Abstract
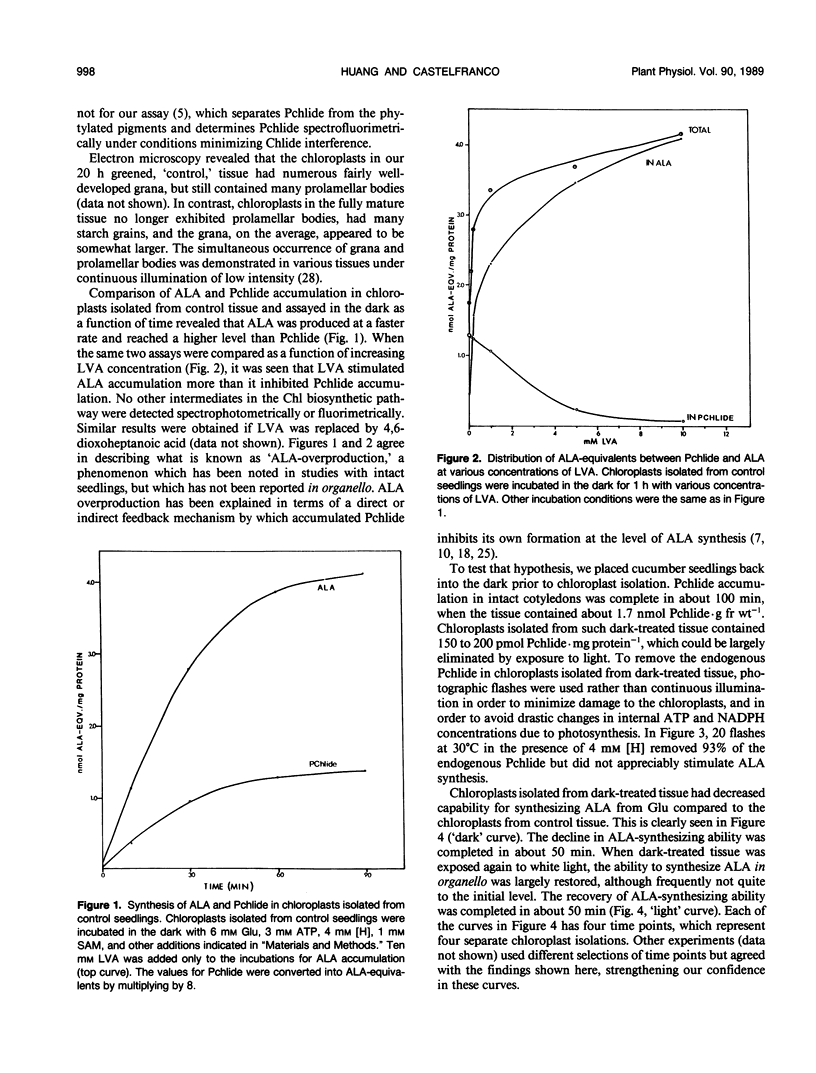
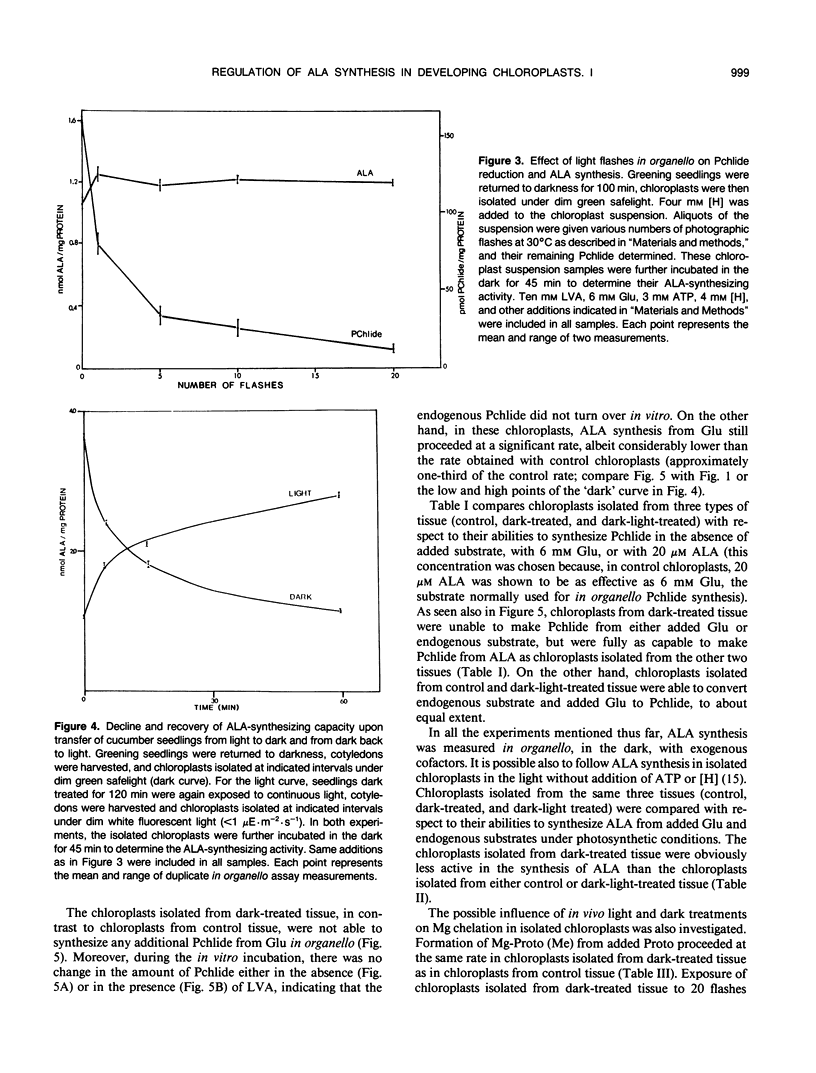
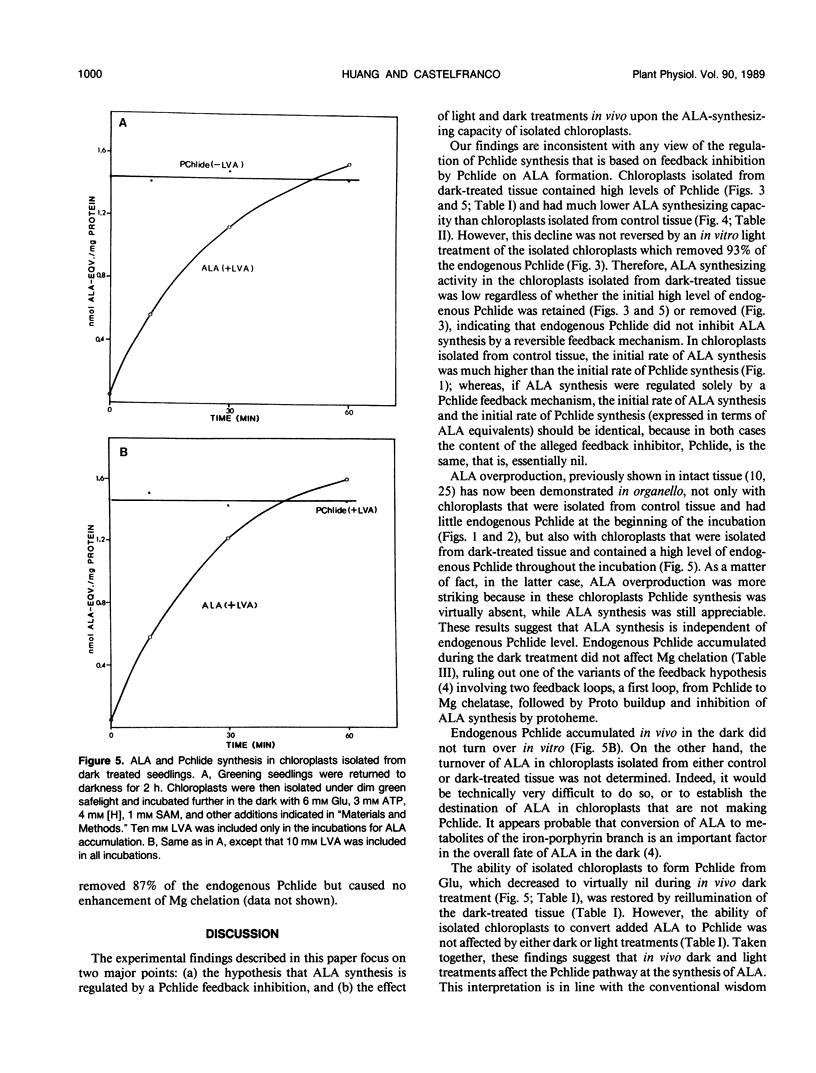
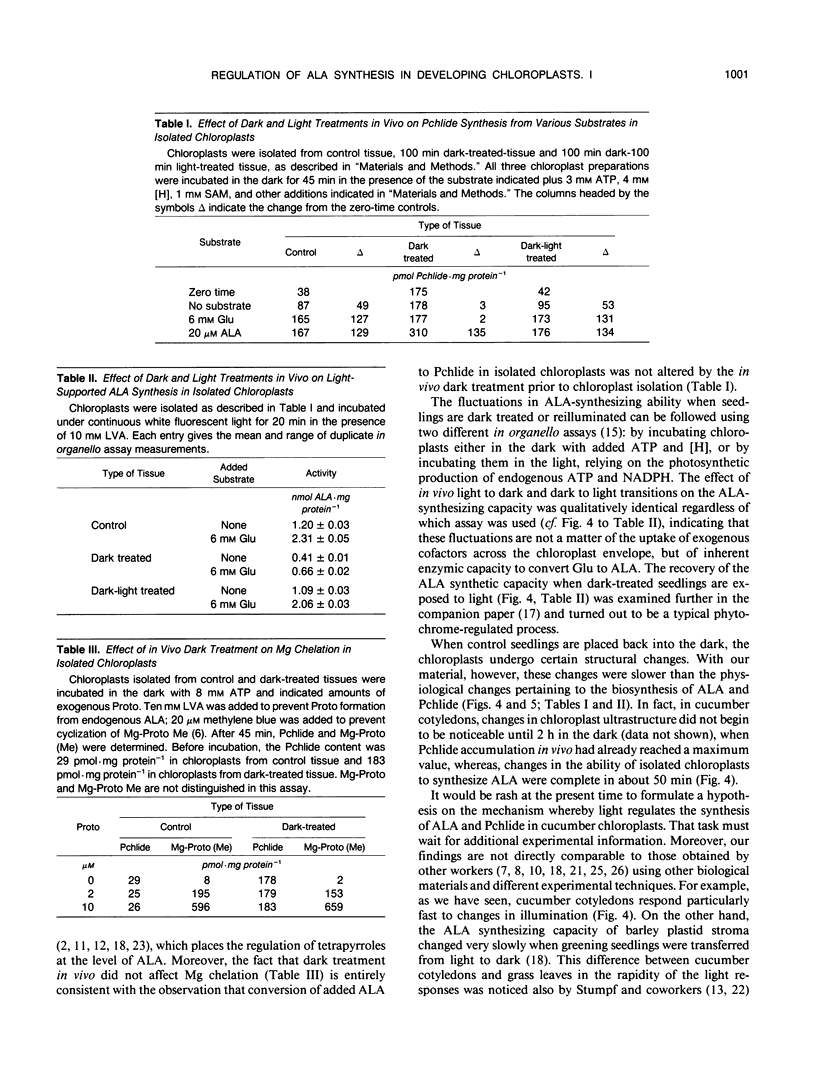
Intact chloroplasts isolated from greening cucumber (Cucumis sativus L. var Beit Alpha) cotyledons regenerated protochlorophyllide (Pchlide) in the dark with added cofactors from either exogenous glutamate or endogenous substrates. No other intermediates of the chlorophyll biosynthetic pathway accumulated. When inhibitors of 5-aminolevulinic acid (ALA) dehydratase were added, the Pchlide that failed to form was replaced by an excessive amount of ALA. When greening seedlings were returned to the dark, ALA-synthesizing activity in the isolated chloroplasts decreased dramatically and recovered if the dark-treated seedlings were again exposed to continuous white light prior to chloroplast isolation. Both the decline and the recovery of ALA-synthesizing activity were complete in approximately 50 minutes. Changes in chloroplast structure during in vivo light to dark and dark to light transitions (as evidenced by electron microscopy) were much slower. Exposing isolated chloroplasts from dark-treated seedlings to short white flashes before incubation transformed nearly all the endogenous Pchlide, but hardly stimulated ALA synthesis, suggesting that Pchlide does not act as a feed-back inhibitor on ALA synthesis. Chloroplasts isolated from dark-treated tissue did not form Pchlide from glutamate when incubated in the dark