Abstract
Background
There are conflicting reports on the factors that increase the likelihood of patients dying from follicular thyroid carcinoma (FTC). Therefore, it is critical to identify risk factors of patients with FTC. This study aimed to identify the factors that increase the risk of death of patients with FTC and help clinicians make better treatment and follow-up decisions.
Methods
A systematic literature review was conducted in PubMed and Web of Science databases for relevant studies published before January 31, 2023. Their reference lists were also analyzed. Two reviewers extracted data and evaluated the quality of eligible studies independently. Studies on patients who had open thyroidectomy procedures with or without neck dissection were included in this review. The RevMan 5.3 software was used to analyze the data.
Results
This meta-analysis included thirteen studies with a total of 2075 patients. The following variables were associated with an increased risk of death in FTC patients: age > 45 years, male, tumor diameter > 4 cm, multifocality, extrathyroidal extension (ETE), widely invasive (WI), cervical lymph node metastasis (CLNM), distant metastases (DM) and non-radical resection tumor. Lobectomy and no radioactive iodine (RAI) treatment was not associated with the death of FTC patients.
Conclusion
Clinicians should pay closer attention to the following significant risk factors associated with the death of FTC patients: age (> 45), male, multifocality, tumor diameter > 4 cm, ETE, WI, non-radical resection tumor, CLNM, and DM. Individualized initial treatment and close follow-up are needed FTC patients who have these risk factors.
Keywords: Follicular thyroid carcinoma, Death, Risk factors, Cervical lymph node metastasis, Non-radical resection.
Introduction
Follicular thyroid carcinoma (FTC) accounts for approximately 10% of all thyroid cancers [1]. FTC and papillary thyroid carcinoma (PTC) have been classified as differentiated thyroid cancers because they emerge from the follicular thyroid cell lineage [2]. On the other hand, there is a marked distinction between the biological characteristics and clinical manifestations of FTC and PTC [3]. Compared to PTC, FTC is considered a more aggressive disease with a poor prognosis. Because of the propensity for capsular and vascular invasion, distant metastases via hematogenous dissemination are more common in FTC [4].
FTC patients typically have a good prognosis, but they may experience local recurrence, distant metastasis, or even death during the follow-up period [5]. According to recent studies, the mortality rate of FTC was 10–30% [6, 7]. Therefore, the most important question among endocrine treating physicians is whether treatments, such as completion thyroidectomy and radioactive iodine (RAI) remnant ablation, should be performed after FTC diagnosis, and what FTC patients can benefit from these treatments in terms of prognosis [8]. The decision to continue treatment should be based on prognostic indicators and risk factors for death [9], even though the pathological tumor node metastasis classification has been established in patients with well-differentiated thyroid carcinoma. Indeed, due to the low number of FTC cases, FTC and PTC are frequently analyzed together in many published studies [10]. However, this joint analysis is inaccurate because the biological behaviors of PTC and FTC are differ [11, 12]. Furthermore, prognostic indicators are not identified in FTC and have not yet been validated for FTC [13].
In FTC patients, various prognostic factors were documented, including age at diagnosis, gender, tumor size, extrathyroidal extension (ETE), and the presence of distant metastasis [14]. Furthermore, it has been demonstrated that widely invasive carcinoma has worse outcomes than minimally invasive carcinoma [15]. Despite this, there are numerous contradictory reports on the risk factors for FTC death [16–18]. Therefore, a meta-analysis was conducted to investigate the risk factors for death in FTC patients and aid clinical decision-making for appropriate treatment and follow-up.
Materials and methods
The Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines were used to conduct this meta-analysis [19].
Search strategy
A systematic literature search was conducted in PubMed and Web of Science databases for relevant studies published before January 31, 2023. The keywords included thyroid cancer OR thyroid carcinoma, follicular OR FTC, risk factors, death OR mortality, outcome, survival, prognosis OR prognostic factors. Two authors carried out the selection process independently (Zhang T and Dong WW). All disagreements were resolved by the two authors through discussions and consensus, or referred to a third author.
Selection criteria
The meta-analysis included prospective or retrospective studies published in English that included primary FTC patients who underwent thyroid and lymphadenectomy surgery. Participants in the included studies were diagnosed using intraoperative or postoperative pathology. Furthermore, the studies included demographic and clinical data for thyroidectomy patients that could be extracted. Review articles, conference abstracts, editorials, letters, and single case reports, on the other hand, were excluded. Duplicate studies were also excluded, as were those without reported outcomes.
Data extraction and quality assessment
The two investigators independently extracted relevant data from the articles in a standardized format. The data included the first author’s name, year of publication, country of origin, research design, number of cases, potential risk factors, and other corresponding data (Fig. 1). The potential risk factors included demographic variables (age, gender), tumor specific variables (tumor diameter, multifocality, ETE, pathologic subtype (widely invasive (WI), or minimally invasive (MI))), disease extent (cervical lymph node metastasis (CLNM), distant metastasis (DM)), and treatment variables (operation type, margin status, radioactive iodine (RAI)). The Newcastle-Ottawa quality assessment scale was applied to evaluate the quality of studies [20, 21].
Fig. 1.
