Abstract
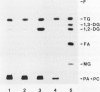
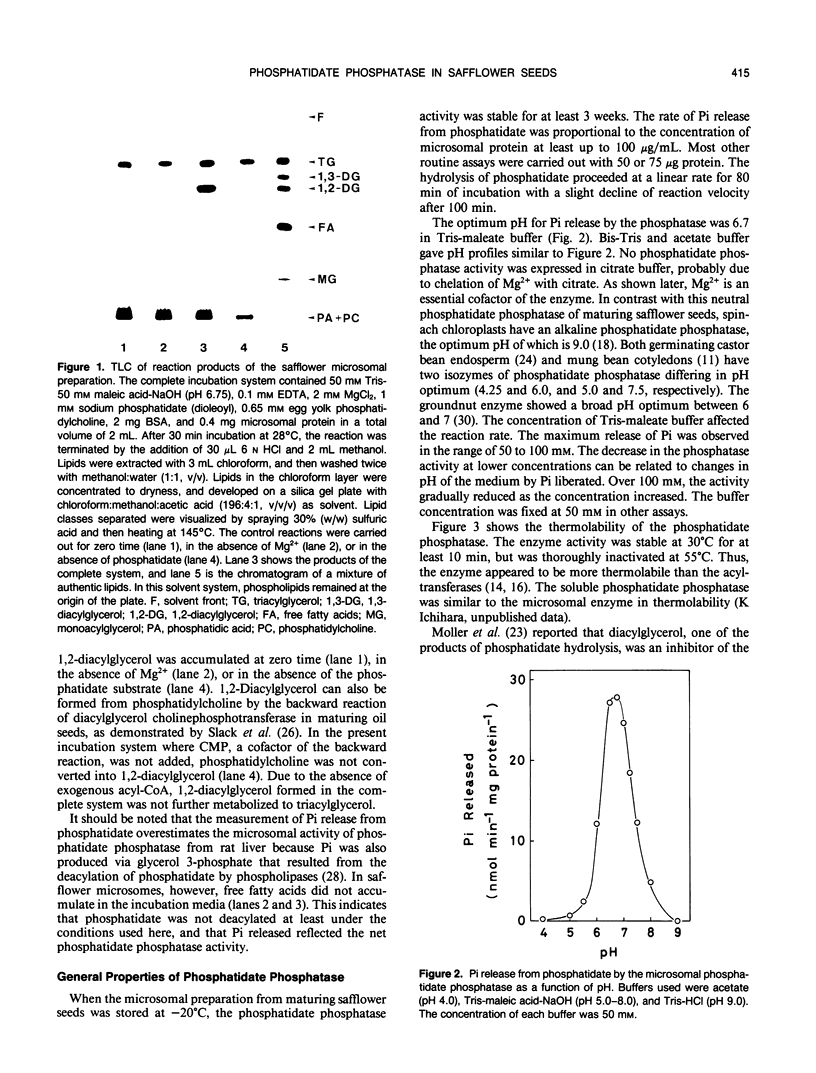
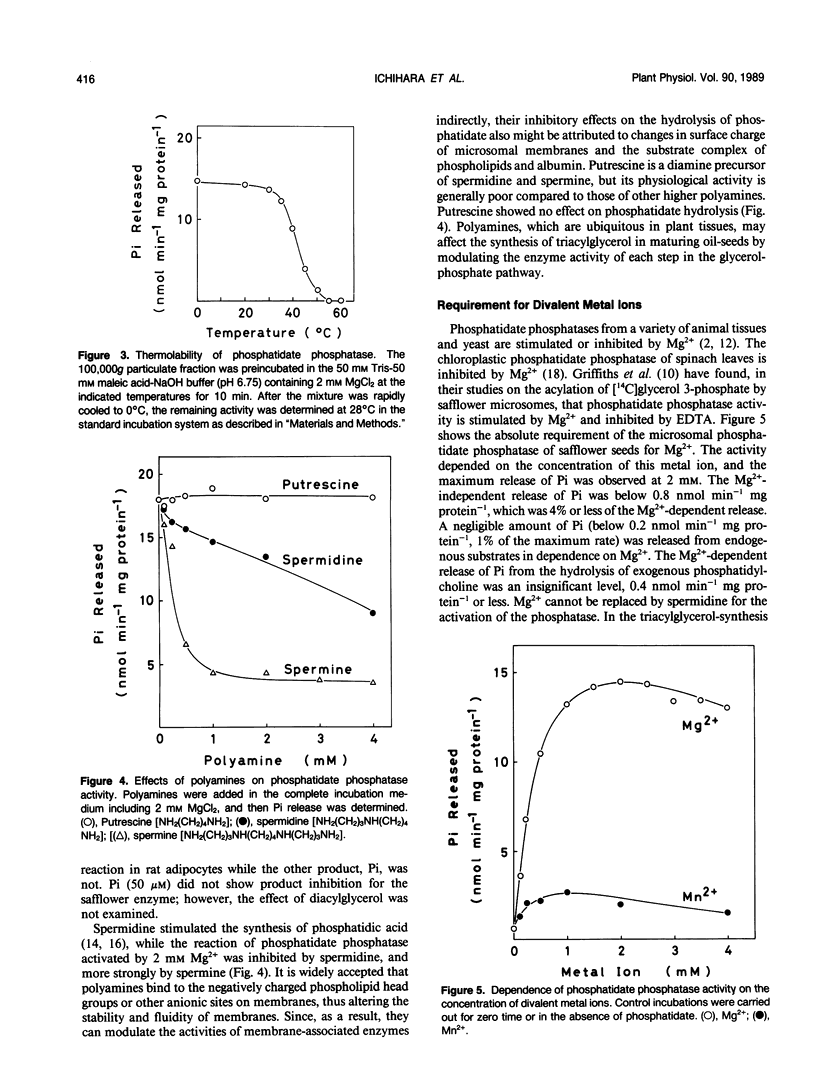
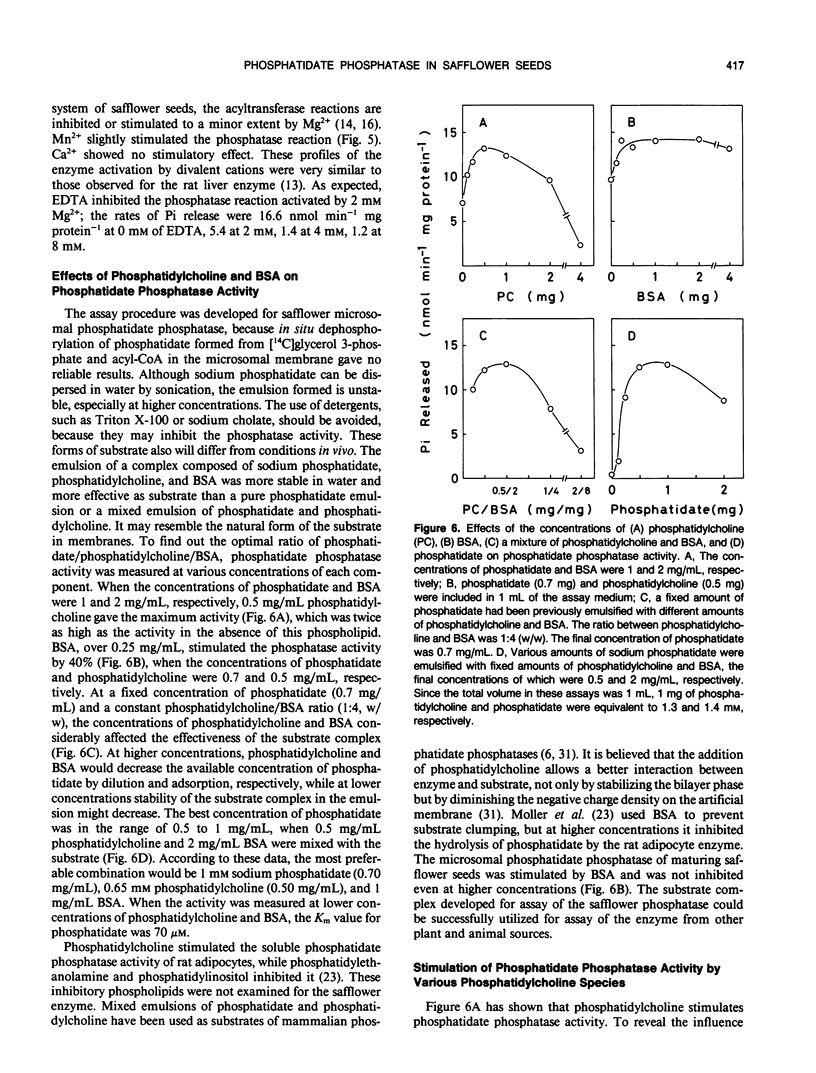
An assay system comprising sodium phosphatidate, phosphatidylcholine, and bovine serum albumin has been developed for the reproducible determination of phosphatidate phosphatase activity in maturing seeds of safflower (Carthamus tinctorius L.). The activity was detected in both membrane and soluble fractions, and the microsomal phosphatidate phosphatase was characterized. The optimum pH for Pi release was 6.7, and the activity depended on the concentration of Mg2+. Phosphatidylcholine and bovine serum albumin stimulated the phosphatase reaction. This phosphatase was highly specific for phosphatidate; lysophosphatidate, and water-soluble phosphate esters did not serve as substrate. The specific activity was approximately 20 nanomoles per minute per milligram of protein, which was close to that of glycerol-phosphate acyltransferase and higher than that of diacylglycerol acyltransferase. Furthermore, the activity per seed was enough to account for the rate of triacylglycerol accumulation in vivo. The step of diacylglycerol formation by phosphatidate phosphatase does not appear to be rate-limiting for triacylglycerol synthesis during seed maturation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRON E. J., STUMPF P. K. Fat metabolism in higher plants. XIX. The biosynthesis of triglycerides by avocado-mesocarp enzymes. Biochim Biophys Acta. 1962 Jul 2;60:329–337. doi: 10.1016/0006-3002(62)90408-0. [DOI] [PubMed] [Google Scholar]