with added cofactors; moreover, the endogenous Pchlide did not turn over in organello. However, these chloroplasts did synthesize Pchlide from added ALA at the normal rate and synthesized ALA from glutamate at a reduced, but still significant, rate. Mg chelation was not affected by in vivo dark treatment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Castelfranco P. A., Weinstein J. D., Schwarcz S., Pardo A. D., Wezelman B. E. The Mg insertion step in chlorophyll biosynthesis. Arch Biochem Biophys. 1979 Feb;192(2):592–598. doi: 10.1016/0003-9861(79)90130-9. [DOI] [PubMed] [Google Scholar]
- Chereskin B. M., Wong Y. S., Castelfranco P. A. In Vitro Synthesis of the Chlorophyll Isocyclic Ring : Transformation of Magnesium-Protoporphyrin IX and Magnesium-Protoporphyrin IX Monomethyl Ester into Magnesium-2,4-Divinyl Pheoporphyrin A(5). Plant Physiol. 1982 Oct;70(4):987–993. doi: 10.1104/pp.70.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhr R., Harel E., Klein S., Meller E. Control of delta-Aminolevulinic Acid and Chlorophyll Accumulation in Greening Maize Leaves upon Light-Dark Transitions. Plant Physiol. 1975 Oct;56(4):497–501. doi: 10.1104/pp.56.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fufsler T. P., Castelfranco P. A., Wong Y. S. Formation of Mg-Containing Chlorophyll Precursors from Protoporphyrin IX, delta-Aminolevulinic Acid, and Glutamate in Isolated, Photosynthetically Competent, Developing Chloroplasts. Plant Physiol. 1984 Apr;74(4):928–933. doi: 10.1104/pp.74.4.