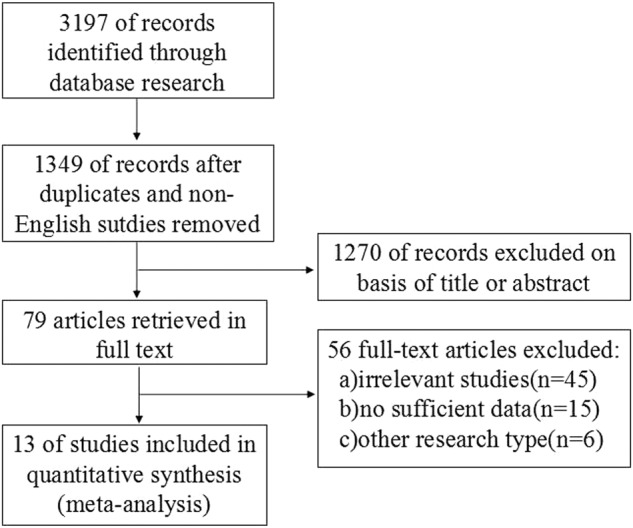
Flowchart of study selection
Data analysis
The data were analyzed using Review Manager version 5.3 (Cochrane Collaborative, Oxford, United Kingdom), and the results were presented as mean difference IV (MD) or odds ratios (ORs) with a 95% confidence interval (CI). A P-value < 0.05 indicated that the observed difference was statistically significant. The Q test and I2 statistics were used to assess data heterogeneity, while Cochran’s Q statistic was used to estimate heterogeneity among studies [19]. A fixed-effects model was used when p > 0.10 and I2 < 50%; otherwise, a random-effects model was used. Begg’s plot was used to assess the potential of publication bias.
Results
The search strategy yielded 3197 potentially relevant studies for meta-analysis. Figure 1 displays a flowchart of the studies that were retrieved and excluded. After excluding studies that did not meet the inclusion criteria, 13 studies [6, 7, 22–32] with 2075 patients were chosen for analysis. This meta-analysis literature search of our study included Hurthle Cell Carcinoma (HCC), because HCC belong to FTC before the 5th edition (2022) of the WHO Classification of Endocrine and Neuroendocrine Tumors. In this study, 91 patients with a diagnosis of HCC were included. The mean follow-up was 3.9–14.4 years. Carcinoma specifific mortality was calculated as the duration from the point of diagnosis to the date of death from FTC. Furthermore, the estimated cumulative incidences of FTC death at 5 and 10 years were 3.5–47.7% and 2.55–34.5%, respectively. The incidence of death in all studies was represented in Table 1.
Table 1.
Characteristics of Eligible Studies
| Author | Year | Country | Study design | Case number | Follow-up period (years) | FTC death | Quality score | ||
|---|---|---|---|---|---|---|---|---|---|
| Death | All | 5 years | 10 years | ||||||
| Lin, JD | 1999 | China | retrospective analysis | 25 | 69 | 7 (0–18) | 47.70% | — | 7 |
| Chow, SM | 2002 | China | retrospective analysis | 37 | 215 | 10.8 (0–20) | 31.20% | — | 9 |
| Passler, C | 2004 | Austria | retrospective analysis | 51 | 168 | 10.8 (0–20) | 30.50% | 21.00% | 8 |
| Lo, CY | 2005 | China | retrospective analysis | 17 | 156 | 14.4 (0.1–38.6) | 6.00% | 12.00% | 8 |
| Pulcrano, M | 2007 | France | retrospective analysis | 12 | 40 | 3.9 (0–19.7) | 30.00% (2.9years)a | — | 6 |
| Asari, R | 2009 | Austria | retrospective analysis | 45 | 207 | 9.7 (1–34) | 18.80% | 22.50% | 9 |
| de Melo TG | 2014 | Brazil | retrospective analysis | 12 | 89 | 9.4 (1–36.6) | 13.48% | — | 7 |
| Kim, HJ | 2014 | Korea | retrospective analysis | 16 | 204 | 3.7 (2.3–8.8) | 6.00% | 15.00% | 9 |
| Stenson, G | 2016 | Sweden | retrospective analysis | 5 | 58 | 11.7 (1.8–25.7) | 3.50% | 34.50% | 7 |
| Lee, YM | 2016 | Korea | retrospective analysis | 4 | 166 | 8.6 (1.1–20.3) | 4.20% | — | 8 |
| HUANG Ji-yuan | 2016 | China | retrospective analysis | 3 | 21 | 0.25–10 | — | 24.60% | 6 |
| Su, DH | 2018 | China | retrospective analysis | 30 | 204 | 8.3 (1–24.4) | 10.60% | 16.50% | 9 |
| Yamazaki, Haruhiko | 2020 | Japan | retrospective analysis | 10 | 478 | 7.7 | — | 2.55% | 9 |
aEnd point of follow-up
Age
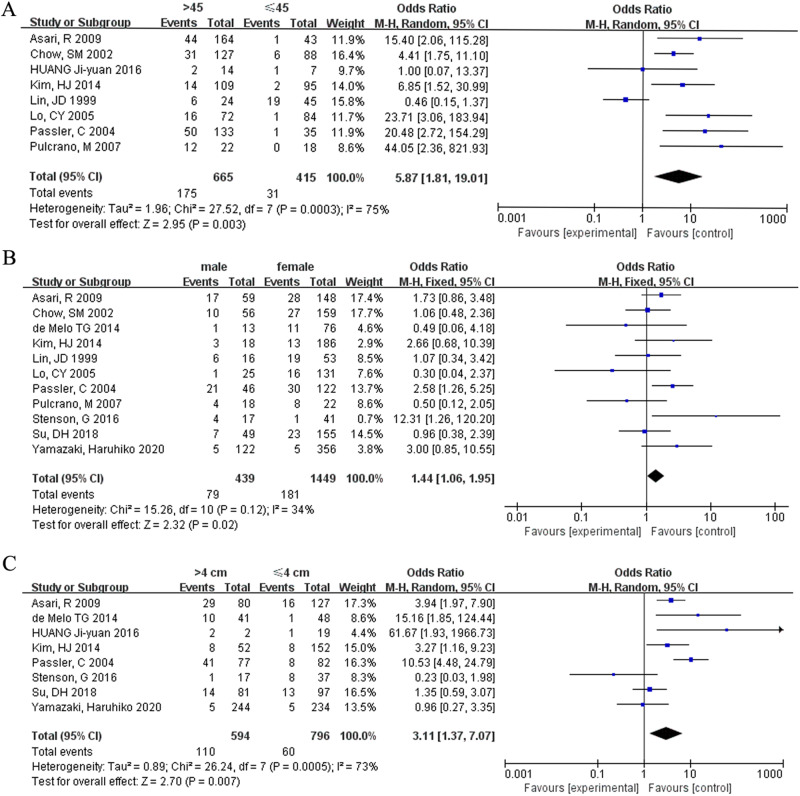
This study included eight studies that looked at age difference in FTC patients aged ≤ 45 and > 45 years. The findings show that age ≤ 45 years was associated with an increased risk of death in FTC patients (OR = 0.17, 95% CI = 0.05–0.55, p = 0.003) (Fig. 2A). Many record data of published articles before 2018, and according to the 6th or 7th edition of the AJCC/TNM staging system, the age boundary is 45 years old. A small number of literature reports that the age boundary is 55 or 60 years old, but due to the number of such literature being less than 3, it cannot be included in the RevMan 5.3 software.
