Abstract
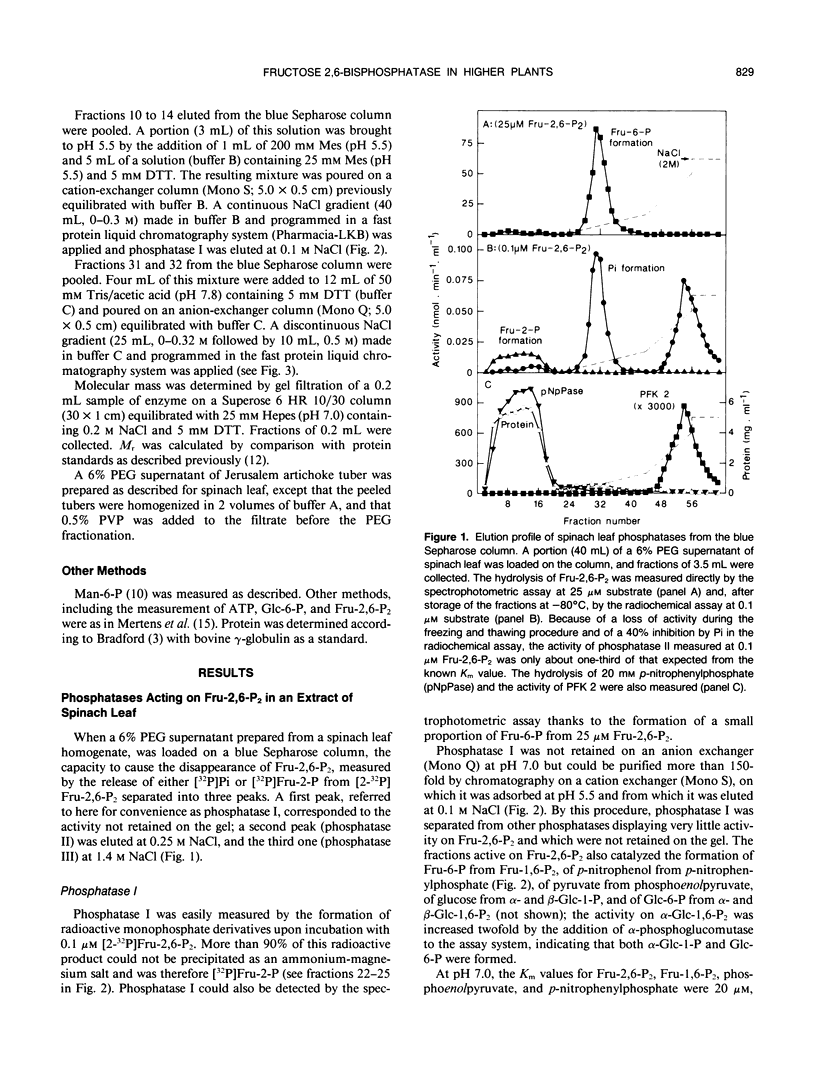
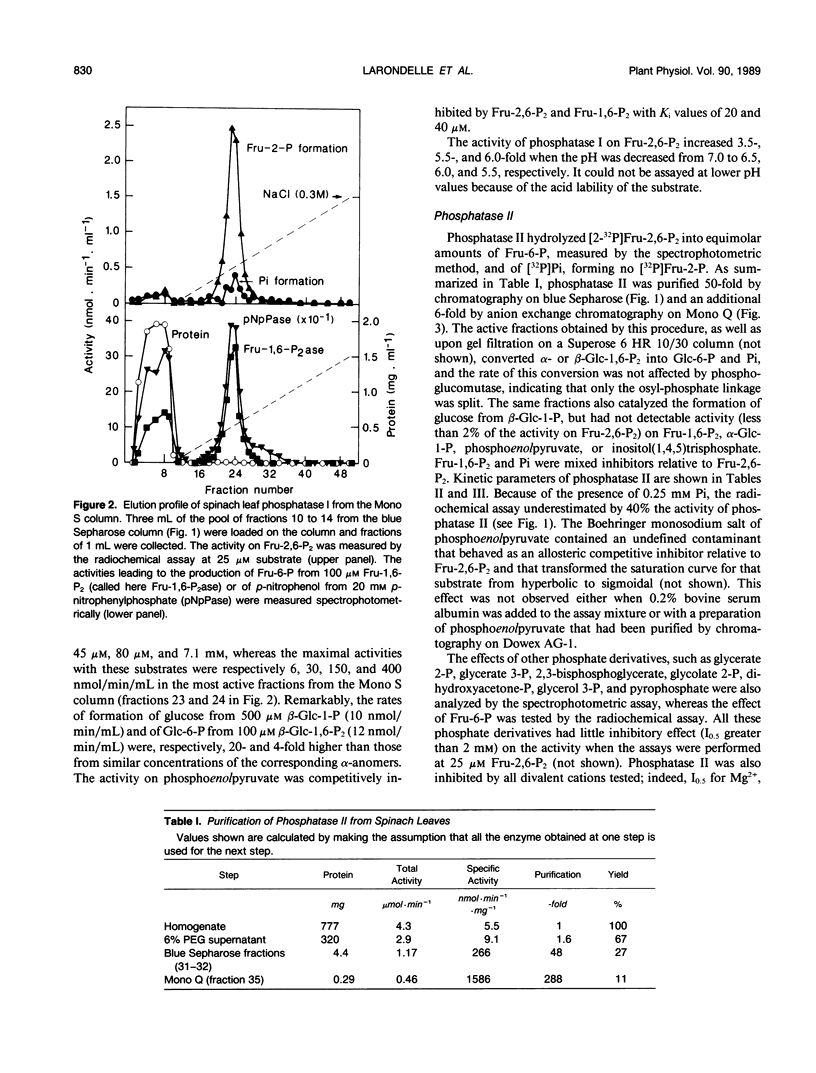
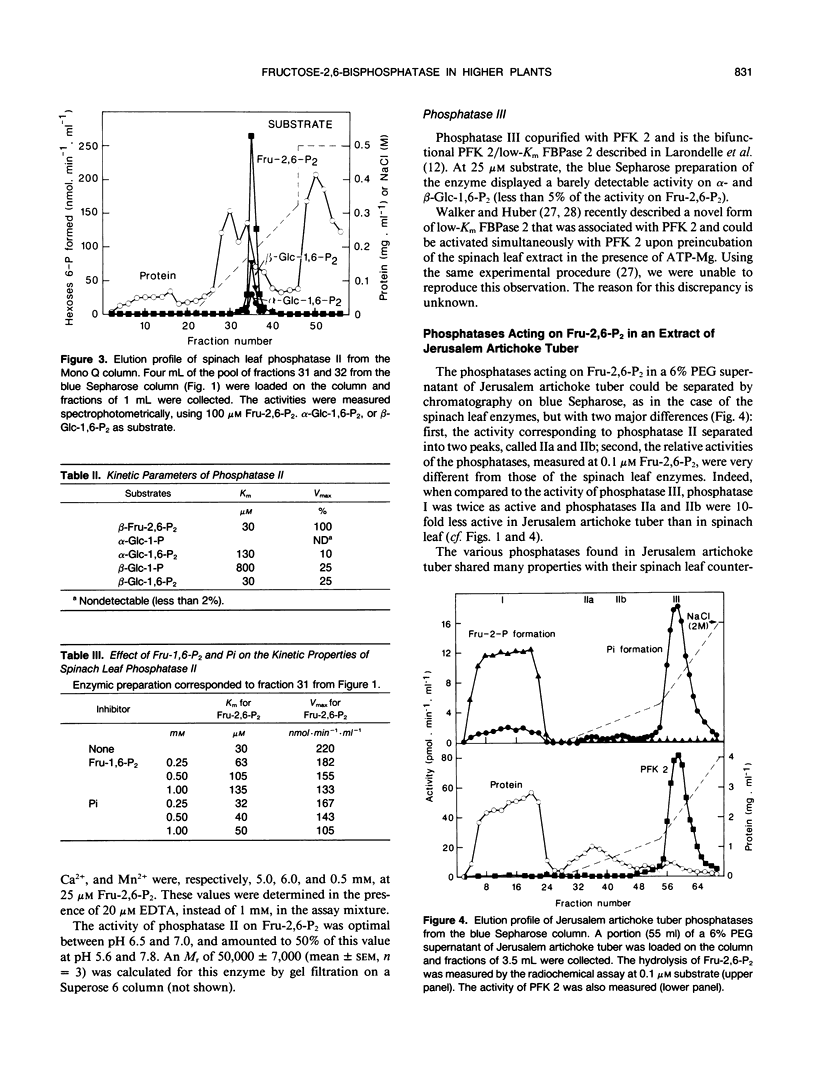
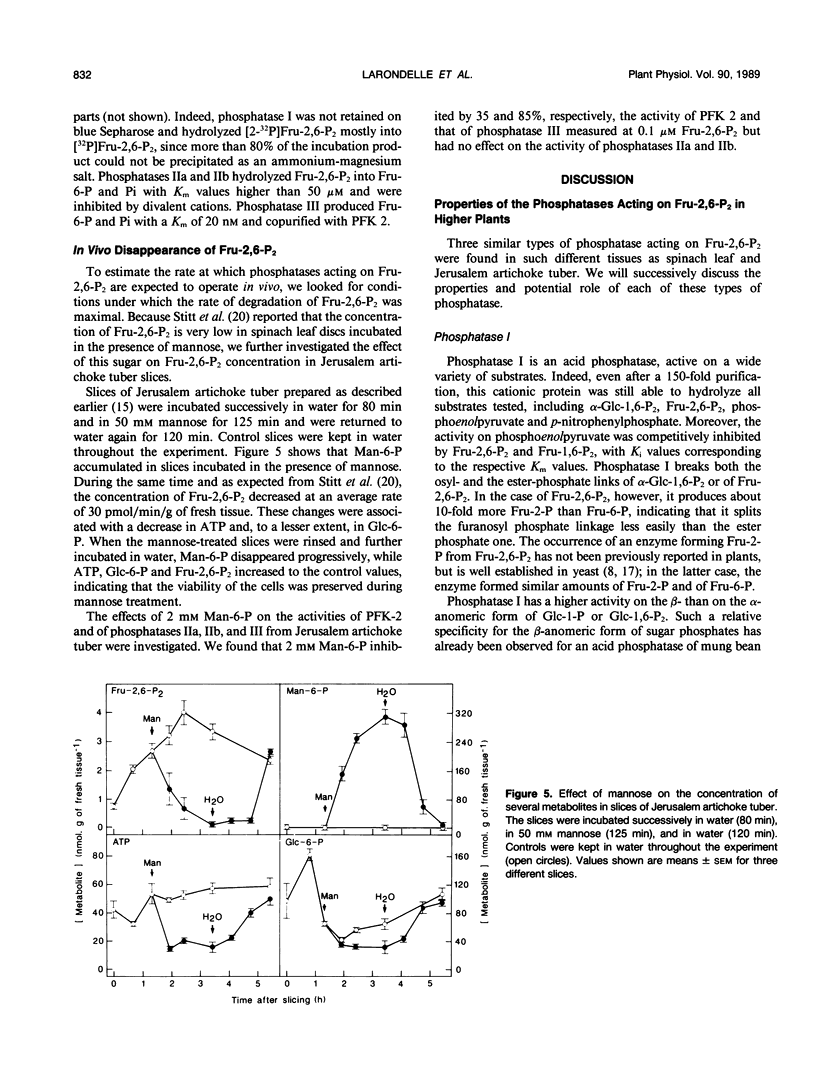
The phosphatases that hydrolyze fructose 2,6-bisphosphate in a crude spinach (Spinacia oleracea L.) leaf extract were separated by chromatography on blue Sepharose, into three fractions, referred to as phosphatases I, II, and III, which were further purified by various means. Phosphatase I hydrolyzed fructose 2,6-bisphosphate, with a Km value of 30 micromolar, to a mixture of fructose 2-phosphate (90%) and fructose 6-phosphate (10%). It acted on a wide range of substrates and had a maximal activity at acidic pH. Phosphatase II specifically recognized the osyl-link of phosphoric derivatives and had more affinity for the β-anomeric form. Its apparent Km for fructose 2,6-bisphosphate was 30 micromolar. It most likely corresponded to the fructose-2,6-bisphosphatase described by F. D. Macdonald, Q. Chou, and B. B. Buchanan ([1987] Plant Physiol 85: 13-16). Phosphatase III copurified with phosphofructokinase 2 and corresponded to the specific, low-Km (24 nanomolar) fructose-2,6-bisphosphatase purified and characterized by Y. Larondelle, E. Mertens, E. Van Schaftingen, and H. G. Hers ([1986] Eur J Biochem 161: 351-357). Three similar types of phosphatases were present in