Abstract
Background
Biofeedback therapy has been used to treat the symptoms of people with chronic constipation referred to specialist services within secondary and tertiary care settings. However, different methods of biofeedback are used within different centres and the magnitude of suggested benefits and comparable effectiveness of different methods of biofeedback has yet to be established.
Objectives
To determine the efficacy and safety of biofeedback for the treatment of chronic idiopathic (functional) constipation in adults.
Search methods
We searched the following databases from inception to 16 December 2013: CENTRAL, the Cochrane Complementary Medicine Field, the Cochrane IBD/FBD Review Group Specialized Register, MEDLINE, EMBASE, CINAHL, British Nursing Index, and PsychINFO. Hand searching of conference proceedings and the reference lists of relevant articles was also undertaken.
Selection criteria
All randomised trials evaluating biofeedback in adults with chronic idiopathic constipation were considered for inclusion.
Data collection and analysis
The primary outcome was global or clinical improvement as defined by the included studies. Secondary outcomes included quality of life, and adverse events as defined by the included studies. Where possible, we calculated the risk ratio (RR) and corresponding 95% confidence interval (CI) for dichotomous outcomes and the mean difference (MD) and 95% CI for continuous outcomes. We assessed the methodological quality of included studies using the Cochrane risk of bias tool. The overall quality of the evidence supporting each outcome was assessed using the GRADE criteria.
Main results
Seventeen eligible studies were identified with a total of 931 participants. Most participants had chronic constipation and dyssynergic defecation. Sixteen of the trials were at high risk of bias for blinding. Attrition bias (4 trials) and other potential bias (5 trials) was also noted. Due to differences between study populations, the heterogeneity of the different samples and large range of different outcome measures, meta‐analysis was not possible. Different effect sizes were reported ranging from 40 to 100% of patients who received biofeedback improving following the intervention. While electromyograph (EMG) biofeedback was the most commonly used, there is a lack of evidence as to whether any one method of biofeedback is more effective than any other method of biofeedback. We found low or very low quality evidence that biofeedback is superior to oral diazepam, sham biofeedback and laxatives. One study (n = 60) found EMG biofeedback to be superior to oral diazepam. Seventy per cent (21/30) of biofeedback patients had improved constipation at three month follow‐up compared to 23% (7/30) of diazepam patients (RR 3.00, 95% CI 1.51 to 5.98). One study compared manometry biofeedback to sham biofeedback or standard therapy consisting of diet, exercise and laxatives. The mean number of complete spontaneous bowel movements (CSBM) per week at three months was 4.6 in the biofeedback group compared to 2.8 in the sham biofeedback group (MD 1.80, 95% CI 1.25 to 2.35; 52 patients). The mean number of CSBM per week at three months was 4.6 in the biofeedback group compared to 1.9 in the standard care group (MD 2.70, 95% CI 1.99 to 3.41; 49 patients). Another study (n = 109) compared EMG biofeedback to conventional treatment with laxatives and dietary and lifestyle advice. This study found that at both 6 and 12 months 80% (43/54) of biofeedback patients reported clinical improvement compared to 22% (12/55) laxative‐treated patients (RR 3.65, 95% CI 2.17 to 6.13). Some surgical procedures (partial division of puborectalis and stapled transanal rectal resection (STARR)) were reported to be superior to biofeedback, although with a high risk of adverse events in the surgical groups (wound infection, faecal incontinence, pain, and bleeding that required further surgical intervention). Successful treatment, defined as a decrease in the obstructed defecation score of > 50% at one year was reported in 33% (3/39) of EMG biofeedback patients compared to 82% (44/54) of STARR patients (RR 0.41, 95% CI 0.26 to 0.65). For the other study the mean constipation score at one year was 16.1 in the balloon sensory biofeedback group compared to 10.5 in the partial division of puborectalis surgery group (MD 5.60, 95% CI 4.67 to 6.53; 40 patients). Another study (n = 60) found no significant difference in efficacy did not demonstrate the superiority of a surgical intervention (posterior myomectomy of internal anal sphincter and puborectalis) over biofeedback. Conflicting results were found regarding the comparative effectiveness of biofeedback and botulinum toxin‐A. One small study (48 participants) suggested that botulinum toxin‐A injection may have short term benefits over biofeedback, but the relative effects of treatments were uncertain at one year follow‐up. No adverse events were reported for biofeedback, although this was not specifically reported in the majority of studies. The results of all of these studies need to be interpreted with caution as GRADE analyses rated the overall quality of the evidence for the primary outcomes (i.e. clinical or global improvement as defined by the studies) as low or very low due to high risk of bias (i.e. open label studies, self‐selection bias, incomplete outcome data, and baseline imbalance) and imprecision (i.e. sparse data).
Authors' conclusions
Currently there is insufficient evidence to allow any firm conclusions regarding the efficacy and safety of biofeedback for the management of people with chronic constipation. We found low or very low quality evidence from single studies to support the effectiveness of biofeedback for the management of people with chronic constipation and dyssynergic defecation. However, the majority of trials are of poor methodological quality and subject to bias. Further well‐designed randomised controlled trials with adequate sample sizes, validated outcome measures (especially patient reported outcome measures) and long‐term follow‐up are required to allow definitive conclusions to be drawn.
Plain language summary
Biofeedback (use of equipment to retrain the muscles around the anus and coordinated pushing) for the treatment of chronic constipation in adults
Chronic constipation (inability to achieve satisfactory bowel emptying for a prolonged period with no apparent medical cause) can be an embarrassing and socially restricting problem. There are many possible causes, including an inability to relax the muscles which control bowel movements. ’Biofeedback’, where computer equipment or a rectal balloon is used to show people how to coordinate and use the muscles properly, is often recommended.
The purpose of this systematic review was to examine the effectiveness and side effects of biofeedback therapy used for the treatment of chronic constipation in adults who are unable to relax the muscles which control bowel movements. This review identified 17 eligible studies that included a total of 931 participants. The studies either compared the effectiveness of different types of biofeedback to one another, or biofeedback to a sham biofeedback (a fake biofeedback treatment) or biofeedback to standard treatment consisting of diet, exercise and laxatives. There is some evidence that biofeedback is superior to treatment with oral diazepam (a sedative known as Valium), sham biofeedback and laxatives. One study of 60 participants found biofeedback with computer equipment to be superior to oral diazepam (a sedative drug that is not usually used to treat constipation). Another study of 77 participants suggests that biofeedback is superior to sham biofeedback or standard therapy consisting of diet, exercise and laxatives. Another study with 109 participants also suggested that biofeedback with computer equipment is superior to conventional treatment with laxatives and dietary and lifestyle advice. Some surgical procedures (partial division of puborectalis and stapled transanal rectal resection) were reported to be superior to biofeedback. However, there was a high risk of side effects in the surgical treatment groups including wound infection, faecal incontinence, pain, and bleeding that required further surgery. One other study of 60 participants did not find a difference in effectiveness between surgery (posterior myomectomy of internal anal sphincter and puborectalis) and biofeedback treatment. Botulinum toxin‐A injection may have short term benefits over biofeedback, but the benefit does not last. No adverse events were reported for biofeedback, although this was not specifically reported in the majority of studies. The results of this review need to be interpreted with caution as they are based on small numbers of patients and the overall quality of the evidence from the studies was rated as low or very low due to lack of precision of the results and the low methodological quality of the studies. Thus no firm conclusions can be made regarding the effectiveness and potential side effects of biofeedback treatment for patients with chronic constipation who are unable to relax the muscles which control bowel movements. Further larger trials are needed to provide better evidence.
Summary of findings
Summary of findings for the main comparison. Balloon sensory training biofeedback versus surgery for chronic constipation.
| Balloon sensory training biofeedback versus surgery for chronic constipation | ||||||
| Patient or population: Patients with anismus and chronic constipation Settings: Outpatient procedure Intervention: Balloon sensory training biofeedback versus surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Balloon sensory training biofeedback versus surgery | |||||
| Number improved at one year | 700 per 10001 | 301 per 1000 (147 to 623) | RR 0.43 (0.21 to 0.89) | 40 (1 study) | ⊕⊝⊝⊝ very low2,3 | |
| Constipation score at one year | The mean constipation score in the control group at one year was 10.5 | The mean constipation score in the intervention group was 5.6 points higher (4.67 to 6.53 higher) | 40 (1 study) |
⊕⊝⊝⊝ very low2,4 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; MD: Mean difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk estimate comes from the control arm of the included trial. 2 High risk of bias in the included study due to open‐label design and risk of other bias 3 Very sparse data (20 events) and wide confidence intervals 4 Sparse data (40 patients)
Summary of findings 2. Balloon sensory training biofeedback versus botulinum toxin‐A for chronic constipation.
| Balloon sensory training biofeedback versus botulinum toxin‐A for chronic constipation | ||||||
| Patient or population: Patients with anismus and chronic constipation Settings: Outpatient procedure Intervention: Balloon sensory training biofeedback versus surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Balloon sensory training biofeedback versus surgery | |||||
| Constipation score at one year | The mean constipation score in the control group at one year was 14.3 | The mean constipation score in the intervention group was 1.8 points higher (0.87 to 2.73 higher) | 40 (1 study) |
⊕⊝⊝⊝ very low1,2 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; MD: Mean difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 High risk of bias in the included study due to open‐label design and risk of other bias 2 Sparse data (40 patients)
Summary of findings 3. EMG biofeedback versus diazepam for chronic constipation.
| EMG biofeedback versus diazepam for chronic constipation | ||||||
| Patient or population: Patients with pelvic floor dyssynergia and chronic constipation Settings: Outpatient procedure Intervention: EMG biofeedback versus diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | EMG biofeedback versus diazepam | |||||
| Number improved at three months | 233 per 10001 | 699 per 1000 (352 to 1393) | RR 3.00 (1.51 to 5.98) | 60 (1 study) | ⊕⊝⊝⊝ very low2,3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; MD: Mean difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk estimate comes from the control arm of the included trial. 2 High risk of bias in the included study due to open‐label design and risk of self‐selection bias among participants. 3 Very sparse data (28 events) and wide confidence intervals.
Summary of findings 4. EMG biofeedback versus STARR procedure for obstructed defecation.
| EMG biofeedback versus STARR procedure for obstructed defecation | ||||||
| Patient or population: Patients with obstructed defecation Settings: Outpatient procedure Intervention: EMG biofeedback versus STARR procedure | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | EMG biofeedback versus STARR | |||||
| Treatment success at one year1 | 815 per 10002 | 334 per 1000 (212 to 530) | RR 0.41 (0.26 to 0.65) | 93 (1 study) | ⊕⊕⊝⊝ low3,4 | |
| Obstructed defecation score at one year | The mean obstructed defecation score in the control group at one year was 4.7 | The mean obstructed defecation score in the intervention group was 5.5 points higher (3.44 to 7.56 higher) | 93 (1 study) | ⊕⊕⊝⊝ low3,5 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; MD: Mean difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Treatment success was defined as a decrease in the obstructed defecation score of > 50% at one year. 2 Control group risk estimate comes from the control arm of the included trial. 3 High risk of bias in the included study due to open‐label design and incomplete outcome data. 4 Sparse data (57 events) and wide confidence intervals. 5 Sparse data (93 patients).
Summary of findings 5. EMG biofeedback versus laxative for chronic constipation.
| EMG biofeedback versus laxative for chronic constipation | ||||||
| Patient or population: Patients with pelvic floor dyssynergia and chronic constipation Settings: Outpatient procedure Intervention: EMG biofeedback versus laxative | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | EMG biofeedback versus laxative | |||||
| Major clinical improvement at six months | 218 per 10001 | 796 per 1000 (473 to 1336) | RR 3.65 (2.17 to 6.13) | 109 (1 study) | ⊕⊕⊝⊝ low2,3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; MD: Mean difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk estimate comes from the control arm of the included trial. 2 High risk of bias in the included study due to open‐label design. 3 Sparse data (55 events) and wide confidence intervals.
Summary of findings 6. Manometry biofeedback versus sham biofeedback for chronic constipation.
| Manometry biofeedback versus sham biofeedback for chronic constipation | ||||||
| Patient or population: Patients with pelvic floor dyssynergia and chronic constipation Settings: Outpatient procedure Intervention: Manometry biofeedback versus sham biofeedback | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Manometry biofeedback versus sham biofeedback | |||||
| Complete spontaneous bowel movements per week at three months | The mean number of complete spontaneous bowel movements per week in the sham control group at three months year was 2.8 | The mean number of complete spontaneous bowel movements in the intervention group was 1.8 movements higher (1.25 to 2.35 higher) | 52 (1 study) |
⊕⊕⊝⊝ low1,2 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; MD: Mean difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 High risk of bias in the included study due to open‐label design and the two groups were not equal at baseline as the biofeedback group had a significantly lower defecation index and relatively greater pelvic floor dysfunction than the sham group. 2 Sparse data (52 patients).
Summary of findings 7. Manometry biofeedback versus standard care for chronic constipation.
| Manometry biofeedback versus standard care for chronic constipation | ||||||
| Patient or population: Patients with pelvic floor dyssynergia and chronic constipation Settings: Outpatient procedure Intervention: Manometry biofeedback versus standard care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Manometry biofeedback versus standard care | |||||
| Complete spontaneous bowel movements per week at three months | The mean number of complete spontaneous bowel movements per week in the control group at three months was 1.9 | The mean number of complete spontaneous bowel movements in the intervention group was 2.7 movements higher (1.99 to 3.41 higher) | 52 (1 study) |
⊕⊕⊝⊝ low1,2 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; MD: Mean difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 High risk of bias in the included study due to open‐label design. 2 Sparse data (52 patients).
Background
Description of the condition
Constipation is a symptom‐based disorder defined as, “unsatisfactory defecation characterized by infrequent stools, difficult stool passage, or both. Difficult stool passage includes straining, a sense of difficulty passing stool, incomplete evacuation, hard lumpy stools, prolonged time to stool or need for manual manoeuvres to pass stool” (Brandt 2005). Chronic constipation is further defined as the presence of these symptoms for at least six months. If investigations exclude all underlying bowel and other pathologies (e.g. neurological or endocrine conditions, adverse effects of medication and immobility), that could be causing the constipation, then the constipation is considered to be functional or idiopathic. Idiopathic constipation accounts for 75% of cases of chronic constipation (Gilliland 1997).
Two main types of idiopathic constipation have been distinguished: slow transit constipation and functional outlet obstruction or evacuation disorders (Bleijenberg 1994), which has also been referred to in the literature as either spastic pelvic floor syndrome, pelvic floor dyssynergia, paradoxical puborectalis contraction or anismus. Outlet obstruction is thought to be due to abnormal use of a normal pelvic floor. During straining, the puborectalis muscle contracts instead of relaxing and the anal canal remains closed, preventing defecation (Bleijenberg 1994; Gilliland 1997). It has also been suggested the problem is due to insufficient propulsive force being generated in the pelvis (Koutsomanis 1995). Slow transit constipation is the result of a failure of peristalsis to move faecal material through the colon at a normal rate. The aetiology of slow transit constipation is unknown, but is likely to be multifactorial and may differ across individuals.
Some patients exhibit both slow transit and functional outlet obstruction (Rieger 1997; Rao 1998). Constipation is a common problem in Western populations, with most studies identifying a prevalence of between 12 to 19% (Higgins 2004). The prevalence of chronic idiopathic constipation is unknown. Chronic idiopathic constipation can result in increased levels of anxiety, depression and poor quality of life (Irvine 2002; Mason 2002; Cheng 2003).
Description of the intervention
Biofeedback is based on behaviour modification (Denis 1996). Gut directed biofeedback retraining usually involves patients being taught to defecate effectively using bracing of the abdominal wall muscles and effective relaxation of the pelvic floor muscles (Emmanuel 2001), with or without attempts to modify sensation in the rectum. However, there is a wide variety of methods and protocols. The first reports of biofeedback being used for the treatment of constipation were published in 1981 and 1983 (Denis 1981; Van Baal 1983).
Biofeedback treatment for idiopathic constipation has since been studied widely and is used to manage chronic idiopathic constipation that is intractable and non‐responsive to dietary manipulation or laxatives. There are three main methods of monitoring the function of the anus and providing biofeedback to patients. These methods include electromyograph (EMG) biofeedback, manometry biofeedback and balloon sensory training (Bassotti 2004). None of these methods have been shown to be consistently more effective than any other method (Chronic Constipation Task Force 2005). During biofeedback sessions patients may also be given basic instruction on gut anatomy and function, as well as behavioural advice about frequency and length of toilet visits, posture on the toilet and dietary habits (Emmanuel 2001).
EMG biofeedback relies upon the display of a recording of electromyographic activity from the external anal sphincter and pelvic floor or abdominal muscles or both on a computer monitor (Bassotti 2004). Recordings may be made from electrodes placed within the anal canal or from adhesive surface electrodes on the patient’s perianal or perineal skin or abdominal muscles or both. The patient learns to relax the pelvic floor muscles initially by watching the recording on a monitor and subsequently learns to 'push' to defecate, while keeping these same muscles relaxed (Bassotti 2004).
Manometry biofeedback requires the insertion of a manometric probe such as a pressure transducer, perfused catheter or balloon, into the anal canal to measure anal canal pressure and contraction and relaxation of the pelvic floor (Bassotti 2004). Contraction and relaxation of the anal sphincters and pelvic floor is then displayed on a computer monitor and training techniques are very similar to those employed during EMG biofeedback.
Sensory training involves the patient learning to discriminate decreasing volumes in the rectum and to expel a simulated stool, usually an air or water‐filled intrarectal balloon. Initially the balloon may be inserted into the rectum, inflated and then withdrawn by the therapist as the patient focuses on the sensations produced and attempts to relax the pelvic floor and anal sphincters (Bassotti 2004). Later during the training the patient is expected to attempt to pass the balloon independently to improve defecation. Two or three different balloon systems are in use and sensory training may be combined with manometry or EMG biofeedback.
How the intervention might work
The majority of biofeedback studies to date have focused on the effectiveness of the therapy for patients with chronic idiopathic constipation due to evacuation disorders, but the effectiveness of biofeedback for chronic idiopathic constipation as a result of slow gut transit has also been investigated (Chiotakaku‐Faliakou 1998; Emmanuel 2001; Battaglia 2004). Patients are enabled to recognise the sensations associated with relaxation of the pelvic floor and anus by a variety of different methods (Denis 1996), learn correct use of abdominal muscles to create an effective pushing force and thus learn to defecate effectively. Evacuating regularly may also stimulate gut transit.
Why it is important to do this review
Non‐randomised studies of biofeedback for idiopathic constipation have reported positive results and suggest that 33% to 90% of patients improve following treatment (Fleshman 1992; Gilliland 1997; Coulter 2002; Heymen 2003; Chiarioni 2008). This has led to the assertion that biofeedback is an effective intervention and the treatment of choice for chronic idiopathic constipation occurring as a result of both evacuation disorders and slow gut transit. However, there is an inherent risk of bias with case series and non‐randomised trials, which do not control for non‐specific effects of an intervention such as incidental advice and patient‐therapist interaction. Non‐randomised studies are more likely to show falsely positive and larger treatment effects than randomised controlled trials (Guyatt 2008a). Potential biases, such as selection bias, confounding and reporting bias, are likely to occur in non‐randomised studies. Effect sizes estimated from such exploratory non‐randomised trials have been used to inform power calculations for a minority of subsequent randomised controlled trials included in this review (Koutsomanis 1995; Glia 1997; Chiarioni 2006; Heymen 2007).
Few investigators have identified the possible 'placebo effect' that the interaction with the biofeedback therapist may produce (Rao 1997). A systematic review is required to summarize the available data on the efficacy of biofeedback for the treatment of chronic idiopathic constipation. The aim of this review was to answer the question: Does biofeedback decrease physical or psychological morbidity and symptom distress and improve quality of life in patients with a diagnosis of chronic idiopathic (functional) constipation?
Objectives
The primary objective was to assess the efficacy and safety of biofeedback for the treatment of chronic idiopathic (functional) constipation in adults.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing one method of biofeedback for constipation with sham treatment, conventional treatment, no treatment or another method of biofeedback were considered for inclusion. There were no language restrictions.
Types of participants
Male or female patients over 18 years of age with chronic idiopathic (functional) constipation receiving care in a variety of healthcare settings (hospital, community) were included. Chronic idiopathic constipation can be defined using the Rome I, II or III criteria. Idiopathic constipation according to the Rome III criteria (Longstreth 2006), consists of two or more of the following symptoms for at least 3 months: 1. straining during at least 25% defecations; 2. lumpy or hard stools in at least 25% defecations; 3. sensation of incomplete evacuation for at least 25% defecations; 4. sensation of anorectal obstruction or blockage in at least 25% defecations; 5. manual manoeuvres to facilitate at least 25% of defecations (e.g. digital evacuation, support of the pelvic floor); 6. fewer than 3 defecations per week; 7. loose stools are rarely present without the use of laxatives; and 8. insufficient criteria for a diagnosis of irritable bowel syndrome (IBS).
To avoid missing studies that did not utilize Rome criteria the American College of Gastroenterology Chronic Constipation Task Force definition of chronic constipation was also utilized (Brandt 2005). Patients with constipation secondary to the use of constipating medication or to conditions such as diabetes mellitus, long‐term neurological conditions, hypothyroidism, tumour, anal fissure as well as acute constipation were excluded.
Types of interventions
Studies of biofeedback treatment for chronic idiopathic constipation were considered for inclusion. All types of visual or auditory biofeedback (e.g. EMG biofeedback, manometry biofeedback, balloon sensory training) were considered. Biofeedback treatments needed to be carried out by a qualified healthcare practitioner (e.g. medical practitioner, nurse, physiotherapist), but could be carried out in a primary, secondary or tertiary care setting.