Fig. 2.
Meta-analysis results for the risk factors of FTC. (A) Age; (B) gender; (C) tumor size
Gender
Eleven studies were included in analyzing the risk factor in FTC patients based on gender (male and female). Male FTC patients died at a significantly higher rate (OR = 0.70, 95% CI = 0.51–0.94, p = 0.02) (Fig. 2B).
Tumor diameter
This analysis included eight studies. Tumor diameter greater than 4 cm was associated with the incidence of death in FTC patients (OR = 0.32, 95% CI = −0.14–0.73, p = 0.007) (Fig. 2C).
Multifocality
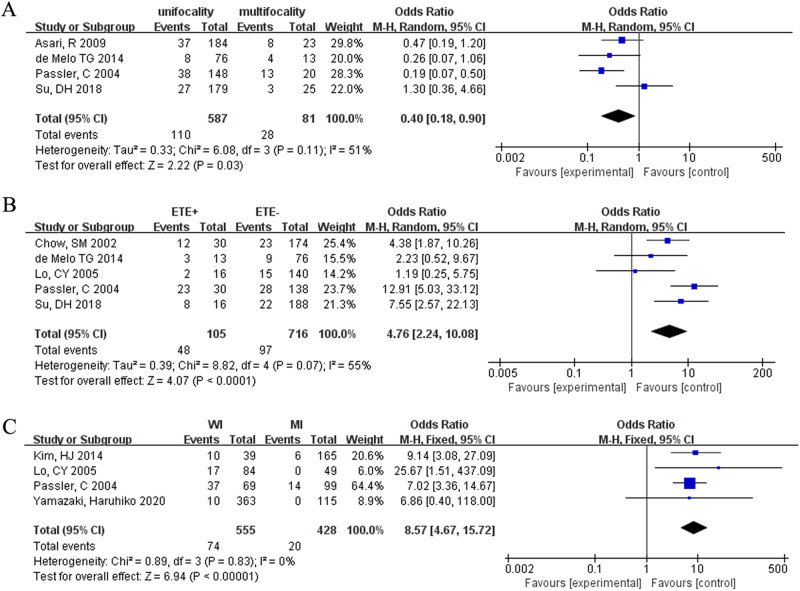
The analysis of tumor multifocality included four studies. In FTC patients, there was a positive correlation between the number of foci and incidence of death (OR = 0.40, 95% CI = 0.18–0.90, p < 0.03) (Fig. 3A).
Fig. 3.
Meta-analysis results for the risk factors of FTC. (A) Multifocality; (B) ETE; (C) histologic subtype
Incidence of ETE
Five studies were included in this analysis. ETE significantly increased the risk of death in FTC patients (OR = 4.76, 95 % CI = 2.24–10.08, p < 0.00001) (Fig. 3B).
Pathologic subtype
Four studies were investigated. Widely invasive FTC patients had an 8.57-fold increased risk of death (OR = 8.57, 95% CI = 4.67–15.77, p < 0.00001) (Fig. 3C).
Surgical type
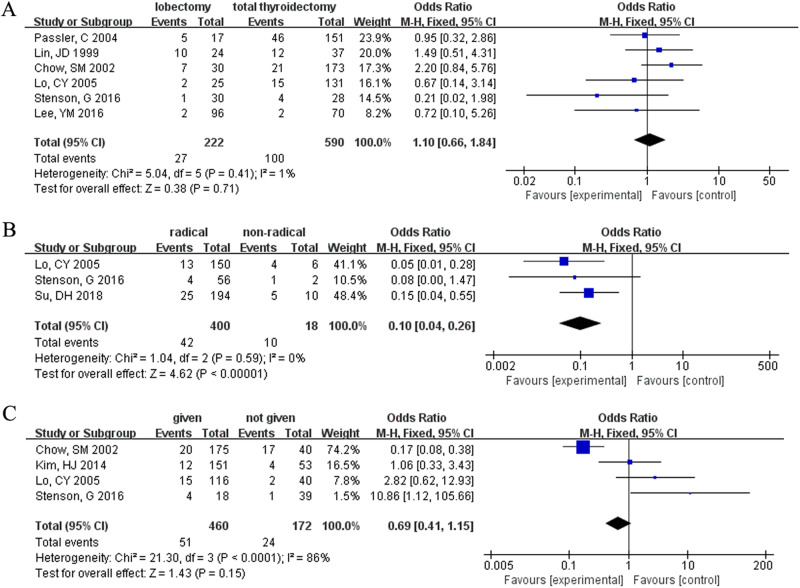
The analysis of risk factors for FTC patients based on surgical type included six studies. However, in FTC patients, neither lobectomy nor total thyroidectomy was associated with death (OR = 1.10, 95% CI = 0.66–1.84, p = 0.71) (Fig. 4A).