a crude extract of Jerusalem artichoke (Helianthus tuberosus) tuber. The concentration of fructose 2,6-bisphosphate decreased at a maximal rate of 30 picomoles per minute and per gram of fresh tissue in slices of Jerusalem artichoke tuber, upon incubation in 50 millimolar mannose. This rate could be accounted for by the maximal extractable activity of the low-Km fructose-2,6-bisphosphatase. A new enzymic method for the synthesis of β-glucose 1,6-bisphosphate from β-glucose 1-phosphate and ATP is described.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asami S., Hara-Nishimura I., Nishimura M., Akazawa T. Translocation of photosynthates into vacuoles in spinach leaf protoplasts. Plant Physiol. 1985 Apr;77(4):963–968. doi: 10.1104/pp.77.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Erneux C., Delvaux A., Moreau C., Dumont J. E. Characterization of D-myo-inositol 1,4,5-trisphosphate phosphatase in rat brain. Biochem Biophys Res Commun. 1986 Jan 14;134(1):351–358. doi: 10.1016/0006-291x(86)90570-x. [DOI] [PubMed] [Google Scholar]
- Eyer P., Hofer H. W., Krystek E., Pette D. Synthesis of glucose 1,6-biphosphate by the action of crystalline rabbit muscle phosphofructokinase. Eur J Biochem. 1971 May 28;20(2):153–159. doi: 10.1111/j.1432-1033.1971.tb01373.x. [DOI] [PubMed] [Google Scholar]
