Abstract
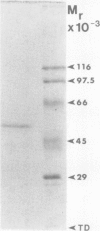
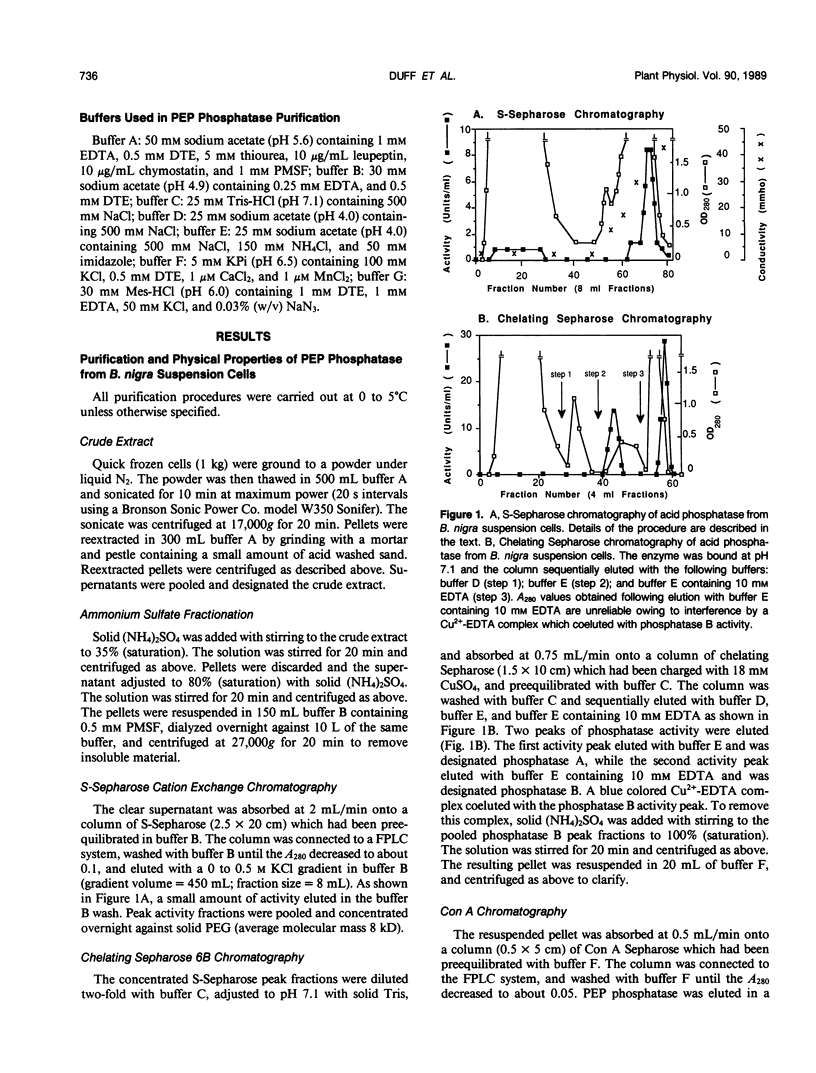
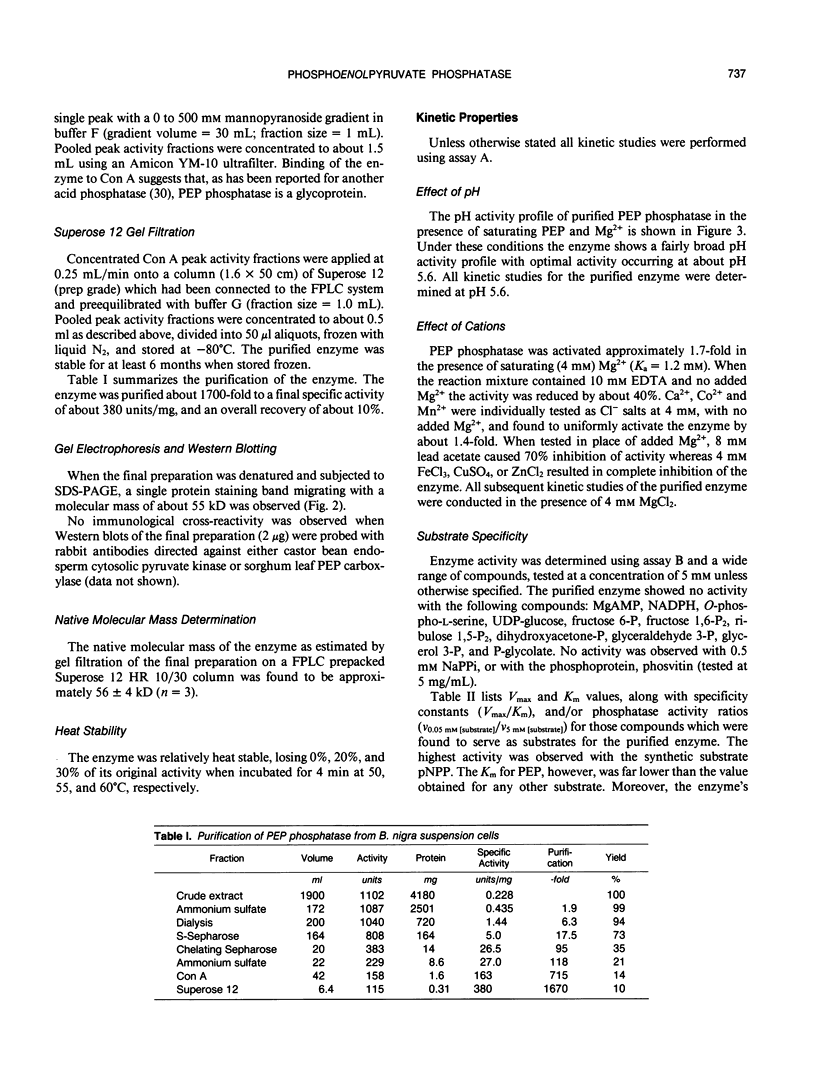
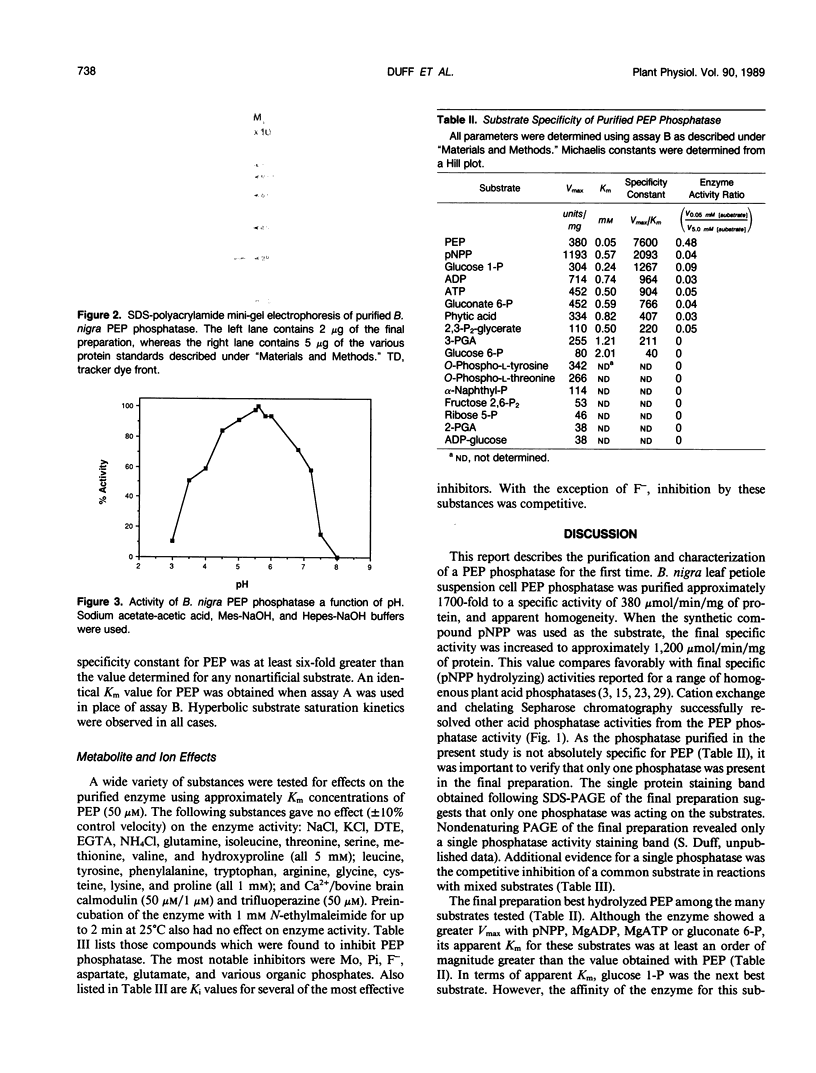
Phosphoenolpyruvate phosphatase from Brassica nigra leaf petiole suspension cells has been purified 1700-fold to apparent homogeneity and a final specific activity of 380 micromole pyruvate produced per minute per milligram protein. Purification steps included: ammonium sulfate fractionation, S-Sepharose, chelating Sepharose, concanavalin A Sepharose, and Superose 12 chromatography. The native protein was monomeric with a molecular mass of 56 kilodaltons as estimated by analytical gel filtration. The enzyme displayed a broad pH optimum of about pH 5.6 and was relatively heat stable. Western blots of microgram quantities of the final preparation showed no cross-reactivity when probed with rabbit polyclonal antibodies prepared against either castor bean endosperm cytosolic pyruvate kinase, or sorghum leaf phosphoenolpyruvate carboxylase. The final preparation exhibited a broad substrate selectivity, showing high activity toward p-nitrophenyl phosphate, adenosine diphosphate, adenosine triphosphate, gluconate 6-phosphate, and phosphoenolpyruvate, and moderate activity toward several other organic phosphates. Phosphoenolpyruvate phosphatase possessed at least a fivefold and sixfold greater affinity and specificity constant, respectively, for phosphoenolpyruvate (apparent Michaelis constant = 50 micromolar) than for any other nonartificial substrate. The enzyme was activated 1.7-fold by 4 millimolar magnesium, but was strongly inhibited by molybdate, fluoride, zinc, copper, iron, and lead ions, as well