Abstract
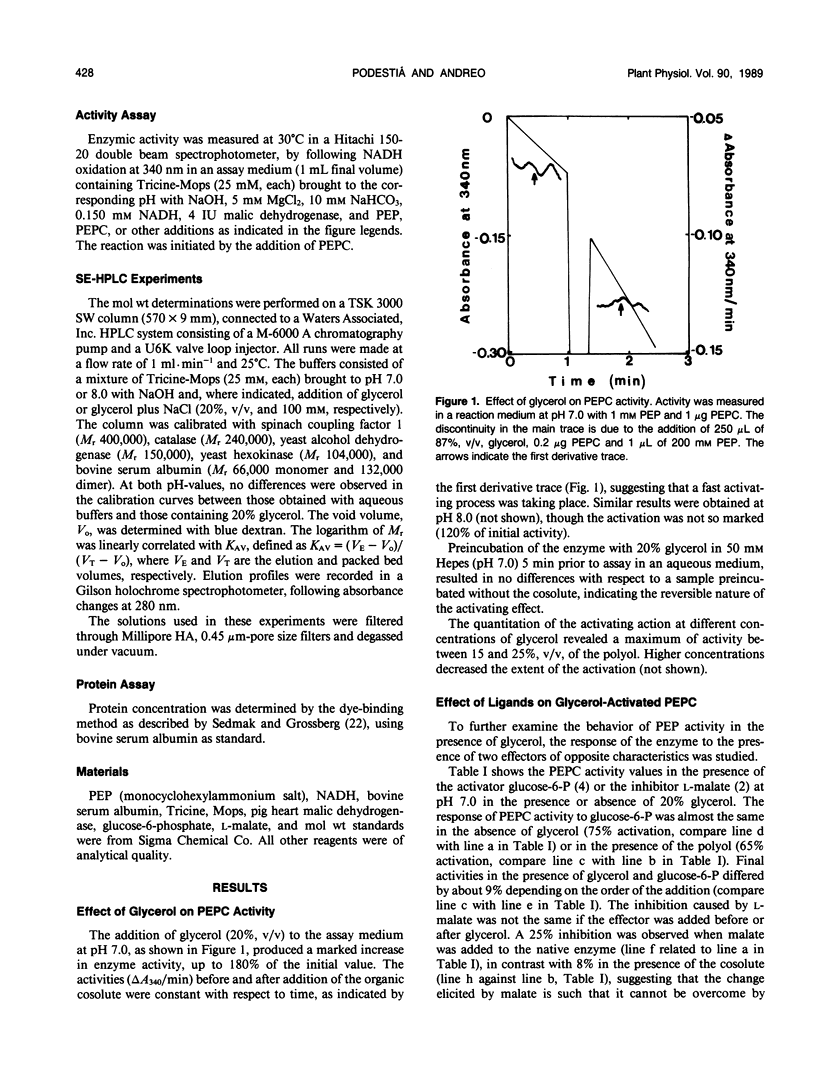
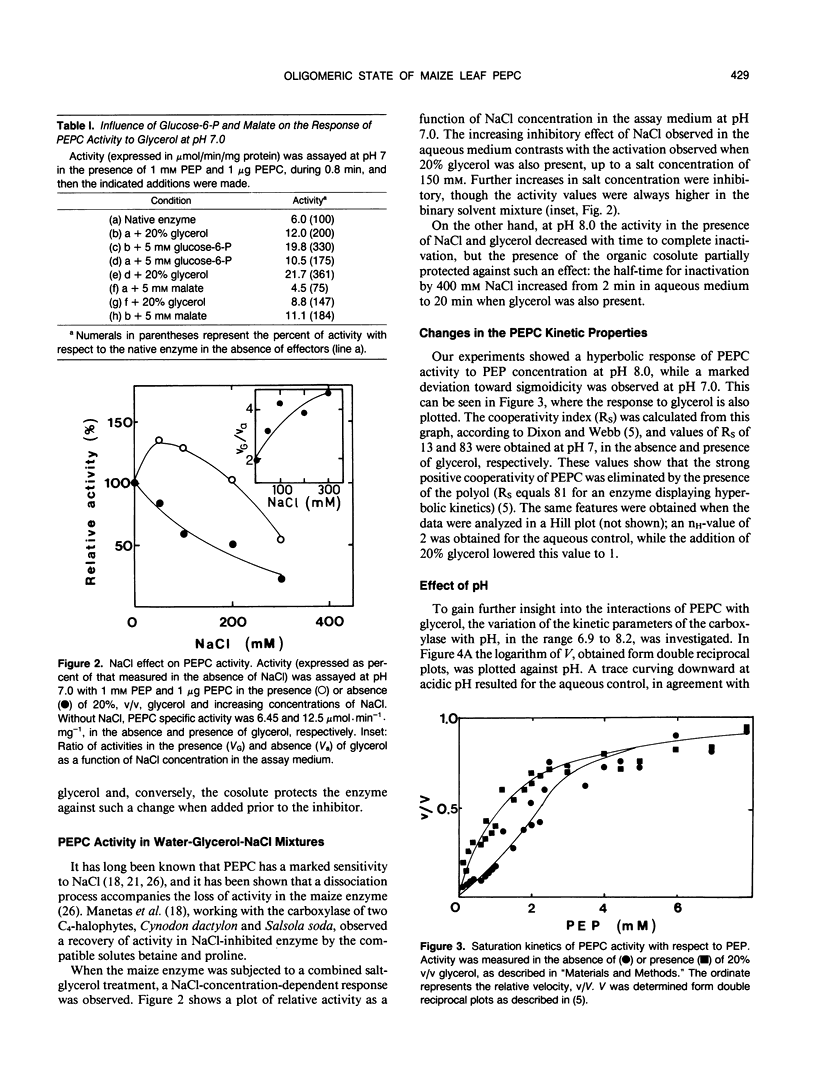
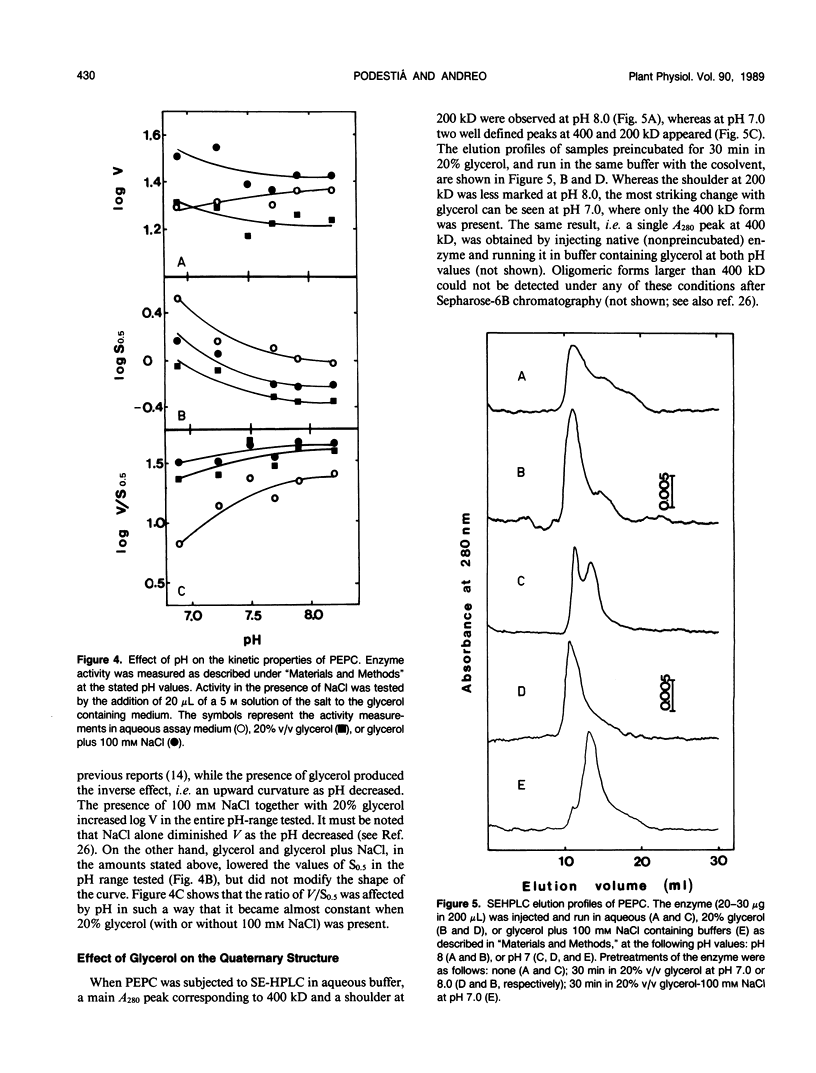
Maize (Zea mays L.) leaf phosphoenopyruvate (PEP) carboxylase activity at subsaturating levels of PEP was increased by the inclusion of glycerol (20%, v/v) in the assay medium. The extent of activation was dependent on H+ concentration, being more marked at pH 7 (with activities 100% higher than in aqueous medium) than at pH 8 (20% activation). The determination of the substrate concentration necessary to achieve half-maximal enzyme activity (S0.5) (PEP) and maximal velocity (V) between pH 6.9 and 8.2 showed a uniform decrease in S0.5 in the presence of glycerol over the entire pH range tested, and only a slight decrease in V at pH values near 8. Including NaCl (100 millimolar) in the glycerol containing assay medium resulted in additional activation, mainly due to an increase in V over the entire range of pH. Glucose-6-phosphate (5 millimolar) activated both the native and the glycerol-treated enzyme almost to the same extent, at pH 7 and 1 millimolar PEP. Inhibition by 5 millimolar malate at pH 7 and subsaturating PEP was considerably lower in the presence of glycerol than in an aqueous medium (8% against 25%, respectively). Size-exclusion high performance liquid chromatography in aqueous buffer revealed the existence of an equilibrium between the tetrameric and dimeric enzyme forms, which is displaced to the tetramer as the pH was increased from 7 to 8. In the presence of glycerol, only the 400 kilodalton tetrameric form was observed at pH 7 or 8. However, dissociation into dimers by NaCl could not be prevented by the polyol. We conclude that the control of the aggregation state by the metabolic status of the cell could be one regulatory mechanism of PEP carboxylase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Budde R. J., Chollet R. In vitro phosphorylation of maize leaf phosphoenolpyruvate carboxylase. Plant Physiol. 