Abstract
Purpose:
This phase Ib study defined the safety, MTD, and recommended phase II dose (RP2D) of regorafenib combined with vincristine and irinotecan (VI). Secondary objectives were evaluation of antitumor activity and pharmacokinetics (PK) of regorafenib and irinotecan.
Patients and Methods:
Patients aged 6 months to <18 years with relapsed/refractory solid malignancies [≥50% with rhabdomyosarcoma (RMS)] received regorafenib (starting dose 72 mg/m2/day) concomitantly or sequentially with vincristine 1.5 mg/m2 on days 1 and 8, and irinotecan 50 mg/m2 on days 1–5 (21-day cycle). Adverse events (AE) and tumor response were assessed. PK (regorafenib and irinotecan) were evaluated using a population PK model.
Results:
We enrolled 21 patients [median age, 10 years; 12, RMS; 5, Ewing sarcoma (EWS)]. The MTD/RP2D of regorafenib in the sequential schedule was 82 mg/m2. The concomitant dosing schedule was discontinued because of dose-limiting toxicities in 2 of 2 patients treated. Most common grade 3/4 (>30% of patients) AEs were neutropenia, anemia, thrombocytopenia, and leukopenia. The overall response rate was 48% and disease control rate [complete response (CR)/partial response/stable disease/non-CR/non-progressive disease] was 86%. Median progression-free survival was 7.0 months [95% confidence interval (CI), 2.9–14.8] and median overall survival was 8.7 months (95% CI, 5.5–16.3). When combined with VI, regorafenib PK was similar to single-agent PK in children and adults (treated with regorafenib 160 mg/day).
Conclusions:
Regorafenib can be combined sequentially with standard dose VI in pediatric patients with relapsed/refractory solid tumors with appropriate dose modifications. Clinical activity was observed in patients with RMS and EWS (ClinicalTrials.gov NCT02085148).
Translational Relevance.
Preclinical and translational studies have shown that regorafenib exhibits potent inhibitory activity against several kinases involved in the etiology of pediatric solid tumors and antitumor activity as a single agent or in combination with standard treatments in preclinical models of these tumors. Regorafenib inhibits key targets relevant in rhabdomyosarcoma (RMS) treatment and has shown preliminary antitumor activity in a phase I monotherapy study. Regorafenib's largely non-myelosuppressive safety profile also makes it a potential candidate for combination with chemotherapy. Our results show that the combination of regorafenib with standard doses of vincristine and irinotecan exhibits clinical activity in pediatric patients with RMS and Ewing sarcoma. The sequential dosing schedule was tolerable with appropriate dosing modifications.
Introduction
Regorafenib is an oral, multi-targeted tyrosine kinase inhibitor (TKI) that targets a broad range of angiogenic, stromal, and oncogenic kinases. Regorafenib is approved for the treatment of adults with metastatic colorectal cancer, advanced gastrointestinal stromal tumors, and hepatocellular carcinoma (1, 2). Kinases inhibited by regorafenib, including VEGFR, platelet-derived growth factor receptor (PDGFR), FGFRs, c-KIT, and RAF-1 (3, 4), are involved in the etiology of multiple pediatric solid tumors (5–12). Antitumor activity of regorafenib alone or in combination with standard treatments has been demonstrated in preclinical models of Ewing sarcoma (EWS), neuroblastoma, medulloblastoma, osteosarcoma, and rhabdomyosarcoma (RMS; ref. 13). Regorafenib moderately inhibited RMS cell lines in vitro and significantly delayed tumor growth in vivo, particularly when combined with DNA-damaging agents or radiotherapy in PDGFRA-amplified tumors (13).
RMS is the most common pediatric soft tissue sarcoma (14). Long-term survival of patients with RMS has improved with the use of multimodal therapy (15); however, patients who do not respond to initial therapy and those who relapse have a poor prognosis (16). New systemic regimens, including targeted therapies with a biologic rationale, are urgently needed. In addition to potent inhibition of VEGFR (3, 4), regorafenib's inhibitory activity against several other biologic targets relevant in RMS, including the key targets, FGFR and PDGFR (17–21), offers an advantage over other TKIs. In addition, its non-myelosuppressive safety profile makes it a potential candidate for combination with chemotherapy (22, 23).