- FELENBOK B., NEUFELD E. F. Hydrolysis of glycopyranosyl phosphates of teh beta-D (or alpha-L) configuration by a plant phosphatase. Arch Biochem Biophys. 1962 Apr;97:116–121. doi: 10.1016/0003-9861(62)90051-6. [DOI] [PubMed] [Google Scholar]
- FRIEDMIN M. Desoxyribose-1-phosphate. II. The isolation of crystalline desoxyribose-1-phosphate. J Biol Chem. 1950 Jun;184(2):449–459. [PubMed] [Google Scholar]
- François J., Van Schaftigen E., Hers H. G. Characterization of phosphofructokinase 2 and of enzymes involved in the degradation of fructose 2,6-bisphosphate in yeast. Eur J Biochem. 1988 Feb 1;171(3):599–608. doi: 10.1111/j.1432-1033.1988.tb13830.x. [DOI] [PubMed] [Google Scholar]
- Kruger N. J., Beevers H. Synthesis and degradation of fructose 2,6-bisphosphate in endosperm of castor bean seedlings. Plant Physiol. 1985 Feb;77(2):358–364. doi: 10.1104/pp.77.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larondelle Y., Mertens E., Van Schaftingen E., Hers H. G. Purification and properties of spinach leaf phosphofructokinase 2/fructose 2,6-bisphosphatase. Eur J Biochem. 1986 Dec 1;161(2):351–357. doi: 10.1111/j.1432-1033.1986.tb10454.x. [DOI] [PubMed] [Google Scholar]
- Macdonald F. D., Chou Q., Buchanan B. B. Ion-exchange chromatography separates activities synthesizing and degrading fructose 2,6-bisphosphate from C3 and C4 leaves but not from rat liver. Plant Physiol. 