Types of outcome measures
Primary outcomes
The primary outcome measures were global or clinical improvement as defined by the included studies (e.g. constipation score, clinical symptoms, frequency of defecation, straining, lumpy or hard stools, sensation of incomplete evacuation, sensation of anorectal blockage, manual manoeuvres to facilitate defecation, pain, and bloating).
Secondary outcomes
Secondary outcome measures included: ‐ anxiety and depression; ‐ quality of life (QoL); ‐ need for rescue medication such as laxatives or rectal evacuants; ‐ gastrointestinal transit time measurement (e.g. using radio‐opaque markers), functional recto‐anal evaluation (proctoscopy, ano‐rectal manometry, defecography) or electromyography; ‐ cost effectiveness; and ‐ any adverse events.
Search methods for identification of studies
Electronic searches
The following databases were searched from inception to December 2013 to obtain relevant studies for this review.
The Cochrane Central Register of Controlled Trials (CENTRAL).
The Cochrane Complementary Medicine Field.
The Cochrane Inflammatory Bowel Disease and Functional Bowel Disorders Group Specialized Register.
MEDLINE.
CINAHL (1982 to present).
British Nursing Index (1984 to present).
EMBASE (1980 to present).
PsychINFO (1989 to present).
SCOPUS.
Science Citation Index Expanded (SCI‐EXPANDED) (1980‐present).
Social Sciences Citation Index (SSCI) (1980‐present).
Conference Proceedings Citation Index ‐ Social Science and Humanities (CPCI‐SSH) (1990‐present).
Conference Proceedings Citation Index ‐ Science (CPCI‐S) (1990‐present).
MESH and keyword terms were modified as necessary for each database. There were no language restrictions. The searches were restricted by publication type to randomised controlled trials and controlled clinical trials by applying the Cochrane highly sensitive search strategy for identifying randomised controlled trials in MEDLINE: Ovid format (Lefebvre 2011). The latest search for this review was conducted on 16 December 2013. The search strategies for each database are reported in Appendix 1.
Searching other resources
The reference lists of identified randomised clinical trials and review articles were checked in order to find randomised trials not identified by the electronic searches. Ongoing trials were searched through the websites www.controlled‐trials.com and www.clinicaltrials.gov. Grey literature was searched through the SIGLE and GreyNet databases and other unpublished literature was obtained through searches of conference proceedings as identified above.
Data collection and analysis
Selection of studies
Two authors (SW and CN) independently reviewed potentially relevant studies to determine if they met the pre‐specified inclusion criteria. Any disagreement between authors was resolved by consensus and if necessary by consultation with the third author (PC).
Data extraction and management
A standardized data extraction sheet was developed to record data on: study quality, study setting, participants (age and sex; how diagnosis was confirmed; inclusion and exclusion criteria), interventions (type of biofeedback, administration, duration, regimen of controlled intervention), outcome measures, attrition, intention‐to‐treat (ITT) analysis, duration of follow‐up and the type and number of any reported adverse events. Two authors (SW and CN) independently extracted the data from each study. Any disagreement was resolved by discussion and consensus with the third author (PC).
Assessment of risk of bias in included studies
The full text of all eligible studies was obtained for independent review by two reviewers (SW and CN). Reviewers were not blinded as to the authors of studies. The methodological quality of each study was assessed and where necessary the study authors were contacted for missing data or clarification of the published data.
The Cochrane risk of bias tool was used to assess the quality of randomised controlled trials (Higgins 2011). Factors assessed included:
random sequence generation;
allocation concealment;
blinding;
incomplete outcome data;
selective outcome reporting; and
other potential sources of bias.
We rated each of these factors as ‘low risk’, ‘high risk’ or ‘unclear risk’. Disagreements were resolved by consensus.
We used the GRADE approach to assess the overall quality of evidence for the primary outcome and selected secondary outcomes of interest. Outcomes from pooling of randomised trials start as high quality evidence, but may be downgraded due to: (1) risk of bias, (2) indirectness of evidence, (3) inconsistency (unexplained heterogeneity), (4) imprecision (sparse data), and (5) reporting bias (publication bias). The overall quality of evidence for each outcome was determined after considering each of these elements, and categorized as high quality (i.e. further research is very unlikely to change our confidence in the estimate of effect); moderate quality (i.e. further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate); low quality (i.e. further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate); or very low quality (i.e. we are very uncertain about the estimate) (Guyatt 2008b; Schünemann 2011).
Measures of treatment effect
The extracted data from the original studies were used to construct two by two tables (e.g. clinical improvement versus no improvement for biofeedback versus control). Where possible we calculated the risk ratio (RR) with corresponding 95% confidence intervals (95% CI) for each dichotomous outcome and the mean difference (MD) with corresponding 95% CI, however this was not possible from the data presented.
Dealing with missing data
The authors of the included studies were contacted, where possible, to obtain any missing data. Where possible an ITT analysis was used whereby any missing outcome data were assumed to be treatment failures.
Data synthesis
Data were analysed using Review Manager (RevMan 5.2). Data from individual trials were to be combined for meta‐analysis if the interventions, patient groups and outcomes were sufficiently similar (determined by consensus). Data were not to be pooled for meta‐analysis if a high degree of heterogeneity was detected (i.e. I2 > 75%). A fixed‐effect model was to be used to pool data in the absence of heterogeneity. A random‐effects model was to be used if significant heterogeneity was detected. The pooled RR and corresponding 95% CI was to be calculated for dichotomous outcomes. For continuous outcomes the pooled MD or SMD and 95% CI were to be calculated as appropriate.
Subgroup analysis and investigation of heterogeneity
If a sufficient number of randomised trials were identified, the following subgroups analyses were planned:
1. duration of disease (less than 5 years, 5 to 10 years, more than 10 years);
2. constipation sub‐type (slow‐transit constipation without evacuation disorder, slow transit constipation with evacuation disorder, normal transit constipation with evacuation disorder); and
3. method of biofeedback (EMG, manometry, sensory training).
Sensitivity analysis
A sensitivity analysis was planned to determine if the findings from the primary analysis were changed by incorporating different trials in the analysis.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies and Characteristics of ongoing studies.
Results of the search
A total of 1232 results were obtained from the electronic searches, of which 98 were duplicated between the databases. The titles and abstracts of the remaining 1134 results were read and 49 papers were considered for inclusion. The full papers of these 49 studies were read and 19 non‐randomised studies, one RCT of biofeedback for anal pain (Chiarioni 2010), and one letter were excluded, leaving a total of 25 individual reports of 17 randomised studies for inclusion in the review (Figure 1). Studies reported as an abstract were only included where no full published paper was available (Hu 2006; Jung 2007).
1.
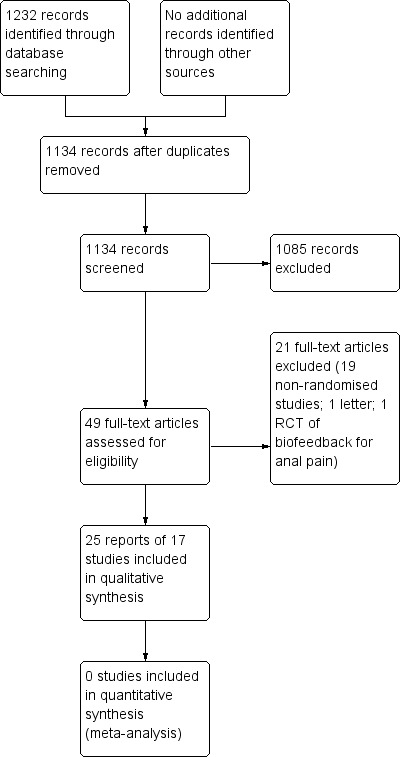
Figure 1: Study flow diagram.
Included studies
Seventeen individual randomised controlled trials (with a total of 931 participants) of biofeedback for chronic idiopathic constipation were identified (See characteristics of included studies). Seven studies were identified comparing biofeedback with 'conventional' non‐surgical treatment (You 2001; Chiarioni 2006; Hu 2006; Heymen 2007; Farid 2009; Simon 2009; Hart 2012). Six studies compared one method of biofeedback with another method of biofeedback (Bleijenberg 1994; Koutsomanis 1995; Glia 1997; Heymen 1999; Chang 2003; Pourmomeny 2010). Two studies compared biofeedback with a less conventional surgical intervention (Lehur 2008; Faried 2010). One study compared manometry biofeedback for constipation with sham treatment (Rao 2007), and reported long‐term follow up separately (Rao 2010). One crossover study compared electrical stimulation to biofeedback (Jung 2007). No studies compared biofeedback with no treatment. It is apparent that the lead author Farid and Faried are the same individual, but it is unclear which is the correct spelling of the name.
Sample sizes
Sample sizes ranged from 21 to 109 randomised subjects with a mean of 48 subjects per study (Bleijenberg 1994; Chiarioni 2006; Hart 2012).
Settings
All seventeen randomised studies were conducted within secondary or tertiary specialist health care settings and all participants had been referred to this service. No studies were identified that had been conducted in primary care settings. It is likely that patients referred to secondary or tertiary settings have more severe constipation, are more needy and bothered by their symptoms, and seek health care more than those in community settings (Simren 2001; Simren 2006). Therefore it would be difficult to generalise the results from these studies to other populations. However, it is likely that the participants in these studies are at least representative of patients referred to specialist centres with chronic idiopathic constipation. The studies were conducted in a range of countries. Four studies were conducted in the US, two in each of Korea and Egypt and one each in Iran, Spain, the Netherlands, the UK, Sweden, Taiwan and Italy. There was only one international study (Lehur 2008), which was conducted at three centres in Europe in Italy, France and the UK. The country of origin was unclear for one study (Hu 2006).
Participants
All participants were diagnosed with chronic idiopathic constipation and most had dyssynergic defecation. Most patients were reported as having failed conservative medical management with dietary fibre and laxatives. However, the failure of conservative medical management was not determined systematically or consistently across all studies. Heymen 2007 utilized a four week 'run‐in' period involving education regarding dietary manipulation, exercise, fluid intake, bowel function and correct defecation technique and only recruited patients who failed to improve after the run‐in period. Chiarioni 2006 administered a 30 day trial of laxatives in patients who were unresponsive to standard treatment. No other study reported on baseline status or previous intervention failure apart from patient self‐report of failure of standard care. There was little consistency in selection criteria for participants and many studies did not report detailed sociodemographics or constipation history for study participants. Eight‐two per cent of participants were women (764/931). Ages ranged from 18 to 82 years, but data were not reported in a format that allowed the calculation of a mean age.
Eight studies used Rome (I, II or III) criteria for chronic constipation or dyssynergic defecation to determine eligibility of participants for inclusion (Glia 1997; Chang 2003; Chiarioni 2006; Hu 2006; Heymen 2007; Rao 2007; Farid 2009; Simon 2009). Eight studies had included only patients with constipation as a result of an evacuation disorder (pelvic floor dyssynergia) (Bleijenberg 1994; Heymen 1999; Chiarioni 2006; Heymen 2007; Rao 2007; Farid 2009; Simon 2009; Hart 2012), one study report stated it included patients with slow transit constipation in addition to pelvic floor dyssynergia (Koutsomanis 1995), while one study included only patients with slow transit constipation (You 2001).
Eight studies reported that the randomised groups were comparable in terms of sociodemographics and constipation history at baseline (Bleijenberg 1994; Koutsomanis 1995; Chang 2003; Chiarioni 2006; Jung 2007; Heymen 2007; Simon 2009; Faried 2010). Six studies failed to report baseline comparability between groups (Glia 1997; Heymen 1999; You 2001; Hu 2006; Farid 2009; Pourmomeny 2010). Rao 2007 reported that patients in the biofeedback group differed significantly from the other groups at baseline for anorectal manometry findings (i.e. higher mean resting sphincter pressure (P = 0.02), mean anal residual pressure (P = 0.0067) and higher threshold for first perception (P = 0.01)) than controls (Rao 2007). Hart 2012 reported baseline characteristics, but did not comment on the comparison between groups, even though some inequalities were evident. Lehur 2008 reported that the baseline 'obstructed defecation score' of the evaluable populations differed between biofeedback and surgical (STARR) treatment groups due to high attrition in the biofeedback arm. However, the statistical significance of this difference was not reported or discussed.
It is noted that Farid 2009 report screening consecutive patients referred to the same institution over the same time period as the Faried 2010 study, with identical demographics reported for both studies. This suggests that the two manuscripts report the results of the same study, however, with major inconsistencies in reporting details. This is a major concern. Attempts to obtain clarification from the authors on this point were unsuccessful and we could find no published comments from other specialist clinicians on these studies.
Interventions
No two studies included used the same protocol for biofeedback.
EMG biofeedback was used in eleven studies (Bleijenberg 1994; Koutsomanis 1995; Heymen 1999; Chang 2003; Chiarioni 2006; Heymen 2007; Jung 2007; Lehur 2008; Simon 2009; Pourmomeny 2010; Hart 2012). Within these studies some investigators used perianal skin surface EMG electrodes (Koutsomanis 1995), while others delivered the EMG training by using a visual display from an anal probe electrode (Bleijenberg 1994; Heymen 1999; Heymen 2007; Jung 2007; Hart 2012). Manometry biofeedback was used in two studies (Glia 1997; Rao 2007). Balloon sensory training was used in three studies (Hu 2006; Farid 2009; Faried 2010). The type of biofeedback used in one study was unclear (You 2001). Three studies incorporated education regarding normal bowel function, dietary manipulation and lifestyle advice and considered this an essential component of the biofeedback intervention (Heymen 1999; Heymen 2007; Farid 2009), while in four studies this was referred to as standard care and therefore a control intervention (Chiarioni 2006; Hu 2006; Rao 2007; Simon 2009).
The number, frequency and duration of biofeedback sessions also varied between studies. The number of biofeedback training sessions ranged from a minimum of five (Chiarioni 2006) to a maximum of fourteen (You 2001). The frequency of biofeedback sessions varied from daily sessions (You 2001) to sessions once every fortnight (Rao 2007; Heymen 2007). Most of the included studies did not report the length of each biofeedback session. The total duration of the biofeedback intervention ranged from two weeks (You 2001) to three months (Rao 2007; Heymen 2007; Hart 2012). See the Characteristics of included studies tables for more details on the interventions used in each study.
Outcomes
Most investigators used some sort of symptom scoring system as an outcome, but these 'scores' did not necessarily assess the same symptoms. Only one study used a validated symptom outcome score with sound psychometric properties to assess the effectiveness of biofeedback (Heymen 2007). This was the patient assessment of constipation symptoms (PAC‐SYM) questionnaire (Frank 1999). Eleven studies included a patient reported outcome measure (PROM) evaluating the patient’s perception of change in, or relief from, symptoms (Bleijenberg 1994; Glia 1997; You 2001; Chang 2003; Chiarioni 2006; Heymen 2007; Rao 2007; Jung 2007; Farid 2009; Faried 2010; Hart 2012). A PROM was used as the primary outcome measure in five of the included studies (Chiarioni 2006; Heymen 2007; Rao 2007; Faried 2010; Hart 2012). In these studies symptom diaries and questionnaires were used to assess the presence of abdominal pain, straining at stool, feeling of incomplete evacuation, frequency of unassisted bowel motions and laxative use. The United States Food and Drug Administration (FDA) has identified complete spontaneous bowel movements (CSBM) as the preferred patient‐reported outcome, recommending this as the primary end‐point for registry trials of constipation treatments. However, this has only recently been recommended and only one study utilized this outcome (Rao 2007).
Some studies specified a pre‐determined level of improvement in symptoms that was required before the patient was considered to have a good clinical outcome (Bleijenberg 1994; Lehur 2008; Farid 2009; Faried 2010). Treatment success was defined as a 50% improvement in post‐treatment scores compared to baseline scores in two studies (Bleijenberg 1994; Lehur 2008). Treatment success was defined as a return to a 'normal bowel habit' in two studies (Farid 2009; Faried 2010). Most studies used a combination of outcome measures, such as symptom assessment, patient global report of satisfaction, anorectal manometry and QoL. Only five studies pre‐specified primary outcome measures (Chiarioni 2006; Heymen 2007; Rao 2007; Lehur 2008; Faried 2010).
The length of follow‐up varied across the 17 included studies. Four studies did not follow participants beyond the completion of the intervention (Heymen 1999; Chang 2003; Hu 2006; Hart 2012). Chiarioni 2006 followed patients for up to 24 months after completion of the intervention. Seven studies reported following patients for one year after completion of the biofeedback intervention (You 2001; Chiarioni 2006; Heymen 2007; Rao 2007; Lehur 2008; Farid 2009; Faried 2010).
Five studies did not report outcomes in a format suitable for the production of forest plots within RevMan (Heymen 1999; Hu 2006; Jung 2007; Simon 2009; Pourmomeny 2010). No meta‐analyses were possible as study populations, interventions and outcomes were too heterogeneous. As a result planned subgroup and sensitivity analyses were not performed. See the Characteristics of included studies tables for more details on the outcome measures used in each study.
Excluded studies
Twenty‐one studies, including 19 non‐randomised trials, one RCT of biofeedback for anal pain (Chiarioni 2010), and one letter (Chiarioni 2007), were excluded. See the Characteristics of excluded studies tables for further details.
Risk of bias in included studies
No studies had a low risk of bias for all categories that were assessed. The overall results of the risk of bias assessment are summarised in Figure 2.
2.
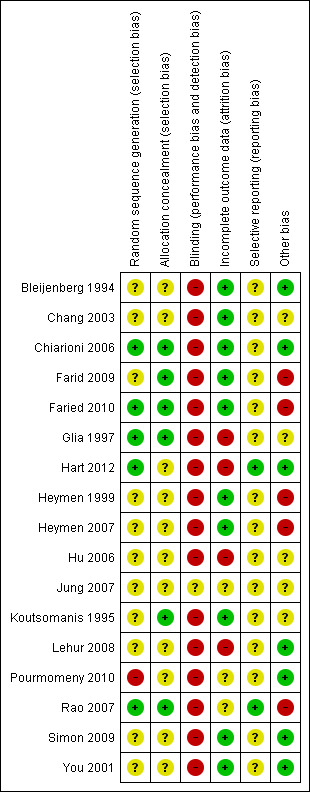
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Overall, random sequence generation was judged to be at low risk of bias in only five studies (Glia 1997; Chiarioni 2006; Rao 2007; Faried 2010; Hart 2012), at high risk of bias in one study (Pourmomeny 2010) and at an unclear risk of bias in the remaining 11 studies. Allocation concealment was judged to be adequate in six studies (Koutsomanis 1995; Glia 1997; Chiarioni 2006; Rao 2007; Farid 2009; Faried 2010), and unclear for the remaining eleven studies. Blinding carried a high risk of bias in all but one study which was rated unclear (Jung 2007). Incomplete outcome data carried a high risk of bias for four studies (Glia 1997; Hu 2006; Lehur 2008; Hart 2012), an unclear risk for three studies (Jung 2007; Rao 2007; Pourmomeny 2010), and a low risk for the remaining ten studies.
Allocation
Seven studies were considered to have adequate random sequence generation, reporting that the allocation sequence was generated randomly by a computer (Koutsomanis 1995; Glia 1997; Rao 2007; Farid 2009; Faried 2010; Hart 2012), or by shuffling sealed opaque envelopes containing the allocation (Chiarioni 2006). However, for the remaining included studies the method used for generation of the allocation sequence was not reported and these studies were rated as unclear risk of bias for this item.
Six studies reported adequate allocation concealment and used sealed opaque envelopes prior to allocation (Koutsomanis 1995; Glia 1997; Chiarioni 2006; Rao 2007; Farid 2009; Faried 2010). In the remaining studies the method used for allocation concealment was not described and these studies were rated as unclear risk of bias for this item.
Blinding
Sixteen of 17 included studies were judged to be at high risk of bias for blinding of participants and personnel. One study compared biofeedback with a sham treatment (Rao 2007), but participants were not blinded as to whether they were receiving biofeedback or sham biofeedback. Blinding was partially maintained between oral diazepam and placebo tablet in one study (Heymen 2007), however, participants were not blinded as to whether they were receiving biofeedback or oral tablets. One study was rated as unclear risk of bias for blinding because it was an abstract publication that did not provide any details (Jung 2007). It is acknowledged that it is difficult to blind either participants or therapists in behavioural studies, however outcome assessors could have remained blind to treatment allocation.
Incomplete outcome data
The use of an intent‐to‐treat analysis was reported in five studies (Chiarioni 2006; Heymen 2007; Rao 2007; Lehur 2008; Farid 2009). Three studies were judged to be at high risk of bias due to high rates of attrition (Glia 1997; Rao 2007; Farid 2009).
Selective reporting
No studies were judged to be at high risk of bias for selective reporting. Two studies were judged to be at low risk for selective reporting because these trials were registered on a clinical trials registry (Rao 2007; Hart 2012). Studies that were not registered on a clinical trials registry were rated as unclear risk of bias for selective reporting. For most studies it was difficult to determine whether outcomes were reported selectively, as the authors had not registered the trial or published the protocol in advance of publishing the findings. Selective outcome reporting was suspected when studies only reported findings from patients who completed the trial and did not report attrition or losses to follow‐up from the study. It was also considered where an ITT analysis was not conducted.