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawke J. C., Stumpf P. K. Desaturation of Oleic and Linoleic Acids by Leaves of Dark- and Light-grown Maize Seedlings. Plant Physiol. 1980 Jun;65(6):1027–1030. doi: 10.1104/pp.65.6.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Bonner B. A., Castelfranco P. A. Regulation of 5-Aminolevulinic Acid (ALA) Synthesis in Developing Chloroplasts : II. Regulation of ALA-Synthesizing Capacity by Phytochrome. Plant Physiol. 1989 Jul;90(3):1003–1008. doi: 10.1104/pp.90.3.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Castelfranco P. A. Regeneration of Magnesium-2,4-Divinylpheoporphyrin a(5) (Divinyl Protochlorophyllide) in Isolated Developing Chloroplasts. Plant Physiol. 1986 Sep;82(1):285–288. doi: 10.1104/pp.82.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannangara C. G., Gough S. P., Bruyant P., Hoober J. K., Kahn A., von Wettstein D. tRNA(Glu) as a cofactor in delta-aminolevulinate biosynthesis: steps that regulate chlorophyll synthesis. Trends Biochem Sci. 1988 Apr;13(4):139–143. doi: 10.1016/0968-0004(88)90071-0. [DOI] [PubMed] [Google Scholar]
- Klein S., Harel E., Ne'eman E., Katz E., Meller E. Accumulation of delta-Aminolevulinic Acid and Its Relation to Chlorophyll Synthesis and Development of Plastid Structure in Greening Leaves. Plant Physiol. 1975 Oct;56(4):486–496. doi: 10.1104/pp.56.4.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. J., Stumpf P. K. Light-dependent Induction of Polyunsaturated Fatty Acid Biosynthesis in Greening Cucumber Cotyledons. Plant Physiol. 