- Blank M. L., Snyder F. Specificities of alkaline and acid phosphatases in the dephosphorylation of phospholipids. Biochemistry. 1970 Dec 8;9(25):5034–5036. doi: 10.1021/bi00827a031. [DOI] [PubMed] [Google Scholar]
- Brindley D. N. Intracellular translocation of phosphatidate phosphohydrolase and its possible role in the control of glycerolipid synthesis. Prog Lipid Res. 1984;23(3):115–133. doi: 10.1016/0163-7827(84)90001-8. [DOI] [PubMed] [Google Scholar]
- Butterwith S. C., Hopewell R., Brindley D. N. Partial purification and characterization of the soluble phosphatidate phosphohydrolase of rat liver. Biochem J. 1984 Jun 15;220(3):825–833. doi: 10.1042/bj2200825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frentzen M., Heinz E., McKeon T. A., Stumpf P. K. Specificities and selectivities of glycerol-3-phosphate acyltransferase and monoacylglycerol-3-phosphate acyltransferase from pea and spinach chloroplasts. Eur J Biochem. 1983 Jan 1;129(3):629–636. doi: 10.1111/j.1432-1033.1983.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Gardiner S. E., Roughan P. G. Relationship between fatty-acyl composition of diacylgalactosylglycerol and turnover of chloroplast phosphatidate. Biochem J. 1983 Mar 15;210(3):949–952. doi: 10.1042/bj2100949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Stobart A. K., Stymne S. The acylation of sn-glycerol 3-phosphate and the metabolism of phosphatidate in microsomal preparations from the developing cotyledons of safflower (Carthamus tinctorius L.) seed. Biochem J. 1985 Sep 1;230(2):379–388. doi: 10.1042/bj2300379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman E. M., Chrispeels M. J. Characteristics and subcellular localization of phospholipase d and phosphatidic Acid phosphatase in mung bean cotyledons. Plant Physiol. 1980 Nov;66(5):1001–1007. doi: 10.1104/pp.66.