Other potential sources of bias
Four studies reported that power calculations were conducted to determine required sample sizes (Koutsomanis 1995; Glia 1997; Chiarioni 2006; Heymen 2007). It is possible that the remaining studies were not sufficiently powered to detect a difference in efficacy between treatment groups. While a total of 931 patients were studied overall, the sample sizes in most studies were small. However, it is acknowledged that it may be difficult to recruit large numbers of patients to behavioural therapy trials. There is also a chance that some of the studies suffered from poor recruitment, although as a CONSORT flowchart was included in only two studies this was difficult to determine (Chiarioni 2006; Heymen 2007). However it seems unlikely that, for example, that Heymen and colleagues set out to undertake a four arm RCT study including only 36 participants (Heymen 1999). It is also interesting to note that Faried 2010 appeared to only see 62 eligible 'consecutive' participants over a period of three years and four months, recruiting 60 of these patients to their study, which suggests a low referral rate or slow recruitment. In addition it is noted that the authors report screening consecutive patients referred to the same institution over the same time period, with identical demographics, for both Farid 2009 (biofeedback and botulinum toxin‐A arms) and for Faried 2010, suggesting that the two manuscripts report the results of the same study, however with major inconsistencies in reporting details. This is a major concern and the findings of these two studies need to be interpreted with caution. One study was stopped early due to poor recruitment which may have been influenced by one of the trial arms involving a surgical intervention and patient preference (Lehur 2008). Both trials that were registered on a recognised online trial database in advance of recruitment did not report the expected enrolment so it is impossible to determine if the target recruitment was achieved or not (Heymen 2007; Rao 2007). Both of these studies included CONSORT flowcharts and subsequent communication confirms that Rao 2007 performed power calculations. However, these details were not reported a priori or within the original study report so it was unclear whether the study recruited to target.
The treatment groups were unequal at baseline in one study with participants who received biofeedback having worse symptoms at baseline than the sham biofeedback group (Rao 2007). These symptoms included a 'significantly lower defecation index' and 'relatively greater pelvic floor dysfunction' in the biofeedback group (Rao 2007). Bias could have been introduced as a result. For example the biofeedback group could have perceived an increased benefit over the sham group as they had worse symptoms to start with and therefore there was more room for improvement. This same study used balloon distention 'to promote awareness for stooling and match the sensory conditioning provided under biofeedback' as a sham treatment, which could have had a therapeutic effect (Rao 2007). As such, the difference in effect size between the two groups may be less than expected than if the sham treatment had no therapeutic effect. The same issue affected Simon 2009, where the control group (EMG assessment of straining) would be considered a form of biofeedback by many, although it was unclear if the patient saw the monitor or not.
Heymen 2007 reported that patients declined to participate due to lack of time to attend the hospital for appointments, travel difficulties, or wanting to have an alternative treatment. This could have led to a sample being recruited that was not representative of the population of interest. In addition, Heymen 2007 did not report if the characteristics of patients who were not given biofeedback following the run‐in period differed from those who remained in the study and went on to receive the intervention. Patients with a more intractable constipation problem could therefore have been entered into the second phase of the study, which tested the biofeedback intervention, and may therefore have been less likely to respond to treatment. This could have led to an underestimation of the effect of the intervention.
In one study there was a disproportionate number of male (n=11/22; 50%) participants compared to the usual population of patients who would be referred to a tertiary treatment centre (Chang 2003). It is not clear whether men respond differently to biofeedback than women, but this sample was certainly not representative of the population of interest.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
Several of the included studies concluded that biofeedback provided a benefit for patients with chronic idiopathic constipation with effect sizes ranging from 40% to 100% of patients (Koutsomanis 1995; Glia 1997). It was not always possible to identify the percentage of patients who improved as some studies only reported that differences were statistically significant and did not report the proportions improved in each group.
Studies comparing one method of biofeedback with no treatment
No studies comparing biofeedback to a no treatment control were found.
Studies comparing one method of biofeedback with sham biofeedback
Rao 2007 investigated the effectiveness of manometry biofeedback compared to a sham treatment and found that patients who received biofeedback had a statistically significant increase in the number of 'complete spontaneous bowel movements' per week compared to baseline (P < 0.02), and sham biofeedback (P < 0.05). The mean number of complete spontaneous bowel movements per week at three months was 4.6 in the biofeedback group compared to 2.8 in the sham biofeedback group (MD 1.80, 95% CI 1.25 to 2.35). Statistically significant improvement in global bowel satisfaction was found in all groups (P < 0.0001) and the dyssynergic pattern was corrected in 79% of biofeedback recipients, 4% of sham and 8.3% of standard treatment recipients respectively (P < 0.001). Long‐term follow‐up from this initial study is reported in a separate paper (Rao 2010).The original paper reported that 77 subjects were randomised to three arms. The longer term follow‐up report excludes the sham arm (Rao 2010). From 77 people originally randomised, the one year follow‐up was reported for only 20 patients.
Studies comparing one method of biofeedback with another treatment for constipation (e.g. laxatives, education, diet manipulation, botulinum toxin‐A, surgery)
Ten studies compared biofeedback with other medical or surgical treatment. These treatments included 'standard care' and laxatives, botulinum toxin‐A injection, diazepam and surgical interventions. Some of these interventions including botulinum toxin‐A injection, diazepam and surgery are not widely used, although they considered to be 'conventional' by the reporting authors.
Hu 2006 reported that balloon sensory training biofeedback was an effective treatment for functional constipation. The study compared balloon sensory training to dietary and lifestyle advice (Hu 2006). After treatment the number of spontaneous bowel movements increased similarly in both groups. There was a decrease in bloating, incomplete evacuation, straining and rescue laxative use in the biofeedback group, but not in the control group. Detailed findings were not reported in this abstract so data extraction was not possible.
Rao 2007 compared manometry biofeedback to standard therapy consisting of diet, exercise and laxatives. Patients who received biofeedback had a statistically significant increase in the number of complete spontaneous bowel movements per week compared to a standard care group (P = 0.006). The mean number of complete spontaneous bowel movements per week at three months was 4.6 in the biofeedback group compared to 1.9 in the standard care group (MD 2.70, 95% CI 1.99 to 3.41).
Chiarioni 2006 reported EMG biofeedback to be significantly superior to conventional treatment with laxatives and dietary and lifestyle advice. This study found that at both 6 and 12 months 80% (43/54) of biofeedback patients reported clinical improvement compared to 22% (12/55) laxative‐treated patients (RR 3.65, 95% CI 2.17 to 6.13). Over 50% of patients on laxatives self‐reported that they were worse or had no change in symptoms compared 15% of the biofeedback group (P < 0.001). The biofeedback group had significantly less straining (P < 0.01) and incomplete evacuation (P < 0.01) compared to patients in the laxative group. Paradoxical contraction on EMG was significantly reduced in the biofeedback group compared to the laxative group (P < 0.001). Patients using digitation were less likely to benefit from biofeedback (P = 0.013) and a logistic regression analysis found digitation to be the only significant independent predictor of clinical improvement at six months. This study also demonstrated that the effects of biofeedback were mostly maintained for up to two years following the completion of treatment, without further training.
Simon 2009 compared EMG biofeedback to a control intervention of 'counselling sessions' focusing on behavioural mechanisms involved in defecation equivalent to the contact time for biofeedback (Simon 2009). The authors reported a statistically significant difference between biofeedback and control in the frequency of defecations per week, EMG activity during straining to defecate and anismus index. No data extraction was possible from this study.
Jung 2007 compared an unspecified biofeedback treatment to electrical stimulation and found no significant differences in clinical improvement between the groups. No data extraction was possible from this abstract.
Hart 2012 compared EMG biofeedback to a control intervention during which participants were trained to relax the temporalis or trapezius muscles using feedback from EMG surface electrodes placed over the corresponding muscles. From 81 patients who met eligibility criteria 10 were randomised to receive biofeedback and 11 were randomised to the control group. The primary outcome was constipation severity using a 'constipation severity index' developed by the authors. Quality of life was assessed as a secondary outcome using the Irritable Bowel Syndrome Quality of Life Scale. There were no statistically significant differences in constipation severity or quality of life between the biofeedback and control groups. The mean constipation severity score in the biofeedback group was 30 compared to 34.9 in the muscle relaxation control group (MD ‐4.90 95% CI ‐17.23 to 7.43). The mean quality of life score in the biofeedback group was 96.1 compared to 96.7 in the control group (MD ‐0.60, 95% CI ‐38.75 to 37.55).
Biofeedback has been compared with both botulinum toxin‐A injections and posterior myomectomy of the internal anal sphincter and puborectalis muscles for people with evacuation difficulty, although it was unclear whether the biofeedback was provided via a manometry or EMG device (You 2001). You 2001 found that 35 of 40 (88%) biofeedback patients had 'satisfactory improvement' in constipation complaints, compared to 20 of 20 Botulinum toxin‐A patients (RR 0.89, 95% CI 0.77 to 1.02). Fourteen patients had some flatus or faecal incontinence following the administration of botulinum toxin‐A. No mortality or morbidity was reported in the myomectomy group. Eight‐eight per cent (35/40) of patients in the biofeedback improved clinically compared to 85% of patients (17/20) in the myomectomy group (RR 1.03, 95% CI 0.83 to 1.28). Thirty‐five biofeedback patients who reported improvement in constipation symptoms had no relapse after two years follow‐up. The authors concluded that biofeedback was superior to both botulinum toxin‐A and myomectomy, but presented no statistical analysis to support this assertion.
Farid 2009 compared the effectiveness of sensory training biofeedback versus botulinum toxin‐A injections into the external anal sphincter and puborectalis muscles. Initial improvement was found in 12 patients (50%) of the biofeedback group, but only maintained in 6 (25%) at 1 year follow‐up. Initial improvement was found in 17 (70.83%) of botulinum toxin‐A group, but only maintained in 8 (33.3%) long‐term. Botulinum toxin‐A was found to perform significantly better than biofeedback initially but there was no statistically significant difference at one year (RR 0.75, 95% CI 0.31 to 1.83). Straining symptoms had improved in both groups post‐treatment (P = 0.04 for biofeedback; P = 0.007 for botulinum toxin‐A), but there was no significant difference between groups. Significant improvement was found for the outcomes manometric relaxation (biofeedback P = 0.04, botulinum toxin‐A P = 0.001), and balloon expulsion (biofeedback P = 0.01, botulinum toxin‐A P = 0.001) but there was no statistically significant difference between treatment groups.
This same group (Faried 2010) also reported a comparison of biofeedback with both botulinum toxin‐A injections and a surgical intervention (bilateral open partial division of puborectalis). Recruitment was slow and there was a risk of selection bias due to patient preference. Initial improvement was found in 50% of biofeedback patients (10/20), compared to 75% of botulinum toxin‐A patients (15/20) and 95% of surgery patients (19/20). Surgery was significantly better than biofeedback at one month (P = 0.006) and one year (P = 0.02). The number of patients with improved constipation at one year was 30% in the biofeedback group compared to 70% in the surgery group (RR 0.43 95% CI 0.21 to 0.89). The mean constipation score at one year was 16.1 in the biofeedback group compared to 10.5 in the surgery group (MD 5.60, 95% CI 4.67 to 6.53). Satisfaction with treatment was reported in 6/20 (30%) of the biofeedback, 7/20 (35%) botulinum toxin‐A and 14/20 (70%) surgery patients (P < 0.05), but there was a high rate of adverse reactions in the surgery group: 3 infections, 2 incontinence, 2 intussusception (n = 7/20) but not reported for other groups. The authors concluded that the surgical intervention was superior to biofeedback, in spite of a high level of adverse reactions to surgery in seven of the twenty patients who underwent the procedure. There was no statistically significant difference in the proportion of botulinum toxin‐A or biofeedback patients reporting improvement in symptoms at one year. Thirty per cent of biofeedback patients reported improvement of symptoms at one year compared to 35% of botulinum toxin‐A patients (RR 0.86, 95% CI 0.35 to 2.10). There was a statistically significant difference in constipation score at one year. The mean constipation score in the biofeedback group was 16.1 compared to 14.3 in the botulinum toxin‐A group (MD 1.80, 95% CI 0.87 to 2.73).
In one other study of biofeedback was compared with a surgical intervention (stapled transanal rectal resection (STARR)) (Lehur 2008). Successful treatment, defined as a decrease in the obstructed defecation score of > 50% at one year was reported in 33% of biofeedback patients compared to 82% of STARR patients (RR 0.41, 95% CI 0.26 to 0.65). The mean obstructed defecation score at one year was 10.2 in the biofeedback group compared to 4.7 in the STARR group (MD 5.50, 95% CI 3.44 to 7.56). Benefits of both treatments were apparent at the end of treatment. PAC‐QOL improved in both groups at 12 months STARR (P < 0.0001) versus biofeedback (P = 0.002), but adverse events occurred in 8 (15%) STARR patients (infection, pain, incontinence, bleeding, UTI, depression), while only one biofeedback patient experienced anal pain. In some cases the bleeding constituted a serious adverse event, requiring further surgery. The authors concluded that surgery was superior to biofeedback in spite of high level of often serious adverse reactions (infection, pain, faecal incontinence, bleeding and depression) among the patients who underwent surgery. It is notable that 25% of biofeedback patients (13/52) withdrew before the end of treatment, most citing unsatisfactory results, and were excluded from the analysis.
Heymen 2007 conducted the only study comparing the effectiveness of EMG biofeedback with oral diazepam or a placebo tablet. Biofeedback was reported to be significantly better than both diazepam (P < 0.001) and placebo (P = 0.017) at three month follow‐up. Seventy per cent (21/30) of biofeedback patients had improved constipation at three month follow‐up compared to 23% (7/30) of diazepam patients (RR 3.00, 95% CI 1.51 to 5.98). Biofeedback patients had significantly increased unassisted bowel movements (P = 0.016) and QoL scores were improved following biofeedback on both SF‐36 and PAC‐QOL compared to the other two groups, but not significantly. EMG findings showed significantly lower activity during straining following biofeedback (P < 0.001) compared with diazepam.
No further data extraction or pooling of data were possible from these studies.
Studies comparing one method of biofeedback with another method of biofeedback
Six studies comparing one method of biofeedback with another method have been published.
All six were poor quality studies with high risk of bias (Bleijenberg 1994; Koutsomanis 1995; Glia 1997; Heymen 1999; Chang 2003; Pourmomeny 2010). Bleijenberg 1994 found EMG biofeedback to be superior to balloon sensory training for clinical improvement, although the difference was not statistically significant. Seventy‐three per cent (8/11) of patients in the EMG biofeedback group improved clinically compared to 22% (2/9) of patients in the balloon sensory training group (RR 3.27, 95% CI 0.91 to 11.71). The authors concluded that EMG biofeedback was more effective than either manometry or balloon training methods of biofeedback and that education and balloon training alone is not sufficient (Bleijenberg 1994). Further data extraction was not possible due to the high risk of bias and the confusion over the reporting of statistical results from the tests applied in this study (Bleijenberg 1994).
A cross‐over design was reported in another study (Koutsomanis 1995), whereby participants crossed over to the alternate treatment arm if they did not improve after two sessions. There were no statistically significant group differences in the frequency of bowel movements, straining or other clinical symptoms. Sixty‐two per cent (18/29) of patients who underwent EMG biofeedback with computer visual display reported clinical improvement compared to 53% of patients (16/30)who underwent balloon sensory training with no visual feedback (RR 1.16. 95% CI 0.75 to 1.81). Koutsomanis 1995 concluded that EMG biofeedback was equally effective with or without visual biofeedback. However this study compared two different methods of biofeedback rather than the emphasis being on the visual display of muscle contraction.
Glia 1997 found no statistically significant difference in effectiveness between manometry and EMG biofeedback (Glia 1997). Eighty per cent (8/10) of patients who received EMG biofeedback reported improvement in symptoms compared to 60% (6/10) of patients who received manometry biofeedback (RR 1.33, 95% CI 0.74 to 2.41).
Heymen 1999 randomised patients to one of four groups: 1. EMG biofeedback; 2. EMG biofeedback plus balloon sensory training; 3: EMG biofeedback plus home EMG trainer; 4: EMG biofeedback plus balloon sensory training plus home EMG trainer. Heymen 1999 reported a statistically significant increase in unassisted bowel movements in groups 1, 2 and 4, and a significant reduction in the use of cathartics in groups 1, 2 and 3. However, the authors did not compare differences across the four groups but rather compared the effects of treatment within each group individually. The authors concluded that neither the addition of a home training device or balloon sensory training improves outcomes over EMG biofeedback alone (Heymen 1999). No data extraction was possible from this study.
Chang 2003 assessed the effectiveness of biofeedback compared to electrical stimulation using an anal plug and pulse generator. Ten patients underwent EMG biofeedback and 12 patients received electrical stimulation therapy (EST). The primary outcome was bowel satisfaction which was calculated using a visual analogue scale. No statistically significant difference in mean bowel satisfaction scores was found. The mean bowel satisfaction score in the biofeedback group was 59 (+/‐ 28.8) compared to 48.3 (+/‐ 34.1) in the EST group (MD 10.70, 95% CI ‐15.58 to 36.98).
Pourmomeny 2010 reported more rapid balloon expulsion, an increased volume of balloon expelled and an increased reported sense of satisfaction with defecation in the biofeedback group compared to the balloon training only group. However they only report within group comparisons and no data extraction was possible from this study.
Discussion
There was a wide variation among the included studies in the type of participants, interventions, use of outcome measures, duration of treatment and length of follow‐up. Many of the included studies were likely to be underpowered to detect differences between groups. These findings are similar to those of a review of studies of biofeedback for the management of faecal incontinence (Norton 2012).
Studies to date have only been undertaken within specialist or tertiary care settings and populations. Further research is required, particularly within primary care populations. There is now some consensus that the Rome criteria (Thompson 1999; Longstreth 2006), should be used to confirm the diagnosis of chronic idiopathic constipation for inclusion criteria in randomised controlled trials. However, not all of the included studies in this systematic review applied these criteria to the selection of participants. While use of Rome criteria has been recommended to produce homogeneous samples for research, it has recently been shown that the Rome criteria do not adequately differentiate between idiopathic constipation and irritable bowel syndrome (Wong 2010). Some expert opinion suggests that biofeedback should only be offered to those patients with dyssynergic defecation (Rao 2008). Diagnostic criteria for dyssynergia were inconsistent among the studies that included these patients. There was some consensus regarding the results from the different investigations that would be considered diagnostic of the condition, i.e. the patient would be unable to expel a water filled balloon (simulated stool), EMG would reveal increased pelvic floor muscle activity during straining and defecography would reveal a lack of increase in the anorectal angle during straining. However, these investigations were applied inconsistently among the different studies and there is as yet no clear consensus as to which investigations should be used, or indeed the clinical significance of abnormal findings. Some authors also included delayed gut transit as a diagnostic criterion for diagnosis of dyssynergia, although delayed gut transit is not unique to patients with a dyssynergic pattern of defecation and thus cannot be considered diagnostic (You 2001; Rao 2007; Farid 2009; Faried 2010). There is a lack of standardisation of physiological tests (anorectal manometry, balloon expulsion and electromyographical assessment), making results difficult to compare between different centres (Rao 2008; Scott 2008; Bharucha 2010). There is also a lack of data regarding normal values, especially regarding age and gender influences on function (Scott 2008), which makes the use of these tests in research studies less helpful.
Numerous outcome measures were used to assess the effectiveness of biofeedback in the included studies and as yet there is a lack of evidence as to which outcome measures are the most appropriate. This finding is similar to the results of the systematic review by Koh and colleagues who also identified that few trials had clearly defined primary and secondary outcomes (Koh 2008). Many studies also used multiple outcome measures, often without specifying which was the primary outcome measure, which increases the risk of introducing a type I error (false positive result). There is a clear lack of consensus as to the most appropriate outcome measures for assessment of chronic idiopathic constipation, evidenced by the range of outcome measures utilised within the studies included in this review. Due to differences between study populations, the heterogeneity of the different samples and large range of different outcome measures, meta‐analysis was not possible. What is clear, however, is the increasing need to include patient reported outcome measures as the pre‐specified primary outcome measure for assessment of the effectiveness of biofeedback.
The outcome measures used in the included studies fall mainly into three groups: (i) anorectal physiological outcome measures, (ii) symptom diaries and symptom‐based outcome scores or questionnaires and (iii) patient reported global satisfaction with treatment. Anorectal physiological outcome measures include anorectal manometric recordings or EMG recordings of pressure profiles within the anal canal (e.g. resting tone, squeeze pressure and response to straining) and were also used as a primary outcome measure in early non‐randomised biofeedback studies (Lestar 1991; Fleshman 1992). Attempts have been made to identify anorectal physiological tests that are predictive of response to biofeedback and inability to expel a balloon and decreased (more acute) anorectal angle were found to be associated with poor outcomes (Shin 2010). There is little clinical consensus about the appropriateness of many methods for assessing anorectal dysfunction and constipation and the significance of normal and abnormal findings remains unclear (Azpiroz 2002). Many abnormal results are found in asymptomatic subjects (Azpiroz 2002). Anorectal physiology has therefore been criticised as an outcome measure, as these parameters do not always reflect symptomatic improvement (Koutsomanis 1994; Rhee 2000), and abnormalities of anorectal function have been reported in healthy non‐constipated subjects (Rieger 1997; Rao 1998). Given that anorectal measures do not always link to patients’ perceptions of improvement (Papachrysostomou 1994), they should not be used as a primary outcome measure to assess the success of biofeedback therapy. It is also likely that where such measures have been used, findings may not be comparable between different studies because of variations in test equipment and biofeedback techniques.
Using symptom based outcome measures without patient subjective reports of improvement can lead to differences of opinion between patients and researchers. This was demonstrated in a non‐randomised study of biofeedback where a patient considered the treatment to be a failure in spite of an increase in the number of unassisted bowel movements and cessation of laxative use (Wexner 1992). Perceptions of improvement can be influenced by patients’ expectations of outcome and may well be linked to perceived quality of life. However, only two of the included studies assessed the impact of biofeedback on quality of life (Heymen 2007; Lehur 2008). Stool frequency has been commonly used as an outcome measure, either individually or with other symptoms. It has been shown that 40% of constipated patients in one non‐randomised study passed pellets more than once a day before biofeedback and after the intervention bowel frequency was actually reduced (Rao 1998). If such patients were included in a study assessing biofeedback therapy, their frequencies may skew the mean data. Thus bowel frequency may not be the most suitable outcome measure to assess the efficacy of biofeedback. The US FDA has identified complete spontaneous bowel movements (CSBM) as the preferred patient‐reported outcome, recommending this as the primary end‐point for registry trials of constipation treatments. Only one study in this review utilized this outcome (Rao 2007), although many included studies pre‐dated this recommendation. There is no international consensus on the use of CSBM as the primary end‐point and this is not a requirement outside of the USA.