Fig. 4.
Meta-analysis results for the risk factors of FTC. (A) Operation type; (B) surgical margin; (C) radioactive iodine
Surgical margin (microscopical)
This analysis included three studies. In FTC, tumor non-radical resection was associated with a higher risk factor for death than radical resection (OR = 0.10, 95% CI = 0.04–0.26, p < 0.00001) (Fig. 4B).
RAI
A total of four studies were included in our database, and the death of FTC patients was not associated with the administration of RAI (OR = 0.69, 95% CI = 0.41–1.15, p = 0.15) (Fig. 4C).
CLNM
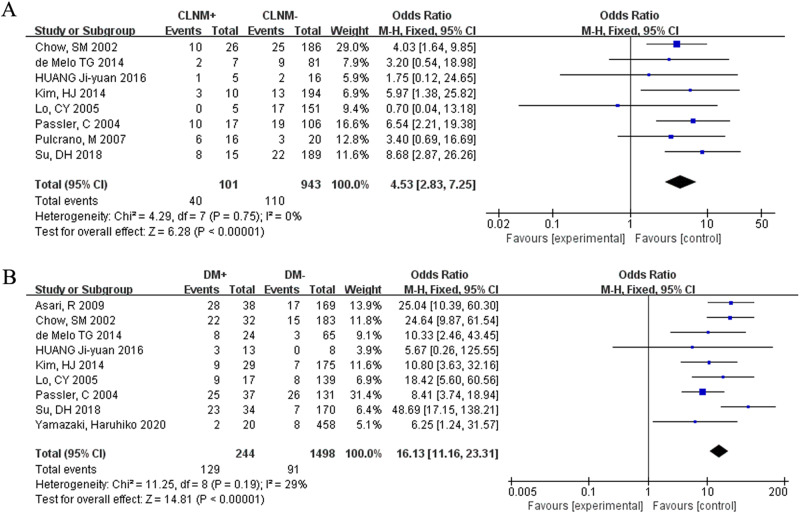
The influence of CLNM on death in FTC patients was assessed in six studies. CLNM was linked to a high rate of death (OR = 4.53, 95% CI = 2.83–7.25, p < 0.00001) (Fig. 5A).
Fig. 5.
Meta-analysis results for the risk factors of FTC. (A) CLNM; (B) distant metastasis
DM
There were nine studies included in this analysis. In FCT patients, DM was correlated with a high risk of death (OR = 16.13, 95% CI = 11.16–23.31, p < 0.00001) (Fig. 5B).
Discussion
Although FTC is generally thought have a good prognosis, some studies have described fatal FTC cases [33–35]. Furthermore, several studies have identified some clinical indicators as poor prognostic factors of FTC for long-term patient survival, which remains debatable, such as age, gender, tumor size, ETE, and so on [36–38]. Few studies evaluated these characteristics with the recommended surgical procedure. Thus, the risk factors of death in patients with FTC should be carefully considered and evaluated, particularly in patients undergoing preoperative evaluation. Predictive risk factors for FTC death are helpful for clinicians in assessing the clinicopathological status of FTC and informing the clinical decision-making of treating physicians [8, 39]. The current study is the first meta-analysis to investigate the risk factors for death of FTC patients, and the findings will assist with evidence-based decisions.
Several studies have revealed the risk factors for FTC death. M C Coburn et al. [40] found that older age at diagnosis was strongly associated with increased mortality. The 10-year survival rate for the older age group was 48%, while the younger age group was 92%. Jukkola A et al. [41] suggested that males had a higher death rate than females.
Similarly, in a study, Xuan V et al. [42] discovered that the tumor diameter of the deceased cases was significantly larger than that of the survivors. Most studies [24, 43] found that multifocality is an independent risk factor for death, with FTC mortality in multifocality being significantly higher than in unifocal cancers. FTC is classified into two major categories in the third edition of the WHO classification based on their degree of invasiveness. WI FTC shows widespread infiltration into adjacent thyroid tissues and blood vessels, whereas MI FTC demonstrates limited capsular and vascular invasion. Invasiveness is not readily visible with MI FTC and can only be determined under a microscope. WI FTC is thought to have a worse prognosis than MI [3]. A retrospective study of 318 FTC patients found that FTC-related ETE (10.37% of cases) had a significantly higher mortality rate than non-ETE [44]. Similarly, univariate and multivariate analysis revealed that WI FTC was significantly associated with death; FTC mortality was 20% (5/25) in the WI group and 0% (0/48) in the MI group [45].
The link between CLNM and death is still debatable. According to some studies, CLNM is not an independent risk factor for death in FTC [46, 47]. Other studies, however, report that CLNM was considered a risk factor [5, 38]. For example, Witte J et al. [48] proposed that FTC be considered when selecting patients for prophylactic central neck lymph node dissection. Ito Y and Slook O et al. conducted a multivariate analysis, and their findings showed that CLNM is an independent risk factor for death in FTC [13, 49]. FTC tends to invade blood vessels, leading to hematogenous dissemination, which increases the likelihood that it will metastasize to distant organs rather than to regional lymph nodes. Patients with FTC who have distant metastases at the time of diagnosis have a poor prognosis [16]. Despite the general belief that FTC has a good prognosis, Su DH et al. reported that most FTC patients have distant metastases [6]. A recent multicenter study with a large cohort demonstrated that distant metastases have an independent risk prognostic value in FTC clinical outcomes [50].