1986 Dec;82(4):1107–1114. doi: 10.1104/pp.82.4.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton A. B. How crowded is the cytoplasm? Cell. 1982 Sep;30(2):345–347. doi: 10.1016/0092-8674(82)90231-8. [DOI] [PubMed] [Google Scholar]
- Furbank R. T., Hatch M. D. Mechanism of c(4) photosynthesis: the size and composition of the inorganic carbon pool in bundle sheath cells. Plant Physiol. 1987 Dec;85(4):958–964. doi: 10.1104/pp.85.4.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekko K., Timasheff S. N. Mechanism of protein stabilization by glycerol: preferential hydration in glycerol-water mixtures. Biochemistry. 1981 Aug 4;20(16):4667–4676. doi: 10.1021/bi00519a023. [DOI] [PubMed] [Google Scholar]
- Gekko K., Timasheff S. N. Thermodynamic and kinetic examination of protein stabilization by glycerol. Biochemistry. 1981 Aug 4;20(16):4677–4686. doi: 10.1021/bi00519a024. [DOI] [PubMed] [Google Scholar]
- Hague D. R., Sims T. L. Evidence for Light-stimulated Synthesis of Phosphoenolpyruvate Carboxylase in Leaves of Maize. Plant Physiol. 1980 Sep;66(3):505–509. doi: 10.1104/pp.66.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C., Sugiyama T., Akazawa T. Light modulation of maize leaf phosphoenolpyruvate carboxylase. Plant Physiol. 1986 Oct;82(2):550–554. doi: 10.1104/pp.82.2.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C., Sugiyama T. Changes in Sensitivity to Effectors of Maize Leaf Phosphoenolypyruvate Carboxylase during Light/Dark Transitions. Plant Physiol. 1986 Jun;81(2):674–677. doi: 10.1104/pp.81.