Rao 2007 investigated the effectiveness of manometry biofeedback compared to a sham treatment and found that patients who received biofeedback had a statistically significant increase in the number of 'complete spontaneous bowel movements' per week compared to baseline and sham biofeedback. However, the sham intervention described by the authors as, "intermittent balloon distensions were performed with the rectal probe to promote awareness for stooling and match the sensory conditioning provided under biofeedback" (Rao 2007), might be considered a form of balloon sensory training, which has been incorporated in some biofeedback programmes. For example, Heymen 1999 included balloon sensory training in which "patients with constipation who had high sensory thresholds (i.e. poor recognition of the urge cue) were trained to perceive decreasing volumes of distention" (Heymen 1999). Similarly, this technique was reported in a non‐randomised study that was excluded from this review (Chiotakaku‐Faliakou 1998). Specialist centres in the UK use balloon sensory training in conjunction with EMG biofeedback as a component of therapy. The Rao 2007 study may be better described as a trial of one method of biofeedback versus another. Further research comparing EMG and other methods of biofeedback to a credible sham treatment is required.
Ten studies compared biofeedback with other medical or surgical treatment. These treatments included 'standard care' and laxatives, botulinum toxin‐A injection, diazepam and surgical interventions, but findings need to be interpreted with caution due to the high risk of bias in most studies. Some of these interventions including botulinum toxin‐A injection, diazepam and surgery are not widely used, although they were considered to be 'conventional' by the reporting authors.
Rao 2007 compared manometry biofeedback to standard therapy consisting of diet, exercise and laxatives. The authors found a statistically significant increase in the number of complete spontaneous bowel movements per week at three months in the biofeedback group compared to the standard care group. Chiarioni 2006 reported EMG biofeedback to be significantly superior to conventional treatment with laxatives and dietary and lifestyle advice. The results of these studies should be interpreted with caution due to the small numbers of patients enrolled and a high risk of bias due to lack of blinding.
Heymen 2007 compared the effectiveness of EMG biofeedback with oral diazepam or a placebo tablet. Clinical improvement at three months was significantly better in the biofeedback group compared to both diazepam (P < 0.001) and placebo tablets (P = 0.017). The authors asserted that diazepam was selected as a control as this intervention was commonly used in clinical practice. However, this intervention has not been reported in any other study, is not widely used and may be an idiosyncratic practice within the clinical setting in which the study was undertaken.
Farid 2009 compared the effectiveness of sensory training biofeedback to botulinum toxin‐A injections into the external anal sphincter and puborectalis muscles. Botulinum toxin‐A injection may have short term benefits over biofeedback, but the benefit does not last. Botulinum toxin‐A was found to perform significantly better than biofeedback at one month but there was no statistically significant difference at one year. The authors concluded that the botulinum toxin‐A injections were significantly more effective than biofeedback initially, in contrast to You 2001, but that this efficacy was not maintained at one year in most patients. These results should be interpreted with caution due to a high risk of selection bias due to patient preference and lack of blinding and the small number of patients enrolled (Farid 2009). While botulinum toxin‐A injections may provide more immediate relief through paralysis of the pelvic floor musculature, the effects are only temporary and deteriorate with time so that injections need to be repeated every three months or so. The authors of this study used only a single injection and treatment was not repeated, so it is unclear if repeated efficacy would be achieved with repeated injections compared with biofeedback. The effect of biofeedback was less immediate, but may be maintained over time without the need for further training beyond the initial intervention. This same group (Faried 2010), also reported a comparison of biofeedback with both botulinum toxin‐A injections and surgery (i.e. bilateral open partial division of puborectalis). Recruitment was slow and there was a risk of selection bias due to patient preference. Although there was a statistically significant difference in constipation score at one year favouring surgery over biofeedback this difference is not likely to have any clinical significance. Farid 2009 reported screening consecutive patients referred to the same institution over the same time period as the Faried 2010 study, with identical demographics reported for both studies. This suggests that the two manuscripts report the results of the same study, however, with major inconsistencies in reporting details. This is a major concern. Attempts to obtain clarification from the authors on this point were unsuccessful and we could find no published comments from other specialist clinicians on these studies.
You 2001 reported no difference in clinical improvement between biofeedback and surgery (i.e. mycomectomy), whereas two studies reported that surgery (i.e. bilateral open partial division of puborectalis or stapled transanal rectal resection) was superior to biofeedback for clinical improvement (Lehur 2008; Faried 2010) and improvement in constipation score (Faried 2010) or obstructed defecation score (Lehur 2008) at one year follow‐up. However, adverse events were much more common in the surgical groups than the biofeedback groups. The results of these studies need to be interpreted with caution due to a high risk of bias in these studies (i.e. selection bias and lack of blinding) and the small numbers of patients enrolled. Further research is required. Even if surgical interventions are shown to be superior to biofeedback, they are invasive, require a general anaesthetic and are clearly not without risk. It is therefore important that patient preference is considered when deciding on treatment options for individual patients.
There is a lack of evidence as to whether any one method of biofeedback is more effective than any other method of biofeedback. Clinicians often take a pragmatic approach and use the methods available locally. Some poor quality studies included in this review suggest that EMG biofeedback could be superior to other methods including balloon sensory biofeedback (Bleijenberg 1994; Koutsomanis 1995), and manometry (Glia 1997). However, the superiority of EMG biofeedback was not statistically significant in these studies and there was a lack of consistency in results, which could be due to the heterogeneity of samples, different methods and biofeedback techniques employed and different outcome measures used (Shin 2010). This echoes the findings of a previous systematic review (Koh 2008). Differences in diagnostic methods, selection criteria, type of treatment (including type of biofeedback, number of sessions and duration of training) may go some way to explaining the variation in results from the literature. Some investigators have shown that biofeedback can be successful without the use of electronic feedback (Koutsomanis 1995). Most, however, insist that biofeedback must incorporate the use of some form of electronic feedback. The funding arrangements for biofeedback in some healthcare systems are such that only treatments with biofeedback that incorporates EMG are paid for by insurers. Investigators possibly have a vested interest in proving that this form of biofeedback is effective. It is clear that there is no consensus or standardised regimen for administering biofeedback and as no two intervention protocols used in the 17 studies were the same, meta‐analysis was not possible.
The results of this review show that there is insufficient evidence from high quality randomised controlled trials to support the effectiveness of biofeedback for the management of chronic idiopathic constipation. Although the studies are not ideally designed, some studies enrolled patients who had failed multiple treatments and had severe symptoms (Chiarioni 2006; Rao 2007). Biofeedback may be appropriate for these patients, although the quality of the evidence is limited. The methodological quality and quality of reporting of randomised controlled trials of biofeedback for idiopathic constipation has improved over time, but there is still a lack of high quality trials without risk of bias to support this intervention and on which to base recommendations. As the quality of trial methods and reporting has improved over time, there is now some evidence that the effect of biofeedback for constipation is specific and more than simply a placebo response, but further studies with low risk of bias are required to confirm these findings.
Summary of main results
We found low or very low quality evidence from randomised controlled trials to support the effectiveness of biofeedback for the management of people with chronic idiopathic constipation and dyssynergic defecation. However, the majority of trials are of poor methodological quality and subject to bias.
Overall completeness and applicability of evidence
There was a wide variation among trial reports in terms of characteristics of participants, characteristics of interventions, choice of outcome measures, duration of treatment and length of follow‐up. Most of the trials were small and probably of insufficient power to detect any differences between intervention groups. The outcome measures used were often insufficiently reported to enable further statistical analyses. Length of follow‐up was inadequate in many of the trials. The way in which data were reported in many of the trials (e.g. by not reporting measures of variance) made a quantitative synthesis of results (meta‐analysis) impossible.
Quality of the evidence
The results of all of the included studies need to be interpreted with caution as GRADE analyses rated the overall quality of the evidence for the primary outcomes (i.e. clinical or global improvement as defined by the studies) as very low (See Table 1; Table 2; Table 3), or low (See Table 4; Table 5; Table 6; Table 7) due to high risk of bias (i.e. open label studies, self‐selection bias, incomplete outcome data, and baseline imbalance) and imprecision (i.e. sparse data).
Most studies were of poor methodological quality with a high risk of bias and reporting in many of the studies did not conform to the CONSORT statement for the quality of methodological reporting of RCTs (Moher 2001). In spite of the lack of high quality evidence, there is expert consensus that biofeedback is the gold standard medical management for patients with chronic idiopathic constipation and dyssynergic defecation (Whitehead 2010).
Potential biases in the review process
We attempted to reduce bias in the review process. A comprehensive literature search was performed to identify all applicable studies. Two review authors independently assessed studies for inclusion, extracted data and assessed study quality.
Agreements and disagreements with other studies or reviews
While several literature reviews of the effectiveness of biofeedback have been published (Enck 1993; Enck 1996; Bassotti 1997; Schiller 2001; Coulter 2002; Heymen 2003; Jorge 2003; Bassotti 2004; Palsson 2004; Chiarelli 2008; Chiarioni 2008; Koh 2008; Rao 2008; Zhou 2008; Enck 2009), and often by the same groups of authors, only four include a systematic approach to searching the literature for randomised controlled trials and are considered here (Chiarelli 2008; Koh 2008; Zhou 2008; Enck 2009). Of these systematic reviews only three include any meta‐analysis of RCTs (Koh 2008; Zhou 2008; Enck 2009), and the work of Zhou and colleagues is only available as an abstract (Zhou 2008). All four of these reviews had methodological limitations.
Zhou 2008 undertook a meta‐analysis of eight trials involving 488 patients and found that biofeedback was an effective method for the management of chronic idiopathic constipation compared to laxatives and non‐biofeedback therapy. They concluded that EMG biofeedback was as effective as manometry biofeedback, but the methods for the review were not fully reported and inclusion and exclusion criteria were not described.
Chiarelli 2008 conducted a somewhat limited review. While a systematic process was followed the review was limited by only including studies published in English and failing to search the Cochrane CENTRAL database of randomised controlled trials, conference abstracts or other trial registers. The date the literature search was conducted was not reported, but the most recent study included was published in 2006. The search strategy was not comprehensive. Chiarelli 2008 did not include studies that were identified by the search strategy for this review (You 2001; Chang 2003; Hu 2006; Jung 2007). Chiarelli 2008 also incorrectly included narrative reviews that were not 'systematic reviews' and these reviews were excluded from the current review.
Koh 2008 conducted the most robust review, searching most of the available relevant electronic databases and placing no language limits on the randomised controlled studies they included. However, their search strategy was not comprehensive enough and they failed to identify several studies that were published prior to their review that were included in this review (You 2001; Chang 2003; Hu 2006; Jung 2007). The seven studies included by the authors were correctly identified as being heterogeneous and yet these studies were combined in a meta‐analysis. This was not appropriate as meta‐analysis should only be conducted for homogeneous studies that are investigating the same population of people, the same intervention and the same outcome measures and clearly this was not the case.
Enck 2009 conducted a limited review, searching only PubMed and no other databases. Their review was also limited to English language papers only and searched for papers published between 1980 and 2008. As with the other reviews, four RCTs of biofeedback for chronic idiopathic constipation were missed by the search (You 2001; Chang 2003; Hu 2006; Jung 2007), and more interestingly the authors included a study published in 2009 which was beyond the date limits of their search (Farid 2009). This brings into question whether the search strategy and limits of the search were strictly applied by the authors. As with the Koh 2008 review a meta‐analysis of heterogeneous studies was undertaken. This meta‐analysis included only four RCTs of biofeedback compared to 'non‐biofeedback' modalities and reported an odds ratio of 3.657 (95% CI 2.127 to 6.290) in favour of biofeedback. As these confidence intervals were narrow and did not cross the line of no effect, the authors concluded that biofeedback was significantly superior to other therapies (P < 0.0001). However, this pooled analysis was not appropriate. The comparator groups for each of the four studies were different and included laxatives (Chiarioni 2006), an oral placebo for diazepam (Heymen 2007), sham biofeedback (Rao 2007), and botulinum toxin‐A injections (Farid 2009). All four studies included in this meta‐analysis were rated as high risk of bias for lack of blinding (Chiarioni 2006; Heymen 2007; Rao 2007; Farid 2009), possible self‐selection bias (Heymen 2007), and participant groups that were not comparable at baseline (Rao 2007).
The consistent findings from these reviews were that biofeedback was superior to control interventions and that the methodological quality of trials of biofeedback for chronic idiopathic constipation was generally poor and that further research was required. These findings are broadly consistent with the outcomes of this review.
Authors' conclusions
Implications for practice.
Currently there is insufficient evidence to allow any firm conclusions regarding the efficacy and safety of biofeedback for the management of people with chronic constipation. We found low or very low quality evidence from single studies to support the effectiveness of biofeedback for the management of people with chronic constipation and dyssynergic defecation. However, the majority of trials are of poor methodological quality and subject to bias. Most studies investigated biofeedback in patients with dyssynergic defecation only and excluded those with isolated slow transit constipation. We found some evidence that suggests biofeedback may be effective for the management of chronic idiopathic constipation and superior to oral diazepam, sham biofeedback and laxatives. Some surgical procedures were reported to be superior to biofeedback although patients who had surgery had a high risk of adverse events (Lehur 2008; Faried 2010). Conflicting results were found regarding the comparative effectiveness of biofeedback and botulinum toxin‐A. One small study suggested that botulinum toxin‐A injection may have short term benefits over biofeedback, but the benefit did not last at one year follow‐up. We found very poor evidence that any one method of biofeedback was superior to any other method of biofeedback. There were no reports of adverse events from biofeedback in any of the studies and it is fairly unlikely that this intervention will cause significant harm.
Implications for research.
There is a need for well‐designed randomised controlled trials with adequate sample sizes, validated outcome measures (especially patient reported outcome measures) and long‐term follow‐up. However, it is unclear which patient reported outcome measures and physiological end‐points are most important. While the concept of complete spontaneous bowel movements has gained popularity, it is not clear that this is the most important outcome from all patients' perspectives. Further work to define patient reported outcome measures, including symptom and disease specific quality of life outcomes that reflect those symptoms that are most bothersome to patients is required. Patient satisfaction with bowel function and treatment may not always correlate with an increase in the number of complete spontaneous bowel movements or other physiological end points, such as gut transit or expulsion of a water‐filled balloon. Further work to define an appropriate sham for biofeedback is also required.
There are now other treatments available for the management of chronic idiopathic constipation, such as prokinetic agents (e.g. prucalopride) and modes of electrical stimulation, which were not available when many of the included studies were conducted. Future studies should compare biofeedback with these interventions and any other newer pharmacological, surgical or behavioural treatments that are developed. Biofeedback should also be compared to established conventional treatments for constipation. Most studies were conducted within secondary or tertiary care populations and there is a need for well‐designed trials in primary care settings.
In addition there is a need for studies assessing the effectiveness of the different components or methods of 'biofeedback', including the information, education and counselling given to patients, exercises, balloon sensory training and EMG or manometry feedback of sphincter and pelvic floor function. Economic outcomes should also be assessed in future studies. Studies that explore the effectiveness of biofeedback for patients with pelvic floor dyssynergia (with or without slow gut transit) and patients with slow gut transit without evidence of dyssynergic defecation are required.
Feedback
Letter to editor from Rao et al
Summary
The Editor
The Cochrane Collaboration Editorial Unit
Attn: Hilary Simmonds
Dear Sir/Madam:
Re: Woodward S, Norton C, Chiarelli P. Biofeedback for treatment of chronic idiopathic constipation in adults. Cochrane Database Syst Rev 2014; Issue 3: CD008486.
We are an international group comprising physiologists, biofeedback therapists, research investigators, gastroenterologists, and colorectal surgeons with a strong interest and published record of original scientific work in the field of dyssynergic defecation and constipation.
We have always appreciated the thorough and meticulous work of the “Cochrane Review” process. However, we believe that this review, although timely, is flawed and is potentially a disservice to the community of health care providers who manage these patients. The authors have made unsupported and arguably incorrect assumptions in their review of the published studies , and at time have misquoted the studies or the interpretation of data. Based on this flawed evidence, the authors conclude that the studies are of low quality or carry significant risk of bias and that there is insufficient evidence to recommend biofeedback treatment.
The randomized controlled trials (RCTs) published in the past 7 years have employed rigorous selection criteria, appropriate diagnosis and well‐defined outcome measures, and they included 370 patients1‐3. These key RCT studies came from several major centers across the world and all groups working independently have concluded that biofeedback therapy is efficacious and superior to appropriate comparison groups. In contrast to the recommendations of this review, we believe that the evidence is compelling that biofeedback therapy is efficacious both in the short‐term and long‐term management of disordered defecation. Major professional societies including the American College of Gastroenterology4, the American Gastroenterological Association5,, and the Rome Foundation6 have reached similar conclusions.
Our concerns with the methods used by the reviewers are as follows:
Heterogeneous groups: In a number of cited studies, the authors have included patients with diverse conditions, many of whom have no clear rationale for biofeedback therapy. These include patients with isolated slow transit constipation, rectoceles, outlet obstruction, rectal mucosal intussusception, and rectal prolapse.
Inclusion of early pilot studies: The inclusion of studies performed decades ago is problematic, as the methodology and analyses were rudimentary, patient selection criteria were unclear and randomization was not performed. The deficiencies of these early studies should not negatively color the description of more recent rigorous studies and should have been excluded.
Estimating Risk of Bias – This is a very useful method if applied correctly. We believe that the authors have in some instances incorrectly interpreted the data and consequently arrived at erroneous conclusions. For example, the study by Rao et. al.1 was a rigorously performed clinical trial that was sponsored by the NIH and to date, is the only sham controlled treatment trial of biofeedback. The study was considered to be a “high risk for bias” and the authors assumed the power calculations were not performed; this was puzzling, as this was an NIH sponsored clinical trial. Indeed, detailed sample size
Calculations formed the basis for the award of the grant. A consort diagram was provided in the manuscript giving a clear disposition of all subjects enrolled and screened, yet the review states that such was not provided. This was also true for the study by Heymen et al3 and Chiarioni et al2 , which were also described as “high risk of bias”; the target enrollment was specified and information was also published on clinical trials.gov. Finally, it was incorrectly stated by the authors that there was bias in subject selection in the Rao study. Subjects were randomly allocated to one of 3 treatments using a permuted blocks method using sealed envelopes and concealed allocation. When the data were compared, there was no difference among the three groups, with regards to demographics, non‐compliance rates, number of therapy sessions, baseline bowel symptoms and in 8 of 12 manometric features. There were minor out significant differences in only 3 manometric features at baseline and these minor differences alone should not constitute grounds for a high risk of bias. Furthermore, the stated main concern was that patients who received biofeedback treatment had more severe symptoms. If so, one could hypothesize that they should have fared worse, but in fact they showed significant improvement in outcomes.
Contacting authors for missing data and clarification: The guidelines for conducting Cochrane reviews include an obligation to contact the authors of studies when important information is missing or unclear. The authors state that they did this. However, authors Rao1, Chiarioni2, and Heymen3 and their research teams were not contacted. If this had been done, it is likely that the reviewers would have drawn different conclusions regarding the risk of bias.
Blinding: The reviewers insist that the masking of active vs. control interventions from both the investigator and the patient, which is a feasible standard for study design in drug trials, should be applied to behavioral therapies such as biofeedback. However, it is not possible to double‐blind a behavioral therapy trial, and alternative criteria have been recommended for managing this source of bias in behavioral trials7. These guidelines include choosing a comparison treatment that is credible to patients, randomizing patients only after eligibility has been confirmed, assessing the expectation of benefit b questionnaire in both the active and control arms of the study after initial exposure to the assigned treatment, and using independent blinded assessors to collect outcome data. These techniques were employed in several of the recent RCTs, but this is not mentioned by the reviewers.
Inclusion of older abstracts: While it seems reasonable to include recent abstracts whose findings have significant implication for treatment, patient management, outcome or safety, it seems inappropriate to include abstracts published in 2006 and 2007, none of which have been published in a peer reviewed manuscript and therefore are unavailable to clinicians and researchers in the Cochrane review.
Surgical Therapies & STARR Procedures: Rectal mucosal intussusception, excessive Pelvic floor descent, rectoceles etc, are not the same conditions as dyssynergic defecation should be omitted from this review.
Reporting of adverse‐events: The authors state that firm conclusions cannot be made regarding potential side‐effects of biofeedback treatment, but not a single trial has ever reported an adverse event and biofeedback is generally described as safe. To posit that this treatment may be unsafe suggests an overall negative bias by the authors.
In summary, there are serious methodological flaws with this systematic review. The conclusions are inaccurate. Contrary to the statement in the review, the signatories to this letter many of whom have authored some of the studies cited in the review were not contacted by the authors of the Cochrane review. Because of the many aforementioned reasons, this review should be retracted. A re‐assessment would be appropriate, either by the original authors or by an independent external set of reviewers
Many thanks for considering our request. Please allow us to reiterate our firm support and confidence in the Cochrane Review process.
Sincerely,
Satish Rao, USA
William Whitehead, USA
Steve Heymen, USA
G. Chiarioni, Italy
A. Wald, USA
A. Bharucha, USA
C. Knowles, UK
R.Felt Bersma, Netherlands
A. Malcolm, Australia
SJ Myung, Korea
S. Gonlachanvit, Thailand
References
1. Rao SS, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham feedback,
and standard therapy for dyssynergic defecation. ClinGastroenterol Hepatol 2007:5:331‐8.
2. Chiarioni G, Whitehead WE, Pezza V, Morelli A, Bassotti G. Biofeedback is superior to laxatives
for normal transit constipation due to pelvic flor dyssynergia. Gastroenterology 2006;130:657‐64.