as by orthophosphate, ascorbate, glutamate, aspartate, and various organic phosphate compounds. It is postulated that phosphoenolpyruvate phosphatase functions to bypass the adenosine diphosphate dependent pyruvate kinase reaction during extended periods of orthophosphate starvation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baysdorfer C., Bassham J. A. Spinach pyruvate kinase isoforms : partial purification and regulatory properties. Plant Physiol. 1984 Feb;74(2):374–379. doi: 10.1104/pp.74.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenblum I., Chain E. An improved method for the colorimetric determination of phosphate. Biochem J. 1938 Feb;32(2):295–298. doi: 10.1042/bj0320295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen C. H., Chen S. C. Evidence of acid phosphatase in the cytoplasm as a distinct entity. Arch Biochem Biophys. 1988 May 1;262(2):427–438. doi: 10.1016/0003-9861(88)90394-3. [DOI] [PubMed] [Google Scholar]
- Ching T. M., Lin T. P., Metzger R. J. Purification and properties of Acid phosphatase from plump and shriveled seeds of triticale. Plant Physiol. 1987 Jul;84(3):789–795. doi: 10.1104/pp.84.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christeller J. T., Tolbert N. E. Phosphoglycolate phosphatase. Purification and properties. J Biol Chem. 1978 Mar 25;253(6):1780–1785. [PubMed] [Google Scholar]
- Doucet J. P., Trifaró J. M. A discontinuous and highly porous sodium dodecyl sulfate-polyacrylamide slab gel system of high resolution. Anal Biochem. 1988 Feb 1;168(2):265–271. doi: 10.1016/0003-2697(88)90317-x. [DOI] [PubMed] [Google Scholar]