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias A. A., Andreo C. S. On the molecular mechanism of maize phosphoenolpyruvate carboxylase activation by thiol compounds. Plant Physiol. 1984 Aug;75(4):983–987. doi: 10.1104/pp.75.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J. A., Chollet R. Light/dark regulation of maize leaf phosphoenolpyruvate carboxylase by in vivo phosphorylation. Arch Biochem Biophys. 1988 Mar;261(2):409–417. doi: 10.1016/0003-9861(88)90357-8. [DOI] [PubMed] [Google Scholar]
- Karabourniotis G., Manetas Y., Gavalas N. A. Detecting Photoactivation of Phosphoenolpyruvate Carboxylase in C(4) Plants : An Effect of pH. Plant Physiol. 1985 Feb;77(2):300–302. doi: 10.1104/pp.77.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond C. B. Salt responses of carboxylation enzymes from species differing in salt tolerance. Plant Physiol. 1972 Feb;49(2):260–263. doi: 10.1104/pp.49.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Uedan K., Sugiyama T. Purification and characterization of phosphoenolpyruvate carboxylase from maize leaves. Plant Physiol. 1976 Jun;57(6):906–910. doi: 10.1104/pp.57.6.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R., Podestá F. E., González D. H., Andreo C. S. Proximity between fluorescent probes attached to four essential lysyl residues in phosphoenolpyruvate carboxylase. A resonance energy transfer study. Eur J Biochem. 1988 May 2;173(3):561–568. doi: 10.1111/j.1432-1033.1988.tb14036.x. [DOI] [PubMed] [Google Scholar]
- Walker G. H., Ku M. S., Edwards G. E. Catalytic activity of maize leaf phosphoenolpyruvate carboxylase in relation to oligomerization. Plant Physiol. 1986 Apr;80(4):848–855. doi: 10.1104/pp.80.4.848. [DOI] [PMC free article] [PubMed] [Google Scholar]