According to KC Loh et al. [51], lobectomy was strongly associated with PTC recurrence and death. According to Rao RS et al. [52], patients who undergo total thyroidectomy have an excellent prognosis. During postoperative follow-up, Kim HJ [29] confirmed that the mortality of FTC was not different between lobectomy and thyroidectomy. Most researchers believed that tumor non-radical resection was significantly related to poor survival of FTC patients [25, 53]. Would highlight that margin positivity may reflect either a very extensive tumor or poor surgical technique that may be the independent driver of poor outcome. Previous research has produced conflicting results regarding whether the death of FTC differs when RAI is administered or not. Jen-Der Lin et al. confirmed that total thyroidectomy with RAI therapy for FTC patients was thought to be unnecessary [54]. Furthermore, Aziz A et al. reported that RAI was associated with FTC patient survival [55]. On the other hand, Hay ID believed that not all FTC patients were inappropriate for RAI [56].
Our forest map analysis revealed that age > 45 years, male, multifocality, tumor diameter > 4 cm, ETE, WI, CLNM, DM and non-radical resection of tumor were risk factors for FTC death. The reason could be that these risk factors are linked to tumor aggressiveness and play a significant role in FTC development. However, lobectomy and failure to receive RAI were not risk factors for FTC death. This finding was contradicted to the published results and that could be attributed to difference in the patient selection criteria and study designs. In this study, there is no evidence that the treatment intensity variables are controlled for the other variables. RAI, for instance, would be expected to be applied to tumors with higher demographic or tumor specific risk. Therefore, an alternative explanation for the findings is that RAI abrogated the poorer outcome for these tumors such that the mortality is now similar to those not treated with RAI. The presence of capsular and vascular invasion of FTC cannot be determined by using preoperative fine-needle aspiration cytology and frozen section pathology because the diagnostic criteria are based on postoperative histological sections [57]. Therefore, even with biomarkers and specific stains, early and accurate diagnosis of FTC is difficult because follicular adenoma and FTC are difficult to be distinguished based on cell morphology [58].
According to our findings, whether a lobectomy or a total thyroidectomy is performed is unimportant. However, it is necessary to ensure complete radical resection. This finding is valuable for treating physicians when FTC is suspected during operation. It is important to note that, complete radical resection, including CLND, should be performed avoiding recurrent laryngeal nerve injury and hypoparathyroidism. Moreover, clinicians should use more individualized initial treatment and closer follow-up for FTC with patients age > 45, male, multifocality, tumor diameter > 4 cm, ETE, WI, CLNM, DM and non-radical resection tumor. Individualized FTC treatment and follow-up could be achieved by combining these clinicopathological risk factors with other imaging techniques.
This study has several limitations. First, the number of studies included was limited due to the lack of raw data from some articles. Second, this meta-analysis included several extensive studies, which may have introduced bias in the overall study results. Third, most patients in the included studies were Asian, which could lead to bias if the findings are applied to all races. This study excluded a randomized controlled trial. Fourth, differences in study populations and objectives among the included studies may have resulted in selective bias. The number of patients with or without CLNM was counted regardless of metastatic site, ipsilateral or bilateral, and central or lateral cervical nodes. Fifth, The cumulative incidence range for 5 years is greater than for 10 years, this is because not every study reports both cumulative incidence outcomes, and there may be selective bias that be due to different case selection criteria for the included studies.
Conclusions
The following significant risk factors for patients dying from FTC were identified in this meta-analysis: age > 45 years, male, multifocality, tumor diameter > 4 cm, ETE, WI, non-radical resection tumor, CLNM, and distant metastases. There was no correlation between lobectomy, RAI treatment and the death of patients with FTC. This finding will help individual management of patients with these risk factors.
Supplementary information
Acknowledgements
This article mainly focuses on the factors that increase the risk factor of death in FTC. Our study showed that age > 45 years, male, tumor diameter (> 4 cm), multifocality, ETE, WI, CLNM, DM and tumor non-radical resection were related to increased risk of death in FTC. Additionally, this finding may support more evidence-based decisions regarding whether FTC patients with these risk factors require additional management and attention. To the best of our knowledge, this is the first meta-analysis to investigate the risk factors for FTC patient death, and the findings may assist with more evidence-based decisions.
Funding
This work was supported by the Scientific Research Foundation of The Education Department of Liaoning Province, China (grant no. QNZR2020009).
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1007/s12020-023-03466-9.