- Fallon H. J., Byrne W. L. 2-phosphoglyceric acid phosphatase: identification and properties of the beef-liver enzyme. Biochim Biophys Acta. 1965 Jul 29;105(1):43–53. doi: 10.1016/s0926-6593(65)80174-6. [DOI] [PubMed] [Google Scholar]
- Gibson D. M., Ullah A. H. Purification and characterization of phytase from cotyledons of germinating soybean seeds. Arch Biochem Biophys. 1988 Feb 1;260(2):503–513. doi: 10.1016/0003-9861(88)90475-4. [DOI] [PubMed] [Google Scholar]
- Goldstein A. H., Danon A., Baertlein D. A., McDaniel R. G. Phosphate Starvation Inducible Metabolism in Lycopersicon esculentum: II. Characterization of the Phosphate Starvation Inducible-Excreted Acid Phosphatase. Plant Physiol. 1988 Jul;87(3):716–720. doi: 10.1104/pp.87.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOYCE B. K., GRISOLIA S. Purification and properties of a nonspecific acid phosphatase from wheat germ. J Biol Chem. 1960 Aug;235:2278–2281. [PubMed] [Google Scholar]
- Lin M., Turpin D. H., Plaxton W. C. Pyruvate kinase isozymes from the green alga, Selenastrum minutum. I. Purification and physical and immunological characterization. Arch Biochem Biophys. 1989 Feb 15;269(1):219–227. doi: 10.1016/0003-9861(89)90103-3. [DOI] [PubMed] [Google Scholar]
- Lin M., Turpin D. H., Plaxton W. C. Pyruvate kinase isozymes from the green alga, Selenastrum minutum. II. Kinetic and regulatory properties. Arch Biochem Biophys. 1989 Feb 15;269(1):228–238. doi: 10.1016/0003-9861(89)90104-5. [DOI] [PubMed] [Google Scholar]
- Mulligan R. M., Tolbert N. E. Properties of a Membrane-bound Phosphatase from the Thylakoids of Spinach Chloroplasts. Plant Physiol. 1980 Dec;66(6):1169–1173. doi: 10.1104/pp.66.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaxton W. C. Purification of pyruvate kinase from germinating castor bean endosperm. Plant Physiol. 