3. Heymen S, Scarlett Y, Jones K, Ringel Y, Drossman D, Whitehead WE. Randomized, controlled
trial shows biofeedback to be superior to alternative treatments for patients with pelvic floor dyssynergia‐type constipation. DisColon Rectum 2007;50:428‐41.
4. Wald AB, A.; Cosman, B.C.; Whitehead, W.E. ACG Clinical Guidelines: Management of Benign
Anoretal Disorders. American Journal of Gastroenterology 2014; In press.
5. Whitehead WE, Bharucha AE. Diagnosis and treatment of pelvic floor disorders: what’s new and
what to do. Gastroenterology 2010;138:123‐5, 5 el‐4.
6. Wald A, Bharucha AE, Enck P, Rao SSC. Functional anorectal disorders. In: Drossman DA,
Corazziari e, Delvaux M, et al., Rome III: The Functional Gastrointestinal Disorders. McLean, Virginia;
Degnon Associates; 2006:639‐85.
7. Whitehead WE. Control groups appropriate for behavioral interventions. Gastroenterology
2004;126:S159‐S63.
Reply
Author and IBD/FBD group response to letter from Rao et al
Our review was clear in its scope: chronic idiopathic constipation of whatever origin. This is the condition for which biofeedback has been trialled and used in clinical practice in most of the world. We did not set out to do a more restricted review of biofeedback for obstructed or dyssynergic defecation and pre‐specified subgroup analyses on patient subgroups was not possible due to an insufficient number of studies. We could consider doing this sub‐group analysis in future updates, but as many studies have not reported these patients separately, this would inevitably be selective to mostly the USA studies. The comments take a very USA‐focused approach, where clinical practice has evolved in some clinics, including those of the signatories, to include only patients who meet the arbitrary definition of “dyssynergic defecation”. This is not the clinical or research practice in most of the rest of the world.
Some of the signatories have a vested interest in the outcome of this review as they have large private practice biofeedback clinics and large grants investigating biofeedback therapy. They are noted to be the authors of the studies they are defending (Heymen, Rao, Chiarioni).
The protocol for the review was published in 2010 in the Cochrane library (Issue 4. Art. No.: CD008486. DOI: 10.1002/14651858.CD008486), giving the signatories ample opportunity to comment on the methods prior to the publication of the review and yet none were received.
We address each of the points from the letter below (see italic text ).
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
The Editor
The Cochrane Collaboration Editorial Unit
Attn: Hilary Simmonds
Dear Sir/Madam:
Re: Woodward S, Norton C, Chiarelli P. Biofeedback for treatment of chronic idiopathic constipation in adults. Cochrane Database Syst Rev 2014; Issue 3: CD008486.
We are an international group comprising physiologists, biofeedback therapists, research investigators, gastroenterologists, and colorectal surgeons with a strong interest and published record of original scientific work in the field of dyssynergic defecation and constipation.
We have always appreciated the thorough and meticulous work of the “Cochrane Review” process. However, we believe that this review, although timely, is flawed and is potentially a disservice to the community of health care providers who manage these patients. The authors have made unsupported and arguably incorrect assumptions in their review of the published studies, and at time have misquoted the studies or the interpretation of data. Based on this flawed evidence, the authors conclude that the studies are of low quality or carry significant risk of bias and that there is insufficient evidence to recommend biofeedback treatment.
Response:We did not conclude that there was insufficient evidence to recommend biofeedback treatment. In the implications for treatment section we provided a general interpretation of the evidence so that it can inform healthcare or policy decisions. Our conclusion was, “Currently there is insufficient evidence to allow any firm conclusions regarding the efficacy and safety of biofeedback for the management of people with chronic constipation. We found low or very low quality evidence from single studies to support the effectiveness of biofeedback for the management of people with chronic constipation and dyssynergic defecation. However, the majority of trials are of poor methodological quality and subject to bias. Further well‐designed randomised controlled trials with adequate sample sizes, validated outcome measures (especially patient reported outcome measures) and long‐term follow‐up are required to allow definitive conclusions to be drawn.”
The randomized controlled trials (RCTs) published in the past 7 years have employed rigorous selection criteria, appropriate diagnosis and well‐defined outcome measures, and they included 370 patients1‐3. These key RCT studies came from several major centers across the world and all groups working independently have concluded that biofeedback therapy is efficacious and superior to appropriate comparison groups. In contrast to the recommendations of this review, we believe that the evidence is compelling that biofeedback therapy is efficacious both in the short‐term and long‐term management of disordered defecation. Major professional societies including the American College of Gastroenterology4, the American Gastroenterological Association5,, and the Rome Foundation6 have reached similar conclusions.
Response:The letter implies that they want a review with only their three studies included. It is against all the principles of Cochrane to select studies in this way. All of the studies that were included in the review including the three cited studies met our pre‐specified inclusion criteria that were published in the protocol for the review.
Our concerns with the methods used by the reviewers are as follows:
Heterogeneous groups: In a number of cited studies, the authors have included patients with diverse conditions, many of whom have no clear rationale for biofeedback therapy. These include patients with isolated slow transit constipation, rectoceles, outlet obstruction, rectal mucosal intussusception, and rectal prolapse.
Response:All of the studies included in our review met our pre‐defined inclusion criteria. It is the authors of the included biofeedback studies, not the review authors, who included heterogeneous patient groups. This we believe reflects clinical practice around the world. It should be noted that meta‐analysis was not possible due to differences between study populations, the heterogeneity of the different samplesand the large range of different outcome measures utilized in the included studies. If a sufficient number of randomised trials were identified, we planned a subgroup analysis by constipation sub‐type. However there wasn't a sufficient number of studies to allow such an analysis. This could be considered for future updates of the review.
Inclusion of early pilot studies: The inclusion of studies performed decades ago is problematic, as the methodology and analyses were rudimentary, patient selection criteria were unclear and randomization was not performed. The deficiencies of these early studies should not negatively color the description of more recent rigorous studies and should have been excluded.
Response:All of the studies included in the review met the pre‐defined inclusion criteria which were randomised controlled trials comparing one method of biofeedback for constipation with sham treatment, conventional treatment, no treatment or another method of biofeedback in patients with constipation were considered for inclusion. Arbitrary exclusion by publication date is not warranted. We followed Cochrane methods and searched systematically for all studies reported since the first case series report of biofeedback for this indication in 1980. We were deliberately non‐selective as this was the first Cochrane review on the subject. We did not include any non‐randomised studies. It is notable that some more recent studies also have rudimentary reporting and methods and the limitations of the included studies are clearly described in the review and taken into consideration when drawing conclusions.
Estimating Risk of Bias – This is a very useful method if applied correctly. We believe that the authors have in some instances incorrectly interpreted the data and consequently arrived at erroneous conclusions. For example, the study by Rao et. al.1 was a rigorously performed clinical trial that was sponsored by the NIH and to date, is the only sham controlled treatment trial of biofeedback. The study was considered to be a “high risk for bias” and the authors assumed the power calculations were not performed; this was puzzling, as this was an NIH sponsored clinical trial. Indeed, detailed sample size
Calculations formed the basis for the award of the grant. A consort diagram was provided in the manuscript giving a clear disposition of all subjects enrolled and screened, yet the review states that such was not provided. This was also true for the study by Heymen et al3 and Chiarioni et al2 , which were also described as “high risk of bias”; the target enrollment was specified and information was also published on clinical trials.gov. Finally, it was incorrectly stated by the authors that there was bias in subject selection in the Rao study. Subjects were randomly allocated to one of 3 treatments using a permuted blocks method using sealed envelopes and concealed allocation. When the data were compared, there was no difference among the three groups, with regards to demographics, non‐compliance rates, number of therapy sessions, baseline bowel symptoms and in 8 of 12 manometric features. There were minor out significant differences in only 3 manometric features at baseline and these minor differences alone should not constitute grounds for a high risk of bias. Furthermore, the stated main concern was that patients who received biofeedback treatment had more severe symptoms. If so, one could hypothesize that they should have fared worse, but in fact they showed significant improvement in outcomes.
Response:We believe that our risk of bias assessment is correct.
Rao 2007 was rated as high risk of bias for ‘blinding’ and ‘other bias’. The study was assessed as high risk for blinding for two reasons. This was a three arm trial that included a biofeedback group, a sham biofeedback group and a standard care group. It would be obvious to the patients in the groups that they were receiving biofeedback or standard care. We also had doubts about blinding for patients in the sham biofeedback group. In fact Rao et al state on page p332 of their manuscript, “Although the therapist and patient could not be blinded, the manometry reader was unaware of patient assignment or previous data.” They did not report assessing the success of blinding and there is no report that they intended to assess this outcome. This statement in the paper by the authors themselves strongly suggests that patients were not blinded between the different interventions and that is why we came to the conclusion that the risk of bias for blinding was high. The Rao 2007 study was judged to be at high risk of bias for ‘other bias’ because of baseline differences between the biofeedback and sham groups in the defecation index and pelvic floor dysfunction. Other possible sources of bias in the Rao 2007 study were discussed in the review but did not inform our risk of bias assessment.
Heyman 2007 was rated as high risk of bias for ‘blinding’ and ‘other bias’. The study was assessed as high risk of for blinding because both investigators and patients knew whether the patients were assigned to biofeedback or diazepam. The study was rated a high risk of ‘other bias’ due to possible self‐selection bias.
Chiarioni 2206 was rated as high risk of bias for ‘blinding’ because both investigators and patients knew whether the patients were assigned to biofeedback or laxatives.
Other issues with these three studies are detailed below.
Heymen (2007)compared biofeedback with diazepam and an inert placebo. The lead investigator/therapist telephoned the participants three months after treatment and asked if they “obtained adequate relief”. This outcome measure is open to bias as the participants had by this time spent 6 x 30 minute sessions with the investigator. Futher this outcome does not appear to have been validated. The study included patients with slow transit constipation which the signatories suggest should have been excluded. Diazepam was used as a comparator with no previous published evidence for efficacy. The authors cite their own clinical practice as a justification. We have not been able to find any other studies using this intervention. Diazepam clearly did not work as hypothesised (see their Figure 4 p 437) as dyssynergia worsened rather than improved in these patients, and there was a high drop‐out rate which the authors attribute to side effects. There is no empirical evidence that we are aware of that diazepam relaxes the pelvic floor. The authors report multiple secondary outcomes, mostly with non‐significant differences. Recruitment was stopped early before reaching target recruitment numbers because an interim analysis indicated that 30 patients per group showed a significant difference for the primary outcome between biofeedback and the two pill groups. This is a pity because final completions were only 66 of the 90 needed rendering the study underpowered. The authors have erroneously summed the SF36 scores rather than reporting subscales. The authors conclusions that “instrumented biofeedback is essential to successful treatment” seems over‐stated as there was only a trend in favour of biofeedback compared with diazepam (p=0.067) in number of unassisted bowel movements as highlighted in the abstract as a major outcome. Perhaps if the study had continued to full recruitment it would have yielded more definitive results. The authors state that follow up data collection is continuing, but we have found no published follow up results.
Chiarioni (2006)was on the whole a well conducted study. It did include slow transit constipation which the signatories felt should be excluded (we disagree). There is a sample size calculation, albeit on the un‐supported presumption that 25% difference between groups would be clinically meaningful. However, to highlight in results that biofeedback group had a significantly greater reduction in laxative use than the comparison group whose intervention was laxatives seems a little biased in favour of biofeedback. To claim repeatedly in the paper that this is a “large” study with just over 100 patients seems a little exaggerated.
Rao (2007)report (and do not adequately explain) selective recruitment. Three hundred of 377 screened patients were excluded: 148 did not meet inclusion criteria (unstated which) and 152 refused. Seventy‐seven patients were recruited. There was no a priori sample size justification in the paper nor on any of the updates published on the Clinicaltrials.gov website so it is unclear if target recruitment was met.
The long term follow up of the same study (Rao 2010) is reported largely as if it was a new study (citing “our earlier study” as if it were a different one), with only 2 of the 3 original randomized groups even mentioned (they have omitted to mention the sham group at all in the text and in the CONSORT diagram) and changing the primary outcome measure from the two primary outcomes in the primary study reference to a single primary outcome “complete spontaneous bowel motions” (CSBM), dropping satisfaction, in the manuscript reporting long term follow up results. There is no mention of power in either manuscript nor on the clinicaltrials.gov website. Of 77 patients originally randomized to three groups (or 52 to 2 groups as reported in the manuscript reporting follow up results) only 7/24 completed the laxative arm and 13/28 the biofeedback arm at 12 months (20/52, 38%, in the 2 arms). There is also no statistical comparison presented between the groups at one year, only a within group comparison, in groups which were not well matched at baseline. One of the primary outcomes (satisfaction) in the primary study reference was not significantly different between the groups in the follow up manuscript. It seems that this was selectively dropped as a primary outcome to give a positive report on biofeedback. The ROME group are keen to promote patient based outcome measures in functional bowel disorders. This author seems to be going against this and selecting the arbitrary CSBM as having primacy.
The concept of CSBM (i.e. laxative free defecation) when one of the intervention arms was a laxative intervention, inevitably makes biofeedback look better: the comparison group were instructed to take laxatives and then counted as worse if they did so. Rao et al selected a “sham” that would in some clinical practices be considered active biofeedback. Use of a manometry probe with progressive muscle relaxation and balloon distensions to “promote awareness for stooling and match sensory conditioning provided under biofeedback” is a comparator biofeedback protocol, not a placebo in our opinion. The more severe symptoms at baseline gave greater capacity to benefit in the biofeedback group. We have not been able to find the abstract quoted in the 2010 paper (Am J Gastro 2005), but did find one in Gastroenterology of the same study.
Contacting authors for missing data and clarification: The guidelines for conducting Cochrane reviews include an obligation to contact the authors of studies when important information is missing or unclear. The authors state that they did this. However, authors Rao1, Chiarioni2, and Heymen3 and their research teams were not contacted. If this had been done, it is likely that the reviewers would have drawn different conclusions regarding the risk of bias.
Response:We contacted authors for additional information when we felt the available information was unclear or missing. We attempted to contact Farid/Faried, but received no reply. We also contacted Dr Peyman Adibi regarding the Pourmomeni 2010 study and received a response confirming duplicate publication of a single study. We did not feel it was necessary to contact the authors of Heyman 2007 for further information. Two of the cited studies (Rao and Heymen) had further information available on clinicaltrails.gov and we took this as definitive information and we did not need to contact the authors for further information.
Blinding: The reviewers insist that the masking of active vs. control interventions from both the investigator and the patient, which is a feasible standard for study design in drug trials, should be applied to behavioral therapies such as biofeedback. However, it is not possible to double‐blind a behavioral therapy trial, and alternative criteria have been recommended for managing this source of bias in behavioral trials7. These guidelines include choosing a comparison treatment that is credible to patients, randomizing patients only after eligibility has been confirmed, assessing the expectation of benefit b questionnaire in both the active and control arms of the study after initial exposure to the assigned treatment, and using independent blinded assessors to collect outcome data. These techniques were employed in several of the recent RCTs, but this is not mentioned by the reviewers.
Response:We have nowhere insisted that blinding can or should be done in trials assessing behavioral interventions. However, blinding can be achieved by the use of an appropriate sham and it is possible to blind outcome assessors. We have simply reported on how blinding was utilized in the included studies for the risk of bias assessment. We clearly stated that “it is acknowledged that it is difficult to blind either participants or therapists in behavioural studies, however outcome assessors could have remained blind to treatment allocation.”
Inclusion of older abstracts: While it seems reasonable to include recent abstracts whose findings have significant implication for treatment, patient management, outcome or safety, it seems inappropriate to include abstracts published in 2006 and 2007, none of which have been published in a peer reviewed manuscript and therefore are unavailable to clinicians and researchers in the Cochrane review.
Response:We did not pre‐specify the exclusion of abstract publications in our protocol. Two abstract publications were included in the review (Hu 2006 and Jung 2007) and these studies are clearly described as abstracts in the text and characteristics of included studies table.
Surgical Therapies & STARR Procedures: Rectal mucosal intussusception, excessive Pelvic floor descent, rectoceles etc, are not the same conditions as dyssynergic defecation should be omitted from this review.
Response:The focus of our review focus was on biofeedback for the treatment of chronic constipation not necessarily constipation due to dyssynergic defecation. We included all RCTs that compared biofeedback to sham treatment, conventional treatment, no treatment or another method of biofeedback without selecting what we consider to be appropriate interventions or not. We utilized a pretty liberal interpretation of what constituted ‘conventional treatment’. For example, we might as easily have decided that diazepam was not as appropriate as surgical procedures which are indeed used in clinical practice. We clearly stated our concerns about some of the surgical studies and highlighted the high complication rates that occurred in the surgery groups in the review.
Reporting of adverse‐events: The authors state that firm conclusions cannot be made regarding potential side‐effects of biofeedback treatment, but not a single trial has ever reported an adverse event and biofeedback is generally described as safe. To posit that this treatment may be unsafe suggests an overall negative bias by the authors.
Response:We concluded that there was insufficient evidence to draw any firm conclusions on safety because of the paucity of data on safety outcomes. In the abstract and plain language summary we clearly state “No adverse events were reported for biofeedback, although this was not specifically reported in the majority of studies.” This point is reiterated in the ‘Implications for Practice’ section of the ‘Authors’ conclusions’ where we state “There were no reports of adverse events from biofeedback in any of the studies and it is fairly unlikely that this intervention will cause significant harm.” At no point did we make any suggestion that biofeedback is unsafe.
In summary, there are serious methodological flaws with this systematic review. The conclusions are inaccurate. Contrary to the statement in the review, the signatories to this letter many of whom have authored some of the studies cited in the review were not contacted by the authors of the Cochrane review. Because of the many aforementioned reasons, this review should be retracted. A re‐assessment would be appropriate, either by the original authors or by an independent external set of reviewers
Response:We disagree – this review was subject to substantial editorial and peer review. We contacted authors as necessary but would be delighted to have additional information from the signatories if this is available. We strongly believe that this review provides a balanced assessment of the current state of knowledge regarding the use of biofeedback for the treatment of chronic constipation.
Contributors
Sue Woodward, Christine Norton, Pauline Chiarelli, Nilesh Chande, John K MacDonald
What's new
| Date | Event | Description |
|---|---|---|
| 21 November 2014 | Feedback has been incorporated | Feedback from Rao et al |
| 21 November 2014 | Amended | Feedback from Rao et al and response from authors and IBD/FBD group |
Acknowledgements
Funding for the IBD/FBD Review Group (September 1, 2010 ‐ August 31, 2015) has been provided by the Canadian Institutes of Health Research (CIHR) Knowledge Translation Branch (CON ‐ 105529) and the CIHR Institutes of Nutrition, Metabolism and Diabetes (INMD); and Infection and Immunity (III) and the Ontario Ministry of Health and Long Term Care (HLTC3968FL‐2010‐2235).
Miss Ila Stewart has provided support for the IBD/FBD Review Group through the Olive Stewart Fund.
Appendices
Appendix 1. Search strategies
The search strategies used for each database are outlined below.
The Cochrane Central Register of Controlled Trials (CENTRAL)
biofeedback AND constipation
The Cochrane Complementary Medicine Field
biofeedback AND constipation
The Cochrane Inflammatory Bowel Disease and Functional Bowel Disorders Group Specialized Register
biofeedback AND constipation
MEDLINE
1. exp Constipation/
2. constipation.mp.
3. pelvic floor dyssynergia.mp.
4. exp Pelvic Floor/ or exp Defecation/ or exp Biofeedback, Psychology/ or exp Rectal Diseases/
5. anismus.mp.
6. exp Anus Diseases/ or exp Anal Canal/
7. 1 or 2 or 3 or 4 or 5 or 6
8. exp Biofeedback, Psychology/
9. biofeedback.mp.
10. 8 or 9
11. 7 and 10
12. limit 11 to yr="1966 ‐Current"
13. limit 12 to yr="1980 ‐Current"
14. randomized controlled trial.pt.
15. randomized clinical trial.pt.
16. randomised.ab.
17. placebo.ab.
18. randomly.ab.
19. trial.ab.
20. groups.ab.
21. 14 or 15 or 16 or 17 or 18 or 19 or 20
22. limit 21 to humans
23. 13 and 22
24. limit 23 to "all adult (19 plus years)"
CINAHL
(MH "Constipation") AND (MH "Biofeedback")
British Nursing Index (BNI)
1. exp Constipation/
2. constipation.mp. [mp=title, abstract, heading words]
3. pelvic floor dyssynergia.mp. [mp=title, abstract, heading words]
4. anismus.mp. [mp=title, abstract, heading words]
5. 1 or 2 or 3 or 4
6. biofeedback.mp. [mp=title, abstract, heading words]
7. 5 and 6
8. limit 7 to yr="1980 ‐Current"
9. randomized controlled trial.mp. [mp=title, abstract, heading words]
10. randomized.mp. [mp=title, abstract, heading words]
11. placebo.mp. [mp=title, abstract, heading words]
12. randomly.mp. [mp=title, abstract, heading words]
13. trial.mp. [mp=title, abstract, heading words]
14. 9 or 10 or 11 or 12 or 13
15. 8 and 14
EMBASE
1. exp feedback system/
2. biofeedback.mp.
3. 1 or 2
4. exp constipation/
5. constipation.mp.
6. pelvic floor dyssynergia.mp.
7. anismus.mp.
8. 4 or 5 or 6 or 7
9. 3 and 8
10. limit 9 to yr="1980 ‐Current"
11. limit 10 to human
12. limit 11 to (adult <18 to 64 years> or aged <65+ years>)
13. random$.mp.
14. factorial$.mp.
15. crossover$.mp.
16. cross over$.mp.
17. cross‐over$.mp.
18. placebo$.mp.
19. (doubl$ adj blind$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
20. (singl$ adj blind$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
21. assign$.mp.