1987;85:13–16. doi: 10.1104/pp.85.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald F. D., Cséke C., Chou Q., Buchanan B. B. Activities synthesizing and degrading fructose 2,6-bisphosphate in spinach leaves reside on different proteins. Proc Natl Acad Sci U S A. 1987 May;84(9):2742–2746. doi: 10.1073/pnas.84.9.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purwin C., Laux M., Holzer H. Fructose 2-phosphate, an intermediate of the dephosphorylation of fructose 2,6-bisphosphate with a purified yeast enzyme. Eur J Biochem. 1987 Apr 1;164(1):27–30. doi: 10.1111/j.1432-1033.1987.tb10987.x. [DOI] [PubMed] [Google Scholar]
- Soll J., Wötzel C., Buchanan B. B. Enzyme regulation in c(4) photosynthesis : identification and localization of activities catalyzing the synthesis and hydrolysis of fructose-2,6-bisphosphate in corn leaves. Plant Physiol. 1985 Apr;77(4):999–1003. doi: 10.1104/pp.77.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Gerhardt R., Kürzel B., Heldt H. W. A role for fructose 2,6-bisphosphate in the regulation of sucrose synthesis in spinach leaves. Plant Physiol. 1983 Aug;72(4):1139–1141. doi: 10.1104/pp.72.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Kürzel B., Heldt H. W. Control of Photosynthetic Sucrose Synthesis by Fructose 2,6-Bisphosphate : II. Partitioning between Sucrose and Starch. Plant Physiol. 