References
- 1.Huang G, Lu H, Li M, Lv Q, Chen Q. Association of total cholesterol and atherosclerotic cardiovascular disease in patients with follicular thyroid cancer: A real-world study from Chinese populations. Medicine (Baltimore) 2021;100:e27310. doi: 10.1097/MD.0000000000027310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baloch ZW, Asa SL, Barletta JA, Ghossein RA, Juhlin CC, Jung CK, LiVolsi VA, Papotti MG, Sobrinho-Simões M, Tallini G, Mete O. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr Pathol. 2022;33:27–63. doi: 10.1007/s12022-022-09707-3. [DOI] [PubMed] [Google Scholar]
- 3.D’Avanzo A, Treseler P, Ituarte PH, Wong M, Streja L, Greenspan FS, Siperstein AE, Duh QY, Clark OH. Follicular thyroid carcinoma: histology and prognosis. Cancer. 2004;100:1123–1129. doi: 10.1002/cncr.20081. [DOI] [PubMed] [Google Scholar]
- 4.Gronlund MP, Jensen JS, Hahn CH, Gronhoj CH, Gronhoj C, Buchwald CV. Risk Factors for Recurrence of Follicular Thyroid Cancer: A Systematic Review. Thyroid. 2021;31:1523–1530. doi: 10.1089/thy.2020.0921. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Mo C, Chen L, Kong L, Wu K, Zhu Y, Chen X. Application of competing risk model in the prognostic prediction study of patients with follicular thyroid carcinoma. Updates Surg. 2022;74:735–746. doi: 10.1007/s13304-021-01103-6. [DOI] [PubMed] [Google Scholar]
- 6.Su DH, Chang TC, Chang SH. Prognostic factors on outcomes of follicular thyroid cancer. J Formos Med Assoc. 2019;118:1144–1153. doi: 10.1016/j.jfma.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Yamazaki H, Sugino K, Katoh R, Matsuzu K, Masaki C, Akaishi J, Hames KY, Tomoda C, Suzuki A, Ohkuwa K, Kitagawa W, Nagahama M, Masuda M, Ito K. Outcomes for Minimally Invasive Follicular Thyroid Carcinoma in Relation to the Change in Age Stratification in the AJCC 8th Edition. Ann Surg Oncol. 2021;28:3576–3583. doi: 10.1245/s10434-020-09397-3. [DOI] [PubMed] [Google Scholar]
- 8.Staubitz JI, Musholt PB, Musholt TJ. The surgical dilemma of primary surgery for follicular thyroid neoplasms. Best Pract Res Clin Endocrinol Metab. 2019;33:101292. doi: 10.1016/j.beem.2019.101292. [DOI] [PubMed] [Google Scholar]
- 9.Gillanders SL, O’Neill JP. Prognostic markers in well differentiated papillary and follicular thyroid cancer (WDTC) Eur J Surg Oncol. 2018;44:286–296. doi: 10.1016/j.ejso.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, Caillou B, Ricard M, Lumbroso JD, De Vathaire F, Schlumberger M. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892–2899. doi: 10.1210/jc.2005-2838. [DOI] [PubMed] [Google Scholar]
- 11.Kim M, Kim HI, Jeon MJ, Kim HK, Kim EH, Yi HS, Kim ES, Kim H, Kim BH, Kim TY, Kim SW, Kang HC, Kim WB, Chung JH, Shong YK, Kim TH, Kim WG. Eighth edition of tumor-node-metastasis staging system improve survival predictability for papillary, but not follicular thyroid carcinoma: A multicenter cohort study. Oral Oncol. 2018;87:97–103. doi: 10.1016/j.oraloncology.2018.10.029. [DOI] [PubMed] [Google Scholar]
- 12.Aboelnaga EM, Ahmed RA. Difference between papillary and follicular thyroid carcinoma outcomes: an experience from Egyptian institution. Cancer Biol Med. 2015;12:53–59. doi: 10.7497/j.issn.2095-3941.2015.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito Y, Miyauchi A, Hirokawa M, Yamamoto M, Oda H, Masuoka H, Sasai H, Fukushima M, Higashiyama T, Kihara M, Miya A. Prognostic value of the 8(th) tumor-node-metastasis classification for follicular carcinoma and poorly differentiated carcinoma of the thyroid in Japan. Endocr J. 2018;65:621–627. doi: 10.1507/endocrj.EJ17-0524. [DOI] [PubMed] [Google Scholar]
- 14.Ito Y, Miyauchi A. Prognostic factors of papillary and follicular carcinomas in Japan based on data of kuma hospital. J Thyroid Res. 2012;2012:973497. doi: 10.1155/2012/973497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Podda M, Saba A, Porru F, Reccia I, Pisanu A. Follicular thyroid carcinoma: differences in clinical relevance between minimally invasive and widely invasive tumors. World J Surg Oncol. 2015;13:193. doi: 10.1186/s12957-015-0612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugino K, Ito K, Nagahama M, Kitagawa W, Shibuya H, Ohkuwa K, Yano Y, Uruno T, Akaishi J, Kameyama K, Ito K. Prognosis and prognostic factors for distant metastases and tumor mortality in follicular thyroid carcinoma. Thyroid. 2011;21:751–757. doi: 10.1089/thy.2010.0353. [DOI] [PubMed] [Google Scholar]
- 17.Sugino K, Kameyama K, Nagahama M, Kitagawa W, Shibuya H, Ohkuwa K, Uruno T, Akaishi J, Suzuki A, Masaki C, Matsuzu K, Kawano M, Ito K. Follicular thyroid carcinoma with distant metastasis: outcome and prognostic factor. Endocr J. 2014;61:273–279. doi: 10.1507/endocrj.EJ13-0437. [DOI] [PubMed] [Google Scholar]
