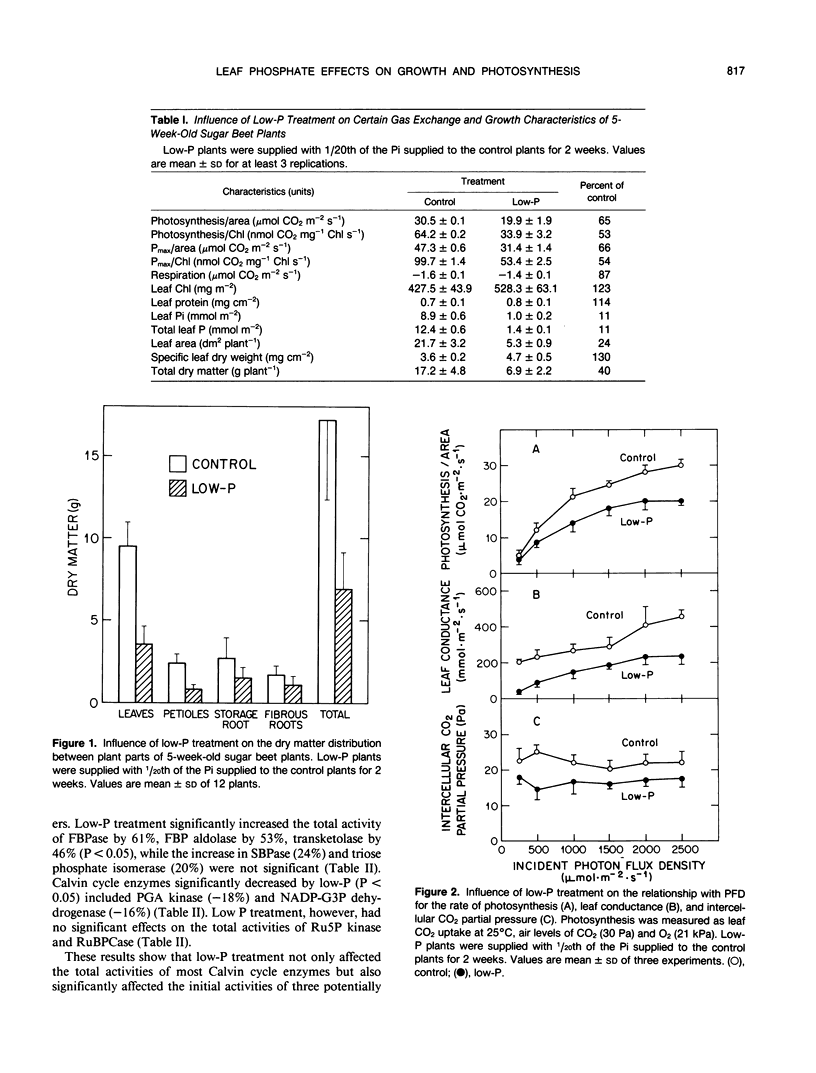
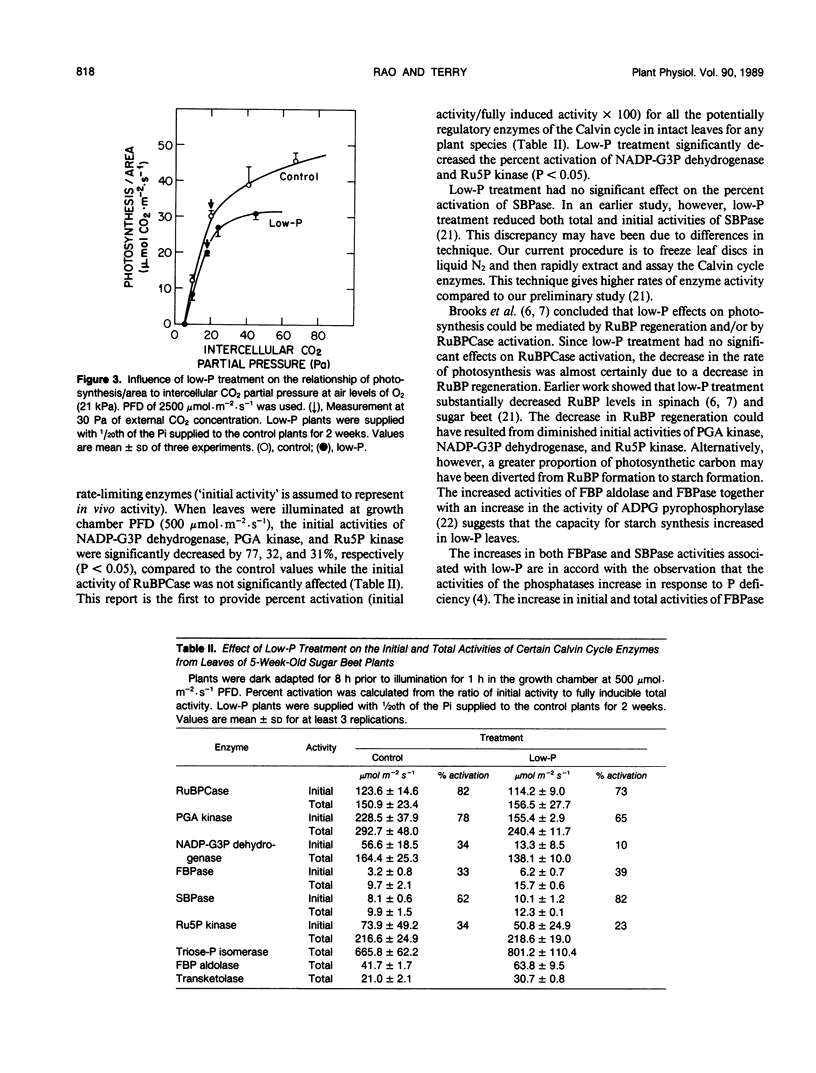
Abstract
Sugar beets (Beta vulgaris L. cv F58-554H1) were cultured hydroponically for 2 weeks in growth chambers with two levels of orthophosphate (Pi) supplied in half strength Hoagland solution. Low-P plants were supplied with 1/20th of the Pi supplied to control plants. With low-P treatment, the acid soluble leaf phosphate and total leaf P decreased by about 88%. Low-P treatment had a much greater effect on leaf area than on photosynthesis. Low-P decreased total leaf area by 76%, dry weight per plant by 60%, and the rate of photosynthesis per area at light saturation by 35%. Low-P treatment significantly decreased the total extractable activity of phosphoglycerate kinase (by 18%) and NADP-glyceraldehyde-3-phosphate dehydrogenase (by 16%), but did not decrease the total activities of ribulose-1,5-bisphosphate (RuBP) carboxylase (RuBPCase) and ribulose-5-phosphate kinase. Low-P treatment decreased the initial activities of three rate-limiting Calvin cycle enzymes, but had no effect on the initial activity of RuBPCase. Furthermore, low-P treatment significantly increased the total extractable activities of fructose-1,6-bisphosphatase (by 61%), fructose-1,6-bisphosphate aldolase (by 53%), and transketolase (by 46%). The results suggest that low-P treatment affected photosynthetic rate through an effect on RuBP regeneration rather than through RuBPCase activity and that the changes in Calvin cycle enzymes with low-P resulted in an increased flow of carbon to starch.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E., Chin H. M., Gupta V. K. Modulation of Chloroplast Fructose-1,6-bisphosphatase Activity by Light. Plant Physiol. 1979 Sep;64(3):491–494. doi: 10.1104/pp.64.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. E. Chloroplast and cytoplasmic enzymes. II. Pea leaf triose phosphate isomerases. Biochim Biophys Acta. 1971 Apr 14;235(1):237–244. doi: 10.1016/0005-2744(71)90051-9. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Fredeen A. L., Rao I. M., Terry N. Influence of Phosphorus Nutrition on Growth and Carbon Partitioning in Glycine max. Plant Physiol. 1989 Jan;89(1):225–230. doi: 10.1104/pp.89.