1984 Jul;75(3):554–560. doi: 10.1104/pp.75.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E. Fructose 2,6-bisphosphate. Adv Enzymol Relat Areas Mol Biol. 1987;59:315–395. doi: 10.1002/9780470123058.ch7. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E., Hers H. G. Formation of fructose 2,6-bisphosphate from fructose 1,6-bisphosphate by intramolecular cyclisation followed by alkaline hydrolysis. Eur J Biochem. 1981 Jul;117(2):319–323. doi: 10.1111/j.1432-1033.1981.tb06339.x. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E., Opperdoes F. R., Hers H. G. Effects of various metabolic conditions and of the trivalent arsenical melarsen oxide on the intracellular levels of fructose 2,6-bisphosphate and of glycolytic intermediates in Trypanosoma brucei. Eur J Biochem. 1987 Aug 3;166(3):653–661. doi: 10.1111/j.1432-1033.1987.tb13563.x. [DOI] [PubMed] [Google Scholar]
- Walker G. H., Huber S. C. ATP-dependent activation of a new form of spinach leaf 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatase. Arch Biochem Biophys. 1987 Oct;258(1):58–64. doi: 10.1016/0003-9861(87)90322-5. [DOI] [PubMed] [Google Scholar]
- van Schaftingen E., Davies D. R., Hers H. G. Fructose-2,6-bisphosphatase from rat liver. Eur J Biochem. 1982 May;124(1):143–149. doi: 10.1111/j.1432-1033.1982.tb05917.x. [DOI] [PubMed] [Google Scholar]