- 18.Wu MH, Lee YY, Lu YL, Lin SF. Risk Factors and Prognosis for Metastatic Follicular Thyroid Cancer. Front Endocrinol (Lausanne) 2022;13:791826. doi: 10.3389/fendo.2022.791826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin JD, Huang MJ, Juang JH, Chao TC, Huang BY, Chen KW, Chen JY, Li KL, Chen JF, Ho YS. Factors related to the survival of papillary and follicular thyroid carcinoma patients with distant metastases. Thyroid. 1999;9:1227–1235. doi: 10.1089/thy.1999.9.1227. [DOI] [PubMed] [Google Scholar]
- 23.Chow SM, Law SC, Mendenhall WM, Au SK, Yau S, Yuen KT, Law CC, Lau WH. Follicular thyroid carcinoma: prognostic factors and the role of radioiodine. Cancer. 2002;95:488–498. doi: 10.1002/cncr.10683. [DOI] [PubMed] [Google Scholar]
- 24.Passler C, Scheuba C, Prager G, Kaczirek K, Kaserer K, Zettinig G, Niederle B. Prognostic factors of papillary and follicular thyroid cancer: differences in an iodine-replete endemic goiter region. Endocr Relat Cancer. 2004;11:131–139. doi: 10.1677/erc.0.0110131. [DOI] [PubMed] [Google Scholar]
- 25.Lo CY, Chan WF, Lam KY, Wan KY. Follicular thyroid carcinoma: the role of histology and staging systems in predicting survival. Ann Surg. 2005;242:708–715. doi: 10.1097/01.sla.0000186421.30982.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pulcrano M, Boukheris H, Talbot M, Caillou B, Dupuy C, Virion A, De Vathaire F, Schlumberger M. Poorly differentiated follicular thyroid carcinoma: prognostic factors and relevance of histological classification. Thyroid. 2007;17:639–646. doi: 10.1089/thy.2007.0029. [DOI] [PubMed] [Google Scholar]
- 27.Asari R, Koperek O, Scheuba C, Riss P, Kaserer K, Hoffmann M, Niederle B. Follicular thyroid carcinoma in an iodine-replete endemic goiter region: a prospectively collected, retrospectively analyzed clinical trial. Ann Surg. 2009;249:1023–1031. doi: 10.1097/SLA.0b013e3181a77b7b. [DOI] [PubMed] [Google Scholar]
- 28.de Melo TG, Zantut-Wittmann DE, Ficher E, da Assumpcao LV. Factors related to mortality in patients with papillary and follicular thyroid cancer in long-term follow-up. J Endocrinol Invest. 2014;37:1195–1200. doi: 10.1007/s40618-014-0131-4. [DOI] [PubMed] [Google Scholar]
- 29.Kim HJ, Sung JY, Oh YL, Kim JH, Son YI, Min YK, Kim SW, Chung JH. Association of vascular invasion with increased mortality in patients with minimally invasive follicular thyroid carcinoma but not widely invasive follicular thyroid carcinoma. Head Neck. 2014;36:1695–1700. doi: 10.1002/hed.23511. [DOI] [PubMed] [Google Scholar]
- 30.Stenson G, Nilsson IL, Mu N, Larsson C, Lundgren CI, Juhlin CC, Hoog A, Zedenius J. Minimally invasive follicular thyroid carcinomas: prognostic factors. Endocrine. 2016;53:505–511. doi: 10.1007/s12020-016-0876-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee YM, Lee YH, Song DE, Kim WB, Sung TY, Yoon JH, Chung KW, Hong SJ. Prognostic Impact of Further Treatments on Distant Metastasis in Patients With Minimally Invasive Follicular Thyroid Carcinoma: Verification Using Inverse Probability of Treatment Weighting. World J Surg. 2017;41:138–145. doi: 10.1007/s00268-016-3608-9. [DOI] [PubMed] [Google Scholar]
- 32.Huang JY, Song WZ, Dai QJ, Guo J. Diagnosis and Treatment of Follicular Thyroid Carcinoma: A Clinical Analysis of 21 Cases. J Chinese. Oncology. 2016;22:907–910. [Google Scholar]
- 33.Janovitz T, Barletta JA. Clinically Relevant Prognostic Parameters in Differentiated Thyroid Carcinoma. Endocr Pathol. 2018;29:357–364. doi: 10.1007/s12022-018-9548-1. [DOI] [PubMed] [Google Scholar]
- 34.Zhang XY, Sun JW, Qiu ZL, Wang Y, Chen XY, Zhao JH, Luo QY. Clinical outcomes and prognostic factors in patients with no less than three distant organ system metastases from differentiated thyroid carcinoma. Endocrine. 2019;66:254–265. doi: 10.1007/s12020-019-01999-6. [DOI] [PubMed] [Google Scholar]
- 35.Sohn SY, Kim HI, Kim YN, Kim TH, Kim SW, Chung JH. Prognostic indicators of outcomes in patients with lung metastases from differentiated thyroid carcinoma during long-term follow-up. Clin Endocrinol. 2018;88:318–326. doi: 10.1111/cen.13489. [DOI] [PubMed] [Google Scholar]
- 36.Rios A, Rodriguez JM, Ferri B, Matínez-Barba E, Febrero B, Parrilla P. Are prognostic scoring systems of value in patients with follicular thyroid carcinoma? Eur J Endocrino. 2013;169:821–827. doi: 10.1530/EJE-13-0372. [DOI] [PubMed] [Google Scholar]
- 37.Yu XF, Wang WB, Teng XD, Wang HY, Chen X, Wang HH, Ma ZM, Fahey TJ, 3rd, Teng LS. Clinicopathological and prognostic analysis of follicular thyroid carcinoma in a single institute over a 15-year period. Eur J Surg Oncol. 2014;40:869–874. doi: 10.1016/j.ejso.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 38.Rios A, Rodriguez JM, Ferri B, Martinez-Barba E, Torregrosa NM, Parrilla P. Prognostic factors of follicular thyroid carcinoma. Endocrinol Nutr. 2015;62:11–18. doi: 10.1016/j.endonu.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Spinelli C, Rallo L, Morganti R, Mazzotti V, Inserra A, Cecchetto G, Massimino M, Collini P, Strambi S. Surgical management of follicular thyroid carcinoma in children and adolescents: A study of 30 cases. J Pediatr Surg. 2019;54:521–526. doi: 10.1016/j.jpedsurg.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 40.Coburn MC, Wanebo HJ. Age correlates with increased frequency of high risk factors in elderly patients with thyroid cancer. Am J Surg. 1995;170:471–475. doi: 10.1016/S0002-9610(99)80332-8. [DOI] [PubMed] [Google Scholar]
