(79)63011-2. [DOI] [PubMed] [Google Scholar]
- Willeford K. O., Wedding R. T. pH Effects on the Activity and Regulation of the NAD Malic Enzyme. Plant Physiol. 1987 Aug;84(4):1084–1087. doi: 10.1104/pp.84.4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]