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W., Chon C. J., Maronde D. Role of orthophosphate and other factors in the regulation of starch formation in leaves and isolated chloroplasts. Plant Physiol. 1977 Jun;59(6):1146–1155. doi: 10.1104/pp.59.6.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobza J., Edwards G. E. Influences of leaf temperature on photosynthetic carbon metabolism in wheat. Plant Physiol. 1987 Jan;83(1):69–74. doi: 10.1104/pp.83.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin J. W., Eidenbock M. P. Carbon Accumulation during Photosynthesis in Leaves of Nitrogen- and Phosphorus-Stressed Cotton. Plant Physiol. 1986 Nov;82(3):869–871. doi: 10.1104/pp.82.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin J. W., Eidenbock M. P. Hydraulic conductance as a factor limiting leaf expansion of phosphorus-deficient cotton plants. Plant Physiol. 1984 Jun;75(2):372–377. doi: 10.1104/pp.75.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci M. E., Anderson J. C. Factors affecting the activation state and the level of total activity of ribulose bisphosphate carboxylase in tobacco protoplasts. Plant Physiol. 1987 Sep;85(1):66–71. doi: 10.1104/pp.85.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. E., Terry N. Limiting Factors in Photosynthesis: V. Photochemical Energy Supply Colimits Photosynthesis at Low Values of Intercellular CO(2) Concentration. Plant Physiol. 1984 May;75(1):82–86. doi: 10.1104/pp.75.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry N., Ulrich A. Effects of phosphorus deficiency on the photosynthesis and respiration of leaves of sugar beet. Plant Physiol. 1973 Jan;51(1):43–47. doi: 10.1104/pp.51.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treeby M. T., van Steveninck R. F., de Vries H. M. Quantitative Estimates of Phosphorus Concentrations within Lupinus luteus Leaflets by Means of Electron Probe X-ray Microanalysis. Plant Physiol. 1987 Oct;85(2):331–334. doi: 10.1104/pp.85.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodrow I. E., Murphy D. J., Walker D. A. Regulation of photosynthetic carbon metabolism. The effect of inorganic phosphate on stromal sedoheptulose-1,7-bisphosphatase. Eur J Biochem. 1983 Apr 15;132(1):121–123. doi: 10.1111/j.1432-1033.1983.tb07335.x. [DOI] [PubMed] [Google Scholar]