- 41.Jukkola A, Bloigu R, Ebeling T, Salmela P, Blanco G. Prognostic factors in differentiated thyroid carcinomas and their implications for current staging classifications. Endocrine-Related Cancer. 2004;11:571–579. doi: 10.1677/erc.1.00826. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen XV, Choudhury KR, Tessler FN, Hoang JK. Effect of Tumor Size on Risk of Metastatic Disease and Survival for Thyroid Cancer: Implications for Biopsy Guidelines. Thyroid. 2018;28:295–300. doi: 10.1089/thy.2017.0526. [DOI] [PubMed] [Google Scholar]
- 43.Lin JD, Hsueh C, Chao TC. Early recurrence of papillary and follicular thyroid carcinoma predicts a worse outcome. Thyroid. 2009;19:1053–1059. doi: 10.1089/thy.2009.0133. [DOI] [PubMed] [Google Scholar]
- 44.Jin M, Kim ES, Kim BH, Kim HK, Yi H-S, Jeon MJ, Kim TY, Kang H-C, Kim WB, Shong YK, Kim M, Kim WG. Clinical Implication of World Health Organization Classification in Patients with Follicular Thyroid Carcinoma in South Korea: A Multicenter Cohort Study. Endocrinol Metab (Seoul) 2020;35:618–627. doi: 10.3803/EnM.2020.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iwasaki H, Toda S, Murayama D, Kato S, Matsui A. Surgical indications and clinical management of benign and malignant follicular thyroid tumors: An algorithmic-based approach. Mol Clin Oncol. 2021;14:32. doi: 10.3892/mco.2020.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin JD, Liou MJ, Chao TC, Weng HF, Ho YS. Prognostic variables of papillary and follicular thyroid carcinoma patients with lymph node metastases and without distant metastases. Endocr Relat Cancer. 1999;6:109–115. doi: 10.1677/erc.0.0060109. [DOI] [PubMed] [Google Scholar]
- 47.Grsc K, Bumber B, Curic Radivojevic R, Leovic D. Prophylactic Central Neck Dissection in Well-differentiated Thyroid Cancer. Acta Clin Croat. 2020;59:87–95. doi: 10.20471/acc.2020.59.s1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Witte J, Goretzki PE, Dieken J, Simon D, Roher HD. Importance of lymph node metastases in follicular thyroid cancer. World J Surg. 2002;26:1017–1022. doi: 10.1007/s00268-002-6668-y. [DOI] [PubMed] [Google Scholar]
- 49.Slook O, Levy S, Slutzky-Shraga I, Tsvetov G, Robenshtok E, Shimon I, Benbassat C, Hirsch D. Long-Term Outcomes and Prognostic Factors in Patients with Differentiated Thyroid Carcinoma and Bone Metastases. Endocr Pract. 2019;25:427–437. doi: 10.4158/EP-2018-0465. [DOI] [PubMed] [Google Scholar]
- 50.Jin M, Kim ES, Kim BH, Kim HK, Yi HS, Jeon MJ, Kim TY, Kang HC, Kim WB, Shong YK, Kim M, Kim WG. Clinical Implication of World Health Organization Classification in Patients with Follicular Thyroid Carcinoma in South Korea: A Multicenter Cohort Study. Endocrinol Metab (Seoul) 2020;35:618–627. doi: 10.3803/EnM.2020.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loh KC, Greenspan FS, Gee L, Miller TR, Yeo PP. Pathological tumor-node-metastasis (pTNM) staging for papillary and follicular thyroid carcinomas: a retrospective analysis of 700 patients. J Clin Endocrinol Metab. 1997;82:3553–3562. doi: 10.1210/jcem.82.11.4373. [DOI] [PubMed] [Google Scholar]
- 52.Rao RS, Parikh HK, Deshmane VH, Parikh DM, Shrikhande SS, Havaldar R. Prognostic factors in follicular carcinoma of the thyroid: a study of 198 cases. Head Neck. 1996;18:118–126. doi: 10.1002/(SICI)1097-0347(199603/04)18:2<118::AID-HED2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 53.Clark OH. Predictors of thyroid tumor aggressiveness. West J Med. 1996;165(3):131–138. [PMC free article] [PubMed] [Google Scholar]
- 54.Lin J-D, Chao T-C, Chen S-T, Huang Y-Y, Liou M-J, Hsueh C. Operative strategy for follicular thyroid cancer in risk groups stratified by pTNM staging. Surg Oncol. 2007;16:107–113. doi: 10.1016/j.suronc.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 55.Aziz A, Masood MQ, Sattar S, Fatima S, Islam N. Follicular Thyroid Carcinoma in a Developing Country: A 10-Year Retrospective Study. Cureus. 2021;13:e16594. doi: 10.7759/cureus.16594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hay ID. Selective use of radioactive iodine in the postoperative management of patients with papillary and follicular thyroid carcinoma. J Surg Oncol. 2006;94:692–700. doi: 10.1002/jso.20696. [DOI] [PubMed] [Google Scholar]
- 57.Zhu Y, Li Y, Jung CK, Song DE, Hang JF, Liu Z, Jain D, Lai CR, Hirokawa M, Kakudo K, Bychkov A. Histopathologic Assessment of Capsular Invasion in Follicular Thyroid Neoplasms-an Observer Variation Study. Endocr Pathol. 2020;31:132–140. doi: 10.1007/s12022-020-09620-7. [DOI] [PubMed] [Google Scholar]
- 58.Mitchell AL, Gandhi A, Scott-Coombes D, Perros P. Management of thyroid cancer: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016;130:S150–S160. doi: 10.1017/S0022215116000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.