1988 Apr;86(4):1064–1069. doi: 10.1104/pp.86.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall D. D., Tolbert N. E. 3-Phosphoglycerate Phosphatase in Plants: III. Activity Associated with Starch Particles. Plant Physiol. 1971 Oct;48(4):488–492. doi: 10.1104/pp.48.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall D. D., Tolbert N. E. 3-Phosphoglycerate phosphatase in plants. I. Isolation and characterization from sugarcane leaves. J Biol Chem. 1971 Sep 10;246(17):5510–5517. [PubMed] [Google Scholar]
- Shaw J. G. Acid phosphatase from tobacco leaves. Arch Biochem Biophys. 1966 Oct;117(1):1–9. doi: 10.1016/0003-9861(66)90118-4. [DOI] [PubMed] [Google Scholar]
- TSUBOI K. K., HUDSON P. B. Acid phosphatase. VI. Kinetic properties of purified yeast and erythrocyte phosphomonoesterase. Arch Biochem Biophys. 1956 Mar;61(1):197–210. doi: 10.1016/0003-9861(56)90332-0. [DOI] [PubMed] [Google Scholar]
- Ullah A. H., Gibson D. M. Purification and characterization of acid phosphatase from cotyledons of germinating soybean seeds. Arch Biochem Biophys. 1988 Feb 1;260(2):514–520. doi: 10.1016/0003-9861(88)90476-6. [DOI] [PubMed] [Google Scholar]
- Van Etten R. L., Saini M. S. Preparation of homogeneous human prostatic acid phosphatase using concanavalin A-sepharose 4-B. Biochim Biophys Acta. 1977 Oct 13;484(2):487–492. doi: 10.1016/0005-2744(77)90104-8. [DOI] [PubMed] [Google Scholar]
- Villareal R. M., Juliano B. O. Some properties of 3-phosphoglycerate phosphatase from developing rice grain. Plant Physiol. 1977 Feb;59(2):134–138. doi: 10.1104/pp.59.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]