22. volunteer$.mp.
23. 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22
24. crossover procedure/
25. double blind procedure/
26. randomized controlled trial/
27. single blind procedure/
28. allocat$.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
29. 23 or 24 or 25 or 26 or 27 or 28
30. 12 and 29
PsychINFO
1. exp Constipation/
2. constipation.mp. [mp=title, abstract, heading word, table of contents, key concepts]
3. pelvic floor dyssynergia.mp. [mp=title, abstract, heading word, table of contents, key concepts]
4. anismus.mp. [mp=title, abstract, heading word, table of contents, key concepts]
5. 1 or 2 or 3 or 4
6. exp Biofeedback/
7. exp Biofeedback Training/
8. biofeedback.mp. [mp=title, abstract, heading word, table of contents, key concepts]
9. 6 or 7 or 8
10. 5 and 9
11. limit 10 to yr="1980 ‐Current"
12. limit 11 to human
13. limit 12 to "300 adulthood "
14. limit 13 to ("0400 empirical study" or "0430 followup study" or "0450 longitudinal study" or "0451 prospective study" or "0452 retrospective study" or 1800 quantitative study or "2000 treatment outcome/randomized clinical trial")
SCOPUS
TITLE‐ABS‐KEY(constipation OR "pelvic floor dyssynergia" OR anismus) AND TITLE‐ABS‐KEY(biofeedback) AND PUBYEAR AFT 1979 AND ("randomized controlled trial")
Science Citation Index Expanded (SCI‐EXPANDED)
biofeedback AND constipation
Social Sciences Citation Index (SSCI)
biofeedback AND constipation
Conference Proceedings Citation Index ‐ Social Science and Humanities (CPCI‐SSH)
biofeedback AND constipation
Conference Proceedings Citation Index ‐ Science (CPCI‐S)
biofeedback AND constipation
Data and analyses
Comparison 1. EMG biofeedback versus balloon sensory biofeedback.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number improved | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
1.1. Analysis.
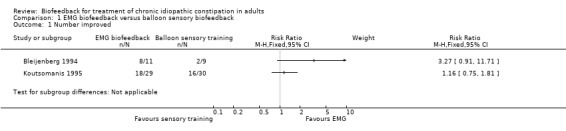
Comparison 1 EMG biofeedback versus balloon sensory biofeedback, Outcome 1 Number improved.
Comparison 2. EMG biofeedback versus manometry biofeedback.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number improved | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
2.1. Analysis.
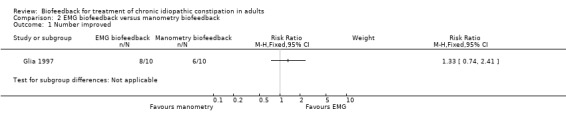
Comparison 2 EMG biofeedback versus manometry biofeedback, Outcome 1 Number improved.
Comparison 3. One method of biofeedback versus surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number improved | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Constipation score | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
3.1. Analysis.
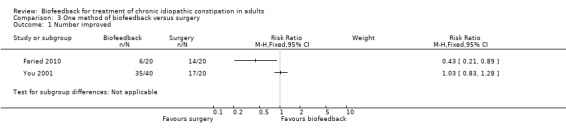
Comparison 3 One method of biofeedback versus surgery, Outcome 1 Number improved.
3.2. Analysis.
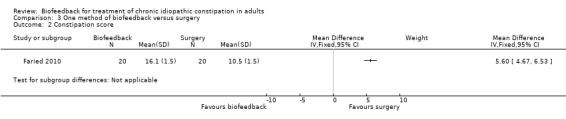
Comparison 3 One method of biofeedback versus surgery, Outcome 2 Constipation score.
Comparison 4. One method of biofeedback versus Botulinum toxin‐A.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number improved | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Constipation score | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
4.1. Analysis.
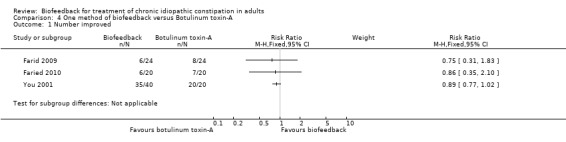
Comparison 4 One method of biofeedback versus Botulinum toxin‐A, Outcome 1 Number improved.
4.2. Analysis.
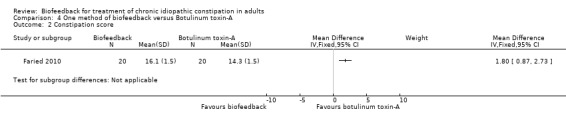
Comparison 4 One method of biofeedback versus Botulinum toxin‐A, Outcome 2 Constipation score.
Comparison 5. EMG biofeedback versus electrical stimulation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bowel satisfaction score | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
5.1. Analysis.
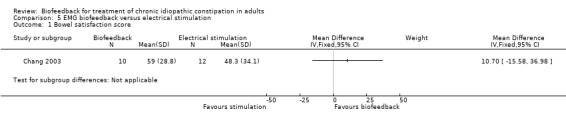
Comparison 5 EMG biofeedback versus electrical stimulation, Outcome 1 Bowel satisfaction score.
Comparison 6. EMG biofeedback versus diazepam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number improved | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
6.1. Analysis.
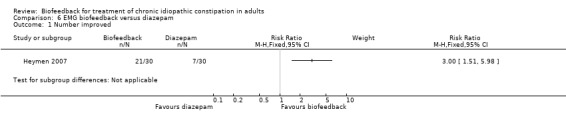
Comparison 6 EMG biofeedback versus diazepam, Outcome 1 Number improved.
Comparison 7. EMG biofeedback versus STARR procedure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Successful treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Obstructed defecation score | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
7.1. Analysis.
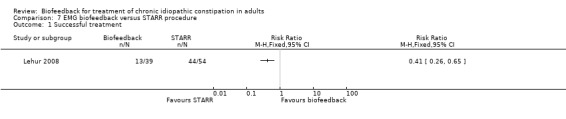
Comparison 7 EMG biofeedback versus STARR procedure, Outcome 1 Successful treatment.
7.2. Analysis.
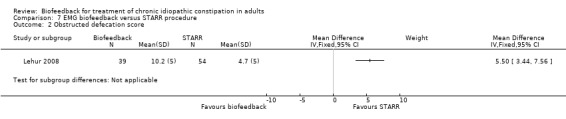
Comparison 7 EMG biofeedback versus STARR procedure, Outcome 2 Obstructed defecation score.
Comparison 8. One method of biofeedback versus control muscle relaxation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Constipation severity index | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2 IBS‐QOL | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
8.1. Analysis.
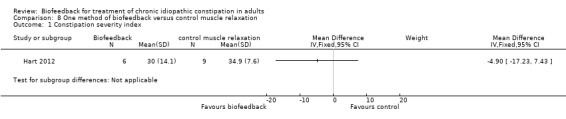
Comparison 8 One method of biofeedback versus control muscle relaxation, Outcome 1 Constipation severity index.
8.2. Analysis.
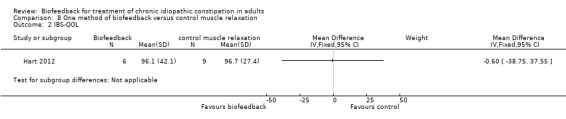
Comparison 8 One method of biofeedback versus control muscle relaxation, Outcome 2 IBS‐QOL.
Comparison 9. EMG biofeedback versus laxative (movicol).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Major clinical improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
9.1. Analysis.
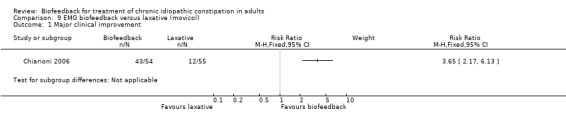
Comparison 9 EMG biofeedback versus laxative (movicol), Outcome 1 Major clinical improvement.
Comparison 10. Manometry biofeedback versus sham.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CSBM | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
10.1. Analysis.
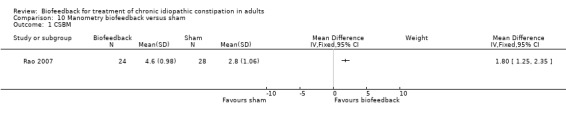
Comparison 10 Manometry biofeedback versus sham, Outcome 1 CSBM.
Comparison 11. Manometry biofeedback versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CSBM | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
11.1. Analysis.
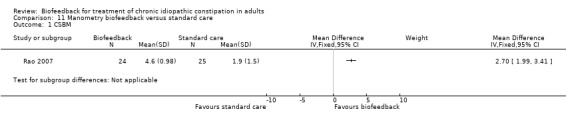
Comparison 11 Manometry biofeedback versus standard care, Outcome 1 CSBM.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bleijenberg 1994.
| Methods | Study design: randomised controlled trial Total study duration: Mean follow‐up 14 months (SD 8.9; range 3 to 28 months) for EMG biofeedback and 9 months (SD 7.0; range 3 to 26 months) for comparison group |
|
| Participants | 21 patients, all with pelvic floor dyssynergia and constipation N = 11 electromyograph (EMG) biofeedback (8 female, 3 male) (mean age 35 years, range 20 to 50 years); duration of symptoms (mean 8 years, range 2 to 11years) N = 10 balloon biofeedback (8 female, 2 male) ‐ 1 drop out (mean age 40 years (range 28 to 47 years)); duration of symptoms (mean 7.5 years, range 2 to 15 years) No difference between groups at pre‐test |
|
| Interventions | No laxatives allowed Weekly outpatient sessions for 8 weeks Intervention group: Phase 1: EMG biofeedback using anal‐plug electrode and digital display Phase 2: simulated defecation of oatmeal porridge Phase 3: toilet instructions ‐ to strain a maximum of 5 times on the toilet after each meal and patient to decide whether 'straining' was correct or not Comparison group: Phase 1: Balloon biofeedback using balloon catheter inflated with 20 mL air inserted 8 cm into rectum, patient to pull balloon out over 10 seconds without straining Phase 2 and 3 same as EMG biofeedback |
|
| Outcomes | EMG quality score (based on depth and amplitude of EMG signal from 0 to 100: 0 = no relaxation during straining, 100 = maximal relaxation) Patient symptom diary (recorded 4 times daily): frequency, difficult evacuation, incomplete evacuation, hard stools, abdominal pain Constipation score (calculated from symptom diary) VAS (0 to 200: 100 = unchanged) of subjective rating of change SCL‐90 ‐ validated psychopathology symptom checklist |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition (one participant only) |
| Selective reporting (reporting bias) | Unclear risk | Not registered on a clinical trials registry |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Chang 2003.
| Methods | Study design: randomised controlled trial Total study duration: 10 to 12 days ‐ no long‐term follow‐up beyond end of treatment |
|
| Participants | 22 patients selected from 130 consecutive referrals who met Rome II criteria for functional constipation and were thought to have 'impaired rectal sensation' defined as rectal sensory threshold volume for desire to defecate of > 90 mL Electrical stimulation: n = 12 (5 male, 7 female) (Age ‐ mean 41 years (range 18 to 71 years) Electromyograph (EMG) biofeedback: n = 10 (6 male 4 female) (Age ‐ mean 53 years (range 28 to 74 years) Duration of symptoms not reported Baseline comparability‐ reported 'no significant difference', but no P values were reported |
|
| Interventions |
Intervention group: Electrical stimulation using anal plug with pulse generator introduced into the anal canal 'Variant stimulation' parameters scheduled individually within pre‐specified range Performed for 20 minutes daily for 10 to 12 sessions Comparison group: EMG biofeedback with visual changes in pressure activity displayed on monitor 10 to 14 sessions lasting 60 to 90 minutes (frequency not reported) |
|
| Outcomes | Symptom questionnaire (bowel frequency and urge, satisfaction with bowel habit, straining, sensation of incomplete evacuation, anal obstruction scored on VAS) Anorectal manometry immediately before and after each treatment Rectal sensation measured using balloon distention |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Unclear risk | Not registered on a clinical trials registry |
| Other bias | Unclear risk | Disproportionate number of male participants compared with usual biofeedback population |
Chiarioni 2006.
| Methods | Study design: randomised controlled trial Total study duration: 12 months for both groups, 24 months for biofeedback group only |
|
| Participants | 109 patients with severe constipation (Rome II criteria) for > 12 months, unresponsive to standard treatment or 30 day trial of fibre All with paradoxical contraction or non‐relaxing pelvic floor on EMG and inability to defecate 50 mL water‐filled balloon Excluded patients with slow transit Biofeedback n = 54 (3 male, 51 female) (Age ‐ mean 33.3 years (SE +/‐ 1.5 years) Controls n = 55 (2 male, 54 female) (Age ‐ mean 35.1 years (SE +/‐ 1.4 years) The authors reported groups to be similar at baseline, but no statistical analysis of comparability was reported |
|
| Interventions |
Intervention group: Biofeedback: 5 weekly 30 minute training sessions EMG biofeedback with contraction and relaxation of pelvic floor displayed on monitor Practice defecation of simulated stool (50 mL water‐filled balloon) while traction applied Comparison group: Laxatives (polyethylene glycol [PEG] 14.6 g/day) plus education Five 30 minute counselling sessions: avoiding unnecessary straining, defecation posture, routine, physiology of constipation, adverse effects of PEG discussed |
|
| Outcomes | Patient response to the question "how would you grade your symptom improvement: worse (0), no improvement (1), mild (2), fair (3) or major improvement (4)" (primary outcome) Bowel diary of stool frequency, laxative use (other than PEG), straining, sense of incomplete evacuation, feeling of blocked defecation (kept for 30 days prior to follow‐up visits following telephone reminder) Anorectal manometry (anal canal pressure) Surface intra‐anal EMG to measure responses to attempted defecation Ability to defecate a 50 ml water‐filled balloon (simulated stool) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Shuffled sealed opaque envelopes |
| Allocation concealment (selection bias) | Low risk | Adequate concealment: sealed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intent‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered on a clinical trials registry |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Farid 2009.
| Methods | Study design: randomised controlled trial Total study duration: 1 year |
|
| Participants | 48 patients ‐ all met Rome II criteria for functional constipation and unresponsive to laxatives or enemas All patients had anismus (non‐relaxing anal sphincter) based on transit time, manometry, balloon expulsion test, defecography and EMG activity of external anal sphincter Biofeedback n = 24 (8 male: 16 female) mean age 39.6 years (range 20 to 69 years), symptom duration 4.8 years (range 1 to 10 years) Botulinum toxin‐A n = 24 (7 male : 17 female) mean age 34.7 years (range 20 to 63), symptom duration 5.9 years (range 2 to 12 years) Baseline comparability between groups was not reported |
|
| Interventions |
Intervention group: Biofeedback twice weekly for 1 month as outpatient. Explained normal bowel and anal physiology Sensory training biofeedback ‐ 'pressure‐based' using balloon expulsion Comparison group: Botulinum toxin‐A injected (Dysport 100 u dissolved in saline (0.5 ml) single injection via insulin syringe) into left and right sides of the puborectalis and external anal sphincters with the patient in left lateral position No anaesthesia was necessary |
|
| Outcomes | Improvement in bowel symptoms: "clinical improvement or success… Patients who returned to normal with regard to their bowel habits” PR assessment of relaxation of puborectalis Symptom questionnaire 1 month post‐treatment and at 1 year (straining, anorectal pain, incomplete evacuation, anal digitation and enema use) assessed using visual analogue scale At 1 month: anorectal manometry, balloon expulsion test, defecography, EMG examination of anal sphincter At 1 year patients asked: “Are you satisfied with the result of procedure performed to you” [sic] |
|
| Notes | It is noted that the authors report screening consecutive patients referred to the same institution over the same time period (Colorectal Surgery Unit of Mansoura University Hospital, Mansoura, Egypt), with identical demographics, for both this study (biofeedback and botulinum toxin‐A arms) and for Faried 2010, suggesting that the two papers report the same study, however with major inconsistencies in reporting details, this is a major concern | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition reported and included in intent‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered on a clinical trials registry |
| Other bias | High risk | See comments in table above |
Faried 2010.
| Methods | Study design: randomised controlled trial Total study duration: 1 year |
|
| Participants | 60 patients seen during recruitment period (> 3 years) met Rome II criteria for functional constipation and unresponsive to laxatives Anismus diagnosed as non‐relaxing external anal sphincter, inability to expel water‐filled balloon, non‐relaxing puborectalis, prolonged evacuation time 17 male, 43 female Mean age 37.53 years (range 20 to 69 years) No difference between groups at baseline NB: demographic data for patients in two groups of this study are identical to the patients in Farid 2009 |
|
| Interventions |
Intervention group: Biofeedback (n = 20) Two times per week for one month (8 sessions), expert therapist Each session 30 minutes as out‐patient Explanation of pelvic floor and own test results Pressure‐based biofeedback learning to expel 50 mL balloon and push down with abdominal muscles using 'trial and error' Continued periodic supervision for 6 months if successful Comparison groups: 1.Botulinum toxin (BTX‐A) (n = 20) into left and right sides of puborectalis and EAS as outpatient 2.Bilateral open partial division of puborectalis (n = 20) under GA |
|
| Outcomes | Primary end point ‐ 'improvement in bowel habits' using symptom questionnaire (unspecified ‐ possibly Cleveland Clinic Score) at 1 month Success defined as bowel habits that 'returned to normal' Secondary endpoints: complications, satisfaction using visual analogue scale (change of at least 2 out of 10 score) post‐operative incontinence, anorectal manometry balloon expulsion test, defecography and EMG of anal sphincter |
|
| Notes | It is noted that the authors report screening consecutive patients referred to the same unit over the same time period, with identical demographics, for both this study (biofeedback and botulinum toxin‐A arms) and for Farid 2009, suggesting that the two papers report the same study, however with major inconsistencies in reporting details This is a major concern |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Unclear risk | Not registered on a clinical trials registry |
| Other bias | High risk | See comments in table above |
Glia 1997.
| Methods | Study design: randomised controlled trial Total study duration: six month follow‐up following five week intervention |
|
| Participants | 26 consecutive patients with functional constipation ‐ based on Rome I criteria ‐ organic lesions excluded Proportion of females or males not reported. Age ‐ mean 55 years (range 28 to 78 years); duration of symptoms ‐ mean 11 years (range 1 to 35 years) 10 patients had rectocele, 1 had enterocele, 11 had intussusception and 4 had rectal prolapse Baseline comparability between groups not reported |
|
| Interventions |
Intervention group: Manometry Biofeedback 1 to 2 sessions per week for maximum of 10 sessions Pressure‐based training using four‐lumen catheter ‐ patients were allowed to view manometric recordings of pressure and encouraged to relax anal sphincter during balloon expulsion Comparison group: EMG biofeedback using bilateral surface electrodes, same frequency as manometry |
|
| Outcomes | Balloon expulsion test of 60 mL water filled balloon. Whole gut transit Anorectal manometry and EMG Bowel symptom diary daily for 1 week Global rating of treatment effect: worse or unaltered or better |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomised sequence generation |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition (six participants from 26, but three from each group) and no intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered on a clinical trials registry |
| Other bias | Unclear risk | Recruitment bias and power unclear |
Hart 2012.
| Methods | Study design: randomised controlled trial Total study duration: 12 weeks ‐ no long‐term follow‐up |
|
| Participants | 21 outpatients with pelvic floor dyssynergia, failed lifestyle modification and other medical interventions Confirmed pelvic floor dyssynergia on anorectal manometry, balloon expulsion and defecography 14 female:1 male. Mean age (SD) 50.1 years (16.8 years) Duration of symptoms not reported n = 10 randomised to biofeedback; n = 11 randomised to control Gender split unequal between groups at baseline |
|
| Interventions |
Intervention group: Anorectal biofeedback by registered nurse Six 1 hour sessions (alternate weeks) ‐ 20 minutes EMG biofeedback (rectal probe) plus coaching for relaxation of pelvic floor muscles Practice pelvic floor exercises between sessions Comparison group: Muscle relaxation of trapezius or temporalis with feedback from registered nurse Six 1 hour sessions (alternate weeks) ‐ EMG with surface electrodes on either site plus coaching for relaxation of the right and left trapezius or right and left temporalis plus breathing technique Practice relaxation and breathing technique between sessions |
|
| Outcomes | Constipation severity instrument (3 subscales: obstructive defecation, colonic inertia, pain) Irritable bowel syndrome quality of life scale (IBS‐QOL) SF‐36 History of childhood sexual or physical abuse using Trauma History Questionnaire (24‐item scale for traumatic events in 3 areas: crime‐related events, general disaster and trauma, unwanted physical and sexual experiences) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | Reported allocation concealed until randomised, although method unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants and therapists not blinded: assessor blinding unclear |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4 dropped out from biofeedback group, 2 from control group: total attrition n = 6 from 21 |
| Selective reporting (reporting bias) | Low risk | Registered prospectively on clinical trials registry |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Heymen 1999.
| Methods | Study design: randomised controlled trial Total study duration: no follow‐up beyond end of treatment |
|
| Participants | 36 patients referred to tertiary centre with pelvic floor dyssynergia, who required laxatives, enemas, digitation or combination to achieve bowel movement (26 female, 10 male) gender split between groups was not reported Age ‐ mean 61 years (range 18 to 82 years); duration of symptoms not reported Baseline comparability not reported |
|
| Interventions | Randomised to four groups All patients in intervention and comparison groups also educated as to normal bowel function, posture, not to prolong defecation attempts beyond 10 to 15 minutes, not to strain and to schedule bowel movements after meals or exercise Intervention group: Group 1: Weekly one hour EMG biofeedback ‐ use of intra‐anal sensor with EMG display of muscle activity Comparison groups: Group 2. EMG biofeedback plus balloon distention sensory training (using flexible manometry catheter with balloon attached and inflated up to 140 mL) 3. EMG biofeedback plus home trainer EMG biofeedback unit 4. EMG biofeedback plus balloon distention sensory training plus home trainer EMG biofeedback unit |
|
| Outcomes | Change in frequency of unassisted bowel movements (UBM) (meaning of UBM not defined) Laxative use |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition reported |
| Selective reporting (reporting bias) | Unclear risk | Not registered on a clinical trials registry |
| Other bias | High risk | Primary outcome was assessed by unblinded therapist making contact with patients by telephone |
Heymen 2007.