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka K., Yamashita S., Numa S. Partial purification, properties, and subcellulsr distribution of rat liver phosphatidate phosphatase. J Biochem. 1975 Mar;77(3):501–509. doi: 10.1093/oxfordjournals.jbchem.a130751. [DOI] [PubMed] [Google Scholar]
- Hosaka K., Yamashita S. Partial purification and properties of phosphatidate phosphatase in Saccharomyces cerevisiae. Biochim Biophys Acta. 1984 Oct 24;796(1):102–109. [PubMed] [Google Scholar]
- Huff J. R., Anderson P. S., Baldwin J. J., Clineschmidt B. V., Guare J. P., Lotti V. J., Pettibone D. J., Randall W. C., Vacca J. P. N-(1,3,4,6,7,12b-hexahydro-2H-benzo[b]furo[2,3-a]quinolizin -2-yl)-N- methyl-2-hydroxyethane-sulfonamide: a potent and selective alpha 2-adrenoceptor antagonist. J Med Chem. 1985 Dec;28(12):1756–1759. doi: 10.1021/jm00150a002. [DOI] [PubMed] [Google Scholar]
- Ichihara K., Asahi T., Fujii S. 1-Acyl-sn-glycerol-3-phosphate acyltransferase in maturing safflower seeds and its contribution to the non-random fatty acid distribution of triacylglycerol. Eur J Biochem. 1987 Sep 1;167(2):339–347. doi: 10.1111/j.1432-1033.1987.tb13342.x. [DOI] [PubMed] [Google Scholar]
- Ichihara K., Takahashi T., Fujii S. Diacylglycerol acyltransferase in maturing safflower seeds: its influences on the fatty acid composition of triacylglycerol and on the rate of triacylglycerol synthesis. Biochim Biophys Acta. 1988 Jan 19;958(1):125–129. doi: 10.1016/0005-2760(88)90253-6. [DOI] [PubMed] [Google Scholar]
- Ichihara K. sn-Glycerol-3-phosphate acyltransferase in a particulate fraction from maturing safflower seeds. Arch Biochem Biophys. 1984 Aug 1;232(2):685–698. doi: 10.1016/0003-9861(84)90589-7. [DOI] [PubMed] [Google Scholar]