1979 Feb;63(2):328–335. doi: 10.1104/pp.63.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler K., Granick S. Controls on chlorophyll synthesis in barley. Plant Physiol. 1970 Aug;46(2):240–246. doi: 10.1104/pp.46.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Stobart A. K., Ameen-Bukhari I. Photoreduction of protochlorophyllide and its relationship to delta-aminolaevulinic acid synthesis in the leaves of dark-grown barley (Hordeum vulgare) seedlings. Biochem J. 1986 Jun 15;236(3):741–748. doi: 10.1042/bj2360741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobart A. K., Ameen-Bukhari I. Regulation of delta-aminolaevulinic acid synthesis and protochlorophyllide regeneration in the leaves of dark-grown barley (Hordeum vulgare) seedlings. Biochem J. 1984 Sep 1;222(2):419–426. doi: 10.1042/bj2220419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J. D., Beale S. I. Enzymatic conversion of glutamate to delta-aminolevulinate in soluble extracts of the unicellular green alga, Chlorella vulgaris. Arch Biochem Biophys. 1985 Mar;237(2):454–464. doi: 10.1016/0003-9861(85)90299-1. [DOI] [PubMed] [Google Scholar]
- Weinstein J. D., Castelfranco P. A. Mg-protoporphyrin-IX and delta-aminolevulinic acid synthesis from glutamate in isolated greening chloroplasts. delta-Aminolevulinic acid sysnthesis. Arch Biochem Biophys. 1978 Mar;186(2):376–382. doi: 10.1016/0003-9861(78)90448-4. [DOI] [PubMed] [Google Scholar]