| Methods | Study design: randomised controlled trial Total study duration: 12 months follow‐up for those who reported adequate relief only |
|
| Participants | 84 patients who met Rome II criteria for pelvic floor dyssynergia and chronic constipation, manometric, EMG or defecographic evidence of non‐relaxing pelvic floor, inadequate propulsive forces during defecation on manometry Evidence of incomplete evacuation Excluded patients currently using diazepam or previous use of biofeedback Still symptomatic following 4 week run in with education regarding diet, exercise, fluid intake, bowel function and correct defecation technique 84 patients (85% female) randomised to biofeedback (n = 30) (mean age 51.4 years, symptom duration 14 years), diazepam (n = 30) (mean age 51.7 years, symptom duration 14.5 years), placebo (n = 24) (mean age 46.1 years, symptom duration 19 years) |
|
| Interventions |
Intervention group: Intensive education intervention plus EMG biofeedback to teach relaxation of pelvic floor during straining to defecate Six x 50 minute biweekly sessions over 3 months using EMG anal plug and function of anal sphincters displayed on monitor Comparison group: (1) Diazepam 5 mg orally one hour before attempted defecation (2) Placebo in place of diazepam one hour before attempted defecation Patients in both of these groups received the same intensive education as the intervention group but no biofeedback |
|
| Outcomes | Primary outcome: report of adequate relief of constipation at 3 months follow‐up assessed by question asked by the therapist during a telephone follow up interview: "compared to before you started the study, have you experienced adequate relief of constipation?" Secondary outcomes: Bowel symptoms ‐ unassisted bowel movements, assisted bowel movements, straining, incomplete evacuation PAC‐SYM PAC‐QOL SF36 Anorectal manometry EMG testing Whole gut transit time |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Both the investigators and the patients knew whether the patients were assigned to biofeedback or to pills Partial blinding between diazepam and placebo only |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intent‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered on a clinical trials registry |
| Other bias | High risk | Possible self‐selection bias among participants |
Hu 2006.
| Methods | Study design: randomised controlled trial Total study duration: 12 weeks |
|
| Participants | 60 patients who met Rome II criteria for chronic idiopathic constipation Symptoms lasting 1 year or more No organic pathology ‐ ruled out via colonoscopy Mean age 44 95% female Randomised biofeedback (n = 30), control (n = 30) |
|
| Interventions | All patients stopped laxatives in both groups Intervention group: Biofeedback ‐ teaching of proper defecation posture, abdominal muscle and pelvic floor exercises, 'pressure' training and balloon expulsion training Each session 30 to 60 minutes every two weeks for six sessions Comparison group: Lifestyle, dietary and general advice only Timing of sessions as biofeedback |
|
| Outcomes | Symptom diary ‐ daily bowel habits and use of rescue laxatives Constipation symptom severity questionnaire ‐ 5 point Likert scale |
|
| Notes | abstract only ‐ never published as full manuscript in peer reviewed literature | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition not reported |
| Selective reporting (reporting bias) | Unclear risk | All patients stopped laxatives in both groups |
| Other bias | Unclear risk | Full manuscript not published |
Jung 2007.
| Methods | Study design: randomised cross‐over design Total study duration: five weeks No long‐term follow‐up beyond end of treatment |
|
| Participants | 40 patients who met Rome II criteria for pelvic floor dyssynergia Intervention group (n = 20) (21 to 77 years, Male/Female 8:12) Comparison group (n = 20) (24 to 70 years, Male/Female 7:13) |
|
| Interventions |
Intervention group: Electrical stimulation therapy (EST) for 2 weeks then Biofeedback for 5 weeks EST performed for 24 minutes (EST parameters not reported) for twelve sessions using anal plug and pulse generator Comparison group: Biofeedback (unspecified method) for 5 weeks then EST for 2 weeks |
|
| Outcomes | Symptom assessment (subjective overall satisfaction, straining, incomplete evacuation, feeling of obstruction) rated from 0 to 10 Patient’s opinion about treatment (success/fail) Anorectal manometry Balloon expulsion substance P expression within rectal mucosa All above recorded before and after each treatment |
|
| Notes | Abstract only ‐ never published as a full manuscript in peer reviewed literature | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Drop outs not reported |
| Other bias | Unclear risk | Not published as a full manuscript |
Koutsomanis 1995.
| Methods | Study design: Randomised controlled trial Total study duration: 2 to 3 month follow‐up |
|
| Participants | 60 patients all unresponsive to standard treatment Included both patients with pelvic floor dyssynergia (n = 47) and slow transit (n = 17) 30 biofeedback (24 female, 6 male) ‐ 1 withdrew and lost to follow‐up, age ‐ mean 40 years (range 20 to 64 years), duration of symptoms 14 years (range 1 to 40 years) 30 muscle training (29 female, 1 male), age ‐ mean 41 years (range 23 to 63 years), duration of symptoms 13 years (range 3 to 40 years) Reported 'no major differences' between groups at baseline, but no statistical comparison presented |
|
| Interventions |
Intervention group Visual biofeedback: Muscle training: Patient taught to direct propulsive forces towards pelvis, while relaxing and protruding lower abdomen to pass balloon filled with 50 mL air, when lying on left side, plus watched EMG trace on a computer screen from surface electrodes placed on the skin over the external anal sphincter at the anal margin. Comparison group Muscle training as for other group without visual display. |
|
| Outcomes | Patient symptom diary (daily for 1 week) Whole gut transit Surface EMG Simulated defecation (50 mL water filled balloon) ‐ successful if balloon passed within 5 minutes All outcomes were assessed at end of treatment and symptom diary only during 2 to 3 month follow‐up |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition (only one participant from intervention group), but no intent‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered on a clinical trials registry |
| Other bias | Unclear risk | Recruitment bias and power unclear |
Lehur 2008.
| Methods | Study design: randomised controlled trial across nine European centres Total study duration: 12 months |
|
| Participants | 119 female patients with 'obstructive defecation syndrome score' >7 (score is the sum of individual scores for 7 symptoms of outlet obstruction), all had confirmed rectocele on defecography, 'Adequate (not defined) external sphincter function on examination STARR group (n = 59) mean age (± SD) 56 years (± 9.2 years: range 34 to 80 years) Biofeedback group (n = 60) mean age 56 years (± 14.3 years: range 24 to 78 years) |
|
| Interventions |
Intervention group: Stapled transanal rectal resection (STARR) under general anaesthetic, with patient in lithotomy position Designed to achieve transanal full‐thickness resection of the lower rectum Used 2 times Ethicon Endo‐surgery PPH01 kits per procedure Same surgeon at each centre, all trained and had conducted 10 STARR procedures previously Comparison group: Biofeedback training two sessions per week for a planned minimum of 10 sessions, but not > 24 given over 3 month period Each session 1 hour of EMG based biofeedback ‐ visual and acoustic feedback via perianal skin electrodes of muscle relaxation during straining Biofeedback 'standardised among different practitioners' |
|
| Outcomes | Primary outcome: obstructed defecation score (ODS) Defined a responder a priori as ≥ 50% reduction in ODS score at one year Secondary outcomes: PAC‐QOL Continence grading scale (interviewer‐led questionnaire) (six questions rated 0‐4) Patient‐reported success (self‐administered questionnaire) (1‐10 scale) Anatomic correction of rectocele in surgery group only Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High drop out (50%) from biofeedback group and not all included in intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered on a clinical trials registry |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Pourmomeny 2010.
| Methods | Study design: randomised controlled trial Total study duration: follow‐up 1 week following treatment completion (6 sessions) |
|
| Participants | 65 out‐patients with dyssynergic defecation (Rome III criteria): symptoms > 6 months Failed to respond to diet and lifestyle modification, psyllium husk and daily exercise 49 women: 16 men EMG Biofeedback group (n = 34) mean age (SD) 38 years (12 years) Balloon‐assisted training group (n = 31) mean age (SD) 36.8 years (10 years) No significant differences between groups at baseline |
|
| Interventions |
Intervention group: EMG biofeedback: trained to increase abdominal pressure and relax rectal muscles Frequency of 6 training sessions not reported Comparison group: Balloon defecation training: asked to expel water‐filled balloon Frequency of six training sessions not reported |
|
| Outcomes | Satisfaction (low, moderate or high) Change in Rome III criteria Ability to expel a rectal balloon (volume and time) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate allocation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis but did not report number of participants who completed or how missing data were dealt with within ITT |
| Selective reporting (reporting bias) | Unclear risk | Not registered on a clinical trials registry |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Rao 2007.
| Methods | Study design: randomised controlled trial Total study duration: 3 month and one year follow‐up (one year follow‐up reported in separate paper (Rao 2010)) |
|
| Participants | 77 patients referred to tertiary centre for constipation and met Rome II criteria for functional constipation. Evidence of pelvic floor dyssynergia on attempted defecation 77 patients (69 female, 8 male) randomised 24 to standard therapy, 28 to biofeedback, 25 to sham biofeedback) Age ‐ mean 43 years (range 18 to 75 years) Mean symptom duration 17 years No difference in demographics between groups at baseline, but biofeedback group had 'significantly lower defecation index' and 'relatively greater pelvic floor dysfunction' (Rao 2007, p.333) than other groups at outset |
|
| Interventions |
Intervention group: Manometry biofeedback: biweekly one hour sessions up to maximum of 6 over a three month period, with visual display of anal sphincter pressures during simulated defecation Also received standard advice: advice on bowel habit, diet, exercise, laxatives and fluid intake, postural and diaphragmatic breathing techniques to improve pushing efforts Comparison groups (2): Sham biofeedback: standard advice plus 6 biweekly I hour relaxation therapy sessions during a period of 3 months Manometry probe placed in rectum and practiced progressive muscle relaxation to audiotape for 20 minutes Also received intermittent balloon distension using the rectal probe to match sensory conditioning component of biofeedback |
|
| Outcomes | Subjective primary outcome measure: number of complete spontaneous bowel movements (CSBM) per week plus global satisfaction on visual analogue scale Physiological primary outcome: dyssynergic pattern of defecation and balloon expulsion time Secondary subjective outcome measures: symptom diary for 1 week: time and consistency of stool (using Bristol Stool Form Scale), straining, incomplete evacuation, digitation. Secondary objective outcomes: anorectal manometry and colonic transit study |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes ‐ adequate concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | High attrition (n = 12 from 77), but performed intention‐to‐treat analysis at 3 months. From 52 patients randomised to biofeedback or standard care, data from 20 are reported at one year |
| Selective reporting (reporting bias) | Low risk | Registered on clinical trials registry |
| Other bias | High risk | The two groups were not equal at baseline as the biofeedback group had a significantly lower defecation index and relatively greater pelvic floor dysfunction than the sham group |
Simon 2009.
| Methods | Study design: Randomised controlled trial Total study duration: follow‐up for two months after end of treatment |
|
| Participants | 30 participants with dyssynergic defecation unresponsive to diet or fibre, fulfil Rome III criteria Paradoxical contraction of anal sphincter during defecation evident Mean age 73.8 years (range 67 to 80 years) 11 male, 19 female Duration of symptoms mean 12.8 years (range 6 to 21 years) No significant difference between groups for frequency of defecation per week (EMG biofeedback n = 15, control n = 15) |
|
| Interventions |
Intervention group: EMG biofeedback ‐ 8 sessions (x2 per week) over 1 month, each lasting 45 minutes EMG during straining displayed via visual and auditory feedback, with patient lying in left lateral position Comparison group: Eight 45 minute counselling sessions ‐ covered behavioural mechanisms involved in defecation, diet, positioning, avoid straining, routine time for defecation Each session ended with EMG assessment during straining to defecate |
|
| Outcomes | Self‐report of bowel frequency, sensation of incomplete evacuation, evacuation difficulty and perianal pain on defecation Latter 3 symptoms rated on a scale (0 = no symptom, 5 = middle symptom, 10 = severe symptom) EMG activity during rest, squeezing and straining to defecate ‐ also used to calculate an 'anismus index' |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported no attrition |
| Selective reporting (reporting bias) | Unclear risk | Not registered on a clinical trials registry |
| Other bias | Low risk | The study appears to be free of other sources of bias |
You 2001.
| Methods | Study design: randomised controlled trial Total study duration: 1 year beyond end of treatment |
|
| Participants | 80 patients with slow transit constipation Age ranged from 20 to 70 years Duration of symptoms and baseline comparability not reported, male: female 4:1 (exact numbers not reported ‐ calculated to be 64 female, 16 male) Distribution of characteristics not reported (20 patients in each of control groups, 40 in biofeedback group) |
|
| Interventions |
Intervention group: Biofeedback using device with anal probe ‐ unclear if this was an EMG or manometry device plus pelvic floor exercises, balloon training Daily intervention for 14 days ‐ not clear if as in‐patient or outpatient Comparison group: (1) botulinum toxin‐A injection to external anal sphincter and puborectalis (2) posterior myomectomy of internal anal sphincter and puborectalis |
|
| Outcomes | Defecation satisfaction level Frequency of bowel movements Transit time Balloon expulsion Anorectal physiology and EMG of anal sphincters and puborectalis |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Unclear risk | Not registered on a clinical trials registry |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Battaglia 2004 | Non‐randomised study |
| Binnie 1992 | Non‐randomised study |
| Chiarioni 2005 | Non‐randomised study |
| Chiarioni 2007 | Letter ‐ not a study |
| Chiarioni 2010 | RCT of biofeedback for anal pain, not chronic idiopathic constipation |
| Chiotakakou‐Faliakou 1998 | Non‐randomised study |
| Dallianas 2000 | Non‐randomised study |
| Emmanuel 2001 | Non‐randomised study |
| Enck 2009 | Meta‐analysis. Not an RCT |
| Ferrara 2001 | Non‐randomised study |
| Horton 2008 | Contacted author Trial abandoned due to poor recruitment |
| Keck 1994 | Non‐randomised study |
| Lin 2005 | Non‐randomised study Included healthy controls |
| NCT00564707 | Trial of biofeedback for anal pain, not constipation |
| Park 2003 | Non‐randomised study Post‐hoc analysis of responders and non‐responders to biofeedback |
| Patankar 1997 | Non‐randomised study |
| Roy 2000 | Non‐randomised study |
| Siproudhis 1995 | Non‐randomised study |
| Wiesel 2001 | Non‐randomised study |
| Yang 2004 | Non‐randomised study |
Characteristics of studies awaiting assessment [ordered by study ID]
Singles 1990.
| Methods | Unobtainable |
| Participants | Unobtainable |
| Interventions | Unobtainable |
| Outcomes | Unobtainable |
| Notes | Unpublished thesis |
Characteristics of ongoing studies [ordered by study ID]
NCT00982839.
| Trial name or title | Rectal Sensory Training ‐ a randomized controlled study of two techniques |
| Methods | Randomised, open label, parallel assignment trial comparing two techniques for rectal hyposensitivity and constipation |
| Participants | Patients categorised as having dyssynergic defecation or slow transit constipation or normal transit constipation |
| Interventions | Intervention: syringe‐assisted sensory conditioning (biofeedback) Comparison: barostat assisted sensory conditioning |
| Outcomes | Primary outcome ‐ rectal sensory thresholds Secondary outcome ‐ satisfaction with bowel function based on visual analogue scale (VAS) |
| Starting date | March 2004 |
| Contact information | Satish SC Rao, MD satish‐rao@uiowa.edu |
| Notes | Expected completion June 2012, but not yet published |
NCT01672216.
| Trial name or title | Multicentre randomized controlled trial to compare the outcome of conservative triple target treatment with EMG‐biofeedback for chronic constipation (3T‐CO) |
| Methods | Parallel group randomised multicentre study with blinded observers |
| Participants | 140 Patients > 18 years with chronic constipation (Rome II criteria) |
| Interventions |
Intervention: Stimulation with a carrier wave of 25 KHz and biphasic modulations of the pulse train of 40 KHz ‐ combination of EMG‐biofeeback plus EMG‐triggered AM‐MF stimulation, carried out at home, with an alternating combination in the morning and EMG‐triggered stimulation in the evening for 20 minute periods. Comparison: EMG‐biofeedback alone, carried out at home, standing, mornings and evenings for 20 minute periods. |
| Outcomes | Primary outcome ‐ Altomare ODS score in its validated form after 3 and 12 months, compared to baseline Secondary outcomes ‐
|
| Starting date | August 2012 |
| Contact information | Dr T Schwander, University of Giessen, Dept of General Surgery, Giessen, Hessen, Germany 35385 |
| Notes | ongoing, but not recruiting participants ‐ estimated completion May 2015 |
Contributions of authors
Two review authors (S. Woodward and C. Norton) wrote the initial review protocol. The same two review authors (SW and CN) examined all citations and abstracts derived from the electronic searches and independently screened the trial reports to identify those that met the selection criteria for the review. Both SW and CN reviewed the methodological quality of the included studies. All three authors interpreted the results and contributed to the writing of the final version of the review.
Declarations of interest
Sue Woodward: SW and CN have designed and recently completed an unfunded RCT comparing biofeedback with reflexology for constipation (being prepared for publication).
Christine Norton: CN has been paid an honorarium by Genesis Lt (UK), who market biofeedback equipment, for speaking at an educational event. CN is partly employed by St Mark’s Hospital, Harrow UK, which provides treatment, including biofeedback, for patients with constipation.