- Joyard J., Douce R. Characterization of phosphatidate phosphohydrolase activity associated with chloroplast envelope membranes. FEBS Lett. 1979 Jun 1;102(1):147–150. doi: 10.1016/0014-5793(79)80947-3. [DOI] [PubMed] [Google Scholar]
- KATES M. Hydrolysis of lecithin by plant plastid enzymes. Can J Biochem Physiol. 1955 Jul;33(4):575–589. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mavis R. D., Finkelstein J. N., Hall B. P. Pulmonary surfactant synthesis. A highly active microsomal phosphatidate phosphohydrolase in the lung. J Lipid Res. 1978 May;19(4):467–477. [PubMed] [Google Scholar]
- Moller F., Green P., Harkness E. J. Soluble rat adipocyte phosphatidate phosphatase activity: characterization and effects of fasting and various lipids. Biochim Biophys Acta. 1977 Feb 23;486(2):359–368. doi: 10.1016/0005-2760(77)90032-7. [DOI] [PubMed] [Google Scholar]
- Moore T. S., Lord J. M., Kagawa T., Beevers H. Enzymes of phospholipid metabolism in the endoplasmic reticulum of castor bean endosperm. Plant Physiol. 1973 Jul;52(1):50–53. doi: 10.1104/pp.52.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturton R. G., Brindley D. N. Problems encountered in measuring the activity of phosphatidate phosphohydrolase. Biochem J. 1978 Apr 1;171(1):263–266. doi: 10.1042/bj1710263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton P. A., Possmayer F. Mg2-dependent phosphatidate phosphohydrolase of rat lung: development of an assay employing a defined chemical substrate which reflects the phosphohydrolase activity measured using membrane-bound substrate. Anal Biochem. 1985 Dec;151(2):479–486. doi: 10.1016/0003-2697(85)90208-8. [DOI] [PubMed] [Google Scholar]