Pauline Chiarelli: PC was part of a team of 15 Australian experts from various healthcare professions who developed the recently published 'Guidelines for the management of constipation and faecal impaction in older adults'. The development of these guidelines was instigated by Norgine Pty Ltd under the auspices of the Continence Foundation of Australia. PC was paid an honorarium for this work.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Bleijenberg 1994 {published data only}
- Bleijenberg G, Kuijpers HC. Biofeedback treatment of constipation: a comparison of two methods. American Journal of Gastroenterology 1994;89(7):1021‐6. [PubMed] [Google Scholar]
Chang 2003 {published data only}
- Chang HS, Myung SJ, Yang SK, Jung HY, Kim TH, Yoon IJ, et al. Effect of electrical stimulation in constipated patients with impaired rectal sensation. International Journal of Colorectal Disease 2003;18(5):433‐8. [DOI] [PubMed] [Google Scholar]
Chiarioni 2006 {published data only}
- Chiarioni G, Whitehead WE, Pezza V, Morelli A, Bassotti G. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology 2006;130(3):657‐64. [DOI] [PubMed] [Google Scholar]
Farid 2009 {published data only}
- Farid M, Monem HA, Omar W, Nakeeb AE, Fikry A, Youssef T, et al. Comparative study between biofeedback retraining and botulinum neurotoxin in the treatment of anismus patients. International Journal of Colorectal Disease 2009;24(1):115‐20. [DOI] [PubMed] [Google Scholar]
Faried 2010 {published data only}
- Faried M, Nakeeb A, Youssef M, Omar W, Monem HA. Comparative study between surgical and non‐surgical treatment of anismus in patients with symptoms of obstructed defecation: a prospective randomized study. Journal of Gastrointestinal Surgery 2010;14(8):1235‐43. [DOI] [PubMed] [Google Scholar]
Glia 1997 {published data only}
- Glia A, Gylin M, Gullberg K, Lindberg G. Biofeedback retraining in patients with functional constipation and paradoxical puborectalis contraction: comparison of anal manometry and sphincter electromyography for feedback. Diseases of the Colon and Rectum 1997;40(8):889‐95. [DOI] [PubMed] [Google Scholar]
Hart 2012 {published data only}
- Hart SL, Lee JW, Berian J, Patterson TR, Rosario A, Varma MG. A randomized controlled trial of anorectal biofeedback for constipation. International Journal of Colorectal Disease 2012;27(4):459‐66. [DOI] [PubMed] [Google Scholar]
Heymen 1999 {published data only}
- Heymen S, Wexner SD, Vickers D, Nogueras JJ, Weiss EG, Pikarsky AJ. Prospective, randomized trial comparing four biofeedback techniques for patients with constipation. Diseases of the Colon and Rectum 1999;42(11):1388‐93. [DOI] [PubMed] [Google Scholar]
Heymen 2007 {published data only}
- Heymen S, Scarlett Y, Jones K, Drossman D, Ringel Y, Whitehead WE. Randomized controlled trial shows biofeedback to be superior to alternative treatments for patients with pelvic floor dyssynergia‐type constipation. Gastroenterology 2005;128(4 Suppl 1):A266. [DOI] [PubMed] [Google Scholar]
- Heymen S, Scarlett Y, Jones K, Ringel Y, Drossman D, Whitehead WE. Randomized controlled trial (RCT) shows biofeedback to be superior to alternative treatments for patients with pelvic floor dyssynergia‐type constipation (PFD). American Journal of Gastroenterology 2005;100(9):S335. [DOI] [PubMed] [Google Scholar]
- Heymen S, Scarlett Y, Jones K, Ringel Y, Drossman D, Whitehead WE. Randomized, controlled trial shows biofeedback to be superior to alternative treatments for patients with pelvic floor dyssynergia‐type constipation. Diseases of the Colon and Rectum 2007;50(4):428‐41. [DOI] [PubMed] [Google Scholar]
- Heymen S, Scarlett Y, Whitehead WE. Twelve‐month follow‐up of randomized controlled trial (RCT) comparing biofeedback to alternative treatments for patients with pelvic floor dyssynergia‐type constipation (PFD). Gastroenterology 2008;134(4 Suppl 1):A65‐6. [DOI] [PubMed] [Google Scholar]
Hu 2006 {published data only}
- Hu WH, Li JH, Chan AO, Wong NY, Wong SH, Hui WM. Biofeedback is an effective treatment for functional constipation: a randomized controlled study. Gastroenterology 2006;130(4 Suppl 2):A287. [Google Scholar]
Jung 2007 {published data only}
- Jung KW, Myung SJ, Byeon JS, Min HJ, Yoon IJ, Ko JE, et al. Combined therapy with electrical stimulation and biofeeback in pelvic floor dyssynergia. Neurogastroenterology and Motility 2007;19(Suppl S3):74. [Google Scholar]
- Jung KW, Myung SJ, Byeon JS, Min HJ, Yoon IJ, Ko JE, et al. Electrical stimulation therapy in pelvic floor dyssynergia: prospective, randomized study combined with biofeedback. Journal of Gastroenterology and Hepatology 2007;22(Suppl 2):A209. [Google Scholar]
Koutsomanis 1995 {published data only}
- Koutsomanis D, Lennard‐Jones JE, Roy AJ, Kamm MA. Controlled randomised trial of visual biofeedback versus muscle training without a visual display for intractable constipation. Gut 1995;37(1):95‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsomanis D, Lennard‐Jones JE, Roy AJ, Kamm MA. Controlled randomized trial of biofeedback versus muscle training alone for intractable constipation. Gut 1994;35(4 Suppl):A184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lehur 2008 {published data only}
- Lehur PA, Stuto A, Fantoli M, Villani RD, Queralto M, Lazorthes F, et al. Outcomes of stapled transanal rectal resection vs. biofeedback for the treatment of outlet obstruction associated with rectal intussusception and rectocele: a multicentre, randomized, controlled trial. Diseases of the Colon and Rectum 2008;51(11):1611‐8. [DOI] [PubMed] [Google Scholar]
Pourmomeny 2010 {published data only}
- Pourmomeni A, Emami MH, Amooshahi M, Adibi P. Comparing biofeedback therapy and balloon defecation training in treatment of dyssynergic defecation. Journal of Isfahan Medical School 2010;28(105). [Google Scholar]
Rao 2007 {published data only}
- Rao SS, Kinkade KJ, Schulze KS, Nygaard II, Brown KE, Stumbo PE, et al. Biofeedback therapy (bt) for dyssynergic constipation ‐ randomized controlled trial. Gastroenterology 2005;128(4 Suppl 1):S1853. [Google Scholar]
- Rao SS, Seaton K, Miller M, Brown K, Nygaard I, Stumbo P, et al. Randomized controlled trial of biofeedback, sham feedback and standard therapy for dyssynergic defecation. Clinical Gastroentereology and Hepatology 2007;5(3):331‐8. [DOI] [PubMed] [Google Scholar]
- Rao SS, Seaton K, Schulze KS, Brown K, Zimmerman B. Effects of biofeedback on psychologic profiles and quality of life in dyssynergic defecation (DD) ‐ a randomized controlled trial. Gastroenterology 2007;132(4 Suppl 2):A522. [Google Scholar]
- Rao SS, Valestin J, Brown CK, Zimmerman B, Schulze K. Long‐term efficacy of biodeefback therapy for dyssynergic defecation: randomized controlled trial. American Journal of Gastroenterology 2010;105(4):890‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simon 2009 {published data only}
- Simon MA, Bueno AM. Behavioural treatment of the dyssynergic defecation in chronically constipated elderly patients: a randomized controlled trial. Applied Psychophysiology and Biofeedback 2009;34(4):273‐7. [DOI] [PubMed] [Google Scholar]
You 2001 {published data only}
- You YT, Wang JY, ChangChien CR, Chen JS, Hsu KC, Tang R, et al. The treatment of outlet obstruction constipation. Formosan Journal of Surgery 2001;34:3‐10. [Google Scholar]
References to studies excluded from this review
Battaglia 2004 {published data only}
- Battaglia E, Serra AM, Buonafede G, Dughera L, Chistolini F, Morelli A, et al. Long‐term study on the effects of visual biofeedback and muscle training as a therapeutic modality in pelvic floor dyssynergia and slow‐transit constipation. Diseases of the Colon and Rectum 2004;47(1):90‐5. [DOI] [PubMed] [Google Scholar]
Binnie 1992 {published data only}
- Binnie NR, Papachrysostomou M, Clare N, Smith AN. Solitary rectal ulcer: the place of biofeedback and surgery in the treatment of the syndrome. World Journal of Surgery 1992;16(5):836‐40. [DOI] [PubMed] [Google Scholar]
Chiarioni 2005 {published data only}
- Chiarioni G, Salandini L, Whitehead WE. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology 2005;129(1):86‐97. [DOI] [PubMed] [Google Scholar]
Chiarioni 2007 {published data only}
- Chiarioni G, Whitehead WE, Bassotti G. Randomized control of biofeedback. Clinical Gastroenterology and Hepatology 2007;5(9):1119. [DOI] [PubMed] [Google Scholar]
Chiarioni 2010 {published data only}
- Chiarioni G, Nardo A, Vantini I, Romito A, Whitehead WE. Biofeedback is superior to electrogalvanic stimulation and massage for treatment of levator ani syndrome. Gastroenterology 2010;138(4):1321‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chiotakakou‐Faliakou 1998 {published data only}
- Chiotakakou‐Faliakou E, Kamm MA, Roy AJ, Storrie JB, Turner IC. Biofeedback provides long‐term benefit for patients with intractable, slow and normal transit constipation. Gut 1998;42(4):517‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dallianas 2000 {published data only}
- Dallianas A, Skandalis N, Rimikis MN, Koutsomanis D, Kardasi M, Archimandritis A. Pelvic floor study in patients with obstructive defecation: influence of biofeedback. Journal of Clinical Gastroenterology 2000;30(2):176‐80. [DOI] [PubMed] [Google Scholar]
Emmanuel 2001 {published data only}
- Emmanuel A, Kamm M. Response to a behavioural treatment, biofeedback, in constipated patients is associated with improved gut transit and autonomic innervation. Gut 2001;49(2):214‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Enck 2009 {published data only}
- Enck P, Voort IR, Klosterhalfen S. Biofeedback therapy in fecal incontinence and constipation. Neurogastroenterology and Motility 2009;21(11):1133‐41. [DOI] [PubMed] [Google Scholar]
Ferrara 2001 {published data only}
- Ferrara A, Jesus S, Gallagher JT, Williamson PR, Larach SW, Pappas D, et al. Time‐related decay of the benefits of biofeedback therapy. Techniques in Coloproctology 2001;5(3):131‐5. [DOI] [PubMed] [Google Scholar]
Horton 2008 {published and unpublished data}
- Horton N. A randomized controlled trial of biofeedback versus laxatives for chronic idiopathic constipation in women. UK Clinical Trials Gateway 2008. [ISRCTN35017966] [Google Scholar]
Keck 1994 {published data only}
- Keck JO, Staniunas RJ, Coller JA, Barrett RC, Oster ME, Schoetz DJ, et al. Biofeedback training is useful in fecal incontinence but disappointing in constipation. Diseases of the Colon and Rectum 1994;37(12):1271‐6. [DOI] [PubMed] [Google Scholar]
Lin 2005 {published data only}
- Lin Z, Lin L, Zhang HJ, Wang MF. Social psychological and behaviour characteristics of patients with functional constipation and the follow‐up of the effects of biofeedback therapy [Chinese]. Chinese Journal of Clinical Rehabilitation 2005;9(28):67‐9. [Google Scholar]
NCT00564707 {published data only}
- NCT00564707. Comparison of biofeedback therapy and botulinum toxin type A injections for treatment of painful levator ani syndrome in women: a randomized, prospective trial. clinicaltrials.gov/ct2/show/NCT00564707 (accessed 22 January 2014).
Park 2003 {published data only}
- Park DH, Myung SJ, Yoon IJ, Kwon OR, Ko JE, Chang HS, et al. Clinical factors associated with response to biofeedback therapy for patients with chronic constipation [Korean]. Korean Journal of Gastroenterlogy 2003;42(4):289‐6. [PubMed] [Google Scholar]
Patankar 1997 {published data only}
- Patankar SK, Ferrara A, Larach SW, Williamson PR, Perozo SE, Levy JR, et al. Electromyographic assessment of biofeedback training for fecal incontinence and chronic constipation. Diseases of the Colon and Rectum 1997;40(8):907‐11. [DOI] [PubMed] [Google Scholar]
Roy 2000 {published data only}
- Roy AJ, Emmanuel AV, Storrie JB, Bowers J, Kamm MA. Behavioural treatment (biofeedback) for constipation following hysterectomy. British Journal of Surgery 2000;87(1):100‐5. [DOI] [PubMed] [Google Scholar]
Siproudhis 1995 {published data only}
- Siproudhis L, Dautreme S, Ropert A, Briand H, Renet C, Beusnel C, et al. Anismus and biofeedback: who benefits?. European Journal of Gastroenterology and Hepatology 1995;7(6):547‐52. [PubMed] [Google Scholar]
Wiesel 2001 {published data only}
- Wiesel PH, Dorta G, Cuypers P, Herranz M, Kreis ME, Schnegg JF, et al. Patient satisfaction after biofeedback for constipation and pelvic floor dyssynergia. Swiss Medical Weekly 2001;131(11‐12):152‐6. [DOI] [PubMed] [Google Scholar]
Yang 2004 {published data only}
- Yang LM, Lin JB, Zhao YL, Liang JL, Lin H, Zhong Z, et al. Effects of biofeedback training by EMG on patients with chronic functional constipation. World Chinese Journal of Digestology 2004;12(3):730‐3. [Google Scholar]
References to studies awaiting assessment
Singles 1990 {published data only}
- Singles JM. Evaluation of a biofeedback‐based behaviour modification program for the treatment of chronic constipation [Thesis]. Norfolk, VA, USA: Old Dominion University, 1990. [Google Scholar]
References to ongoing studies
NCT00982839 {published data only}
- NCT00982839. Rectal sensory training ‐ a randomized controlled study of two techniques. clinicaltrials.gov/ct2/show/NCT00982839 (accessed 22 January 2014).
NCT01672216 {published data only}
- NCT01672216. Multicentre randomized controlled trial to compare the outcome of conservative triple target treatment with EMG‐biofeedback in chronic constipation (3T‐CO). clinicaltrials.gov/ct2/show/NCT01672216 (accessed 22 January 2014).
Additional references
Azpiroz 2002
- Azpiroz F, Enck P, Whitehead WE. Anorectal functional testing: review of collective experience. American Journal of Gastroenterology 2002;97(20):232‐40. [DOI] [PubMed] [Google Scholar]
Bassotti 1997
- Bassotti G, Whitehead WE. Biofeedback, relaxation training and cognitive behaviour modification as treatments for lower functional gastrointestinal disorders. QJM 1997;90(8):545‐50. [DOI] [PubMed] [Google Scholar]
Bassotti 2004
- Bassotti G, Chistolini F, Sietchiping‐Nzepa F, Roberto G, Morelli A, Chiarioni G. Biofeedback for pelvic floor dysfunction in constipation. BMJ 2004;328(7436):393‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bharucha 2010
- Bharucha AE, Wald AM. Anorectal disorders. American Journal of Gastroenterology 2010;105(4):786‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brandt 2005
- Brandt LJ, Prather CM, Quigley EM, Schiller LR, Schoenfeld P, Talley NJ. Systematic review on the management of chronic constipation in North America. American Journal of Gastroenterology 2005;100(Suppl 1):S5‐S21. [DOI] [PubMed] [Google Scholar]
Cheng 2003
- Cheng C, Chan AO, Hui WM, Lam SK. Coping strategies, illness perception, anxiety and depression of patients with idiopathic constipation: a population‐based study. Alimentary Pharmacology and Therapeutics 2003;18(3):319‐26. [DOI] [PubMed] [Google Scholar]
Chiarelli 2008
- Chiarelli P. Systematic review: the management of constipation using physical therapies including biofeedback. Australian and New Zealand Continence Journal 2008;14(1):6‐13. [Google Scholar]
Chiarioni 2008
- Chiarioni G, Whitehead WE. The role of biofeedback in the treatment of gastrointestinal disorders. Gastroenterology and Hepatology 2008;5(7):371‐82. [DOI] [PubMed] [Google Scholar]
Chiotakaku‐Faliakou 1998
- Chiotakakou‐Faliakou E, Kamm MA, Roy AJ, Storrie JB, Turner IC. Biofeedback provides long term benefit for patients with intractable, slow and normal transit constipation. Gut 1998;42(4):517‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chronic Constipation Task Force 2005
- American College of Gastroenterology Chronic Constipation Task Force. An evidence‐based approach to the management of chronic constipation in North America. American Journal of Gastroenterology 2005;100(Suppl 1):S1‐4. [DOI] [PubMed] [Google Scholar]
Coulter 2002
- Coulter ID, Favreau JT, Hardy ML, Morton SC, Roth EA, Shekelle P. Biofeedback interventions for gastrointestinal conditions: a systematic review. Alternative Therapies in Health and Medicine 2002;8(3):76‐83. [PubMed] [Google Scholar]
Denis 1981
- Denis PB, Cayron G, Galmiche JP. Biofeedback; the light at the end of the tunnel? Maybe for constipation. Gastroenterology 1981;80(5 Pt 1):1089‐90. [PubMed] [Google Scholar]
Denis 1996
- Denis P. Methodology of biofeedback. European Journal of Gastroenterology and Hepatology 1996;8(6):530‐3. [DOI] [PubMed] [Google Scholar]
Enck 1993
- Enck P. Biofeedback training in disordered defecation: a critical review. Digestive Diseases and Sciences 1993;38(11):1953‐60. [DOI] [PubMed] [Google Scholar]
Enck 1996
- Enck P, Schafer R. Biofeedback applications in gastroenterology. European Journal of Gastroenterology and Hepatology 1996;8(6):534‐9. [DOI] [PubMed] [Google Scholar]
Fleshman 1992
- Fleshman JW, Dreznik Z, Meyer K, Fry RD, Carney R, Kodner IJ. Outpatient protocol for biofeedback therapy of pelvic floor outlet obstruction. Diseases of the Colon and Rectum 1992;35(1):1‐7. [DOI] [PubMed] [Google Scholar]
Frank 1999
- Frank L, Kleinman L, Farup C, Taylor L, Miner P. Psychometric validation of a constipation symptom assessment questionnaire. Scandanavian Journal of Gastroenterology 1999;34(9):870‐7. [DOI] [PubMed] [Google Scholar]
Gilliland 1997
- Gilliland R, Heymen S, Altomare DF, Park UC, Vickers D, Wexner S. Outcome and predictors of success of biofeedback for constipation. British Journal of Surgery 1997;84(8):1123‐6. [PubMed] [Google Scholar]
Guyatt 2008a
- Guyatt G, Rennie D, Meade MO, Cook DJ. User’s Guide to the Medical Literature: Essentials of Evidence Based Medicine. 2nd Edition. New York: JAMA Evidence, 2008. [Google Scholar]
Guyatt 2008b
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heymen 2003
- Heymen S, Jones KR, Scarlett Y, Whitehead WE. Biofeedback treatment for constipation: a critical review. Diseases of the Colon and Rectum 2003;46(9):1208‐17. [DOI] [PubMed] [Google Scholar]
Higgins 2004
- Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. American Journal of Gastroenterology 2004;99(4):750‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Irvine 2002
- Irvine EJ, Ferrazzi S, Pare P, Thompson WG, Rance L. Health‐related quality of life in functional GI disorders: focus on constipation and resource utilization. American Journal of Gastroenterology 2002;97(8):1986‐93. [DOI] [PubMed] [Google Scholar]
Jorge 2003
- Jorge JMN, Habr‐Gama A, Wexner SD. Biofeedback therapy in the colon and rectal practice. Applied Psychophysiology and Biofeedback 2003;28(1):47‐61. [DOI] [PubMed] [Google Scholar]
Koh 2008
- Koh CE, Young CJ, Young JM, Solomon MJ. Systematic review of randomized controlled trials of the effectiveness of biofeedback for pelvic floor dysfunction. British Journal of Surgery 2008;95(9):1079‐87. [DOI] [PubMed] [Google Scholar]
Koutsomanis 1994
- Koutsomanis D, Lennard‐Jones JE, Kamm M. Prospective study of biofeedback treatment for patients with slow and normal transit constipation. European Journal of Gastroenterology and Hepatology 1994;6(2):131‐7. [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Lestar 1991
- Lestàr B, Pennickx F, Kerremans R. Biofeedback defaecation training for anismus. International Journal of Colorectal Disease 1991;6(4):202‐7. [DOI] [PubMed] [Google Scholar]
Longstreth 2006
- Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 2006;130(5):1480‐91. [DOI] [PubMed] [Google Scholar]
Mason 2002
- Mason HJ, Serrano‐Ikkos E, Kamm MA. Psychological state and quality of life in patients having behavioural treatment (biofeedback) for intractable constipation. American Journal of Gastroenterology 2002;97(12):3154‐9. [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomised trials. Lancet 2001;357(9263):1191‐4. [PubMed] [Google Scholar]
Norton 2012
- Norton C, Cody JD. Biofeedback and/or sphincter exercises for the treatment of faecal incontinence in adults. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD002111.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Palsson 2004
- Palsson OS, Heymen S, Whitehead WE. Biofeedback treatment for functional anorectal disorders: a comprehensive efficacy review. Applied Psychophysiology and Biofeedback 2004;29(3):153‐74. [DOI] [PubMed] [Google Scholar]
Papachrysostomou 1994
- Papachrysostomou M, Smith AN. Effects of biofeedback on obstructive defecation‐‐reconditioning of the defecation reflex?. Gut 1994;35(2):252‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rao 1997
- Rao SS, Welcher KD, Pelsang RE. Effects of biofeedback therapy on anorectal function in obstructive defecation. Digestive Diseases and Sciences 1997;42(11):2197‐205. [DOI] [PubMed] [Google Scholar]
Rao 1998
- Rao SS, Welcher KD, Leistikow JS. Obstructive defecation: a failure of rectoanal coordination. American Journal of Gastroenterology 1998;93(7):1042‐50. [DOI] [PubMed] [Google Scholar]
Rao 2008
- Rao SS. Dyssynergic defecation and biofeedback therapy. Gastroenterology Clinics of North America 2008;37(3):549‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rao 2010
- Rao SS, Valestin J, Brown CK, Zimmerman B, Schulze K. Long‐term efficacy of biodeefback therapy for dyssynergic defecation: randomized controlled trial. American Journal of Gastroenterology 2010;105(4):890‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rhee 2000
- Rhee PL, Choi MS, Kim YH, Son HJ, Kim JJ, Koh KC, et al. An increased rectal maximum tolerable volume and long anal canal are associated with poor short‐term response to biofeedback therapy for patients with anismus with decreased bowel frequency and normal colonic transit time. Diseases of the Colon and Rectum 2000;43(10):1405‐11. [DOI] [PubMed] [Google Scholar]
Rieger 1997
- Rieger NA, Wattchow DA, Sarre RG, Saccone GT, Rich CA, Cooper SJ, et al. Prospective study of biofeedback for treatment of constipation. Diseases of the Colon and Rectum 1997;40(10):1143‐8. [DOI] [PubMed] [Google Scholar]
Schiller 2001
- Schiller LR. Review article: the therapy of constipation. Alimentary Pharmacology and Therapeutics 2001;15(6):749‐63. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Scott 2008
- Scott SM, Gladman MA. Manometric, sensorimotor, and neurophysiologic evaluation of anorectal function. Gastroenterology Clinics of North America 2008;37(3):511‐38. [DOI] [PubMed] [Google Scholar]
Shin 2010
- Shin JK, Cheon JH, Kim ES, Yoon JY, Lee JH, Jeon SM, et al. Predictive capability of anorectal physiologic tests for unfavourable outcomes following biofeedback therapy in dyssynergic defecation. Journal of the Korean Medical Science 2010;25(7):1060‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simren 2001
- Simrén M, Abrahamsson H, Svedlund J, Björnsson ES. Quality of life in patients with irritable bowel syndrome seen in referral centres versus primary care: the impact of gender and predominant bowel pattern. Scandanavian Journal of Gastroenterology 2001;36(5):545‐52. [DOI] [PubMed] [Google Scholar]
Simren 2006
- Simrén M, Svedlund J, Posserud I, Björnsson ES, Abrahamsson H. Health‐related quality of life in patients attending a gastroenterology outpatient clinic: functional versus organic diseases. Clinical Gastroenterology and Hepatology 2006;4(2):187‐95. [DOI] [PubMed] [Google Scholar]
Thompson 1999
- Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Mueller‐Lissner SA. Functional Bowel disorders and functional abdominal pain. Gut 1999;45(Suppl 11):1143‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Baal 1983
- Baal JG, Leguit P, Brummelkamp WH. Relaxation biofeedback conditioning as treatment of a disturbed defecation reflex: Report of a case. Diseases of the Colon and Rectum 1983;27(3):187‐9. [DOI] [PubMed] [Google Scholar]
Wexner 1992
- Wexner SD, Cheape JD, Jorge JMN, Heymen S, Jagelman DG. Prospective assessment of biofeedback for the treatment of paradoxical puborectalis contraction. Diseases of the Colon and Rectum 1992;35(2):145‐50. [DOI] [PubMed] [Google Scholar]
Whitehead 2010
- Whitehead WE, Bharucha AE. Diagnosis and treatment of pelvic floor disorders: what’s new and what to do. Gastroenterology 2010;138(4):1231‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2010
- Wong RK, Palsson OS, Turner MJ, Levy RL, Feld AD, Korff M, et al. Inability of the Rome III criteria to distinguish functional constipation from constipation‐subtype irritable bowel syndrome. American Journal of Gastroenterology 2010;105(10):2228‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2008
- Zhou LK, Chang M, Ke MY. Systematic review of biofeedback for adult chronic constipation. Journal of Gastroenterology and Hepatology 2008;23(Suppl 5):A115. [Google Scholar]