In the dose-escalation part of this phase I study, the MTD and recommended phase II dose (RP2D) of regorafenib monotherapy in children with recurrent or refractory solid malignancies was 82 mg/m2, with a safety profile and mean exposure similar to the recommended dose in adults (24). Preliminary evidence of antitumor activity was observed, including one patient with an unconfirmed partial response (PR) and one with prolonged stable disease (SD) among three patients with RMS. Based on these results, we undertook a phase Ib dose-expansion study to evaluate regorafenib in combination with vincristine and irinotecan (VI) in pediatric patients with recurrent/refractory RMS and other solid tumors.
Patients and Methods
Study design and patients
This open-label, phase Ib, dose-expansion study explored the new combination by incorporating its own dose-escalation component, and was conducted in patients aged 6 months to <18 years with relapsed/refractory solid tumors for which treatment with VI was considered appropriate. The study was approved by ethics committees and medical authorities and/or institutional review boards and conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. All patients and their parents or legal guardians provided written informed consent and age-appropriate assent.
Eligible patients had evaluable or measurable disease, performance status ≥70% (Karnofsky/Lansky), life expectancy ≥12 weeks, and adequate hematologic, renal, and hepatic function. Patients who had received prior vincristine and/or irinotecan were eligible even if they had progressed on these agents. In accordance with the European Medicines Agency–approved regorafenib Pediatric Investigation Plan, ≥50% of patients were required to have RMS.
Patients were excluded if they had previously received regorafenib, chemotherapy within 4 weeks or five elimination half-lives, active infection or uncontrolled hypertension, major surgery or radiotherapy within 28 days, clinically significant bleeding within 30 days, or an arterial or venous thrombotic event within 6 months.
Patients were randomly assigned to one of two cohorts, in which regorafenib was administered concomitantly on days 1–14 (cohort 1a) or sequentially on days 8–21 (cohort 1b) with VI (intravenous irinotecan 50 mg/m2 on days 1–5 and vincristine 1.5 mg/m2 on days 1 and 8) in a 21-day cycle (Supplementary Fig. S1). Prophylactic oral cefixime was recommended. Treatment was continued for up to 12 cycles (treatment beyond 12 cycles was possible after discussion with the sponsor). Patients were enrolled using a “rolling 6” design, in which cohorts of up to six evaluable patients received oral regorafenib 72 or 82 mg/m2/day (60 or 65 mg/m2/day if aged 6 months to <2 years) once daily. These doses were selected based on the results of the dose-escalation (monotherapy) phase of the study, in which escalation was stopped at 93 and 82 mg/m2, established as the MTD in patients aged 3–17 years (24). The starting dose was 72 mg/m2 as regorafenib was being administered in combination with chemotherapy, and the safety of TKI combinations in children had not yet been established. In addition, this dose provided similar exposure to 82 mg/m2, with high interpatient variability. Regorafenib was available as 20-mg tablets or 5- and 20-mg granules.
Study objectives
The primary objective of the dose-expansion part of the study was to define the safety profile, MTD, and RP2D of regorafenib in combination with VI. Secondary objectives were to evaluate antitumor activity and the pharmacokinetics (PK) of regorafenib (in combination with VI) and irinotecan.
Assessments
Adverse events (AE) were summarized using MedDRA v22.1 (severity graded by National Cancer Institute Common Terminology Criteria for Adverse Events v4.0 criteria). Attributions of AE causality were assessed by the investigator, with specific guidance for known regorafenib-related AEs of hand–foot skin reaction (HFSR), hypertension, and transaminase increases. Dose-limiting toxicities (DLT) were defined as treatment-related hematologic toxicity with prespecified severity and/or duration, or non-hematologic grade 3/4 toxicity during cycle 1 (Supplementary Data S1: Definition of DLT; ref. 24).
Tumor assessments were carried out at screening and every 6 weeks until tumor progression or discontinuation due to unacceptable toxicity, withdrawal of consent, or death. After 12 cycles of study treatment, tumor assessment could be carried out every 3 months (±14 days) for one additional year, and thereafter as clinically indicated until disease progression. Best response was evaluated using Response Evaluation Criteria in Solid Tumors v1.1 (25) or International Neuroblastoma Response Criteria for patients with neuroblastoma (26, 27), per investigator's assessment (confirmation of responses was not required for this secondary endpoint). An overall response of SD required a duration of 42 days.
PK
PK of regorafenib administered in combination with VI was evaluated using a population PK model based on adult data (28). To investigate whether regorafenib alters irinotecan clearance through a drug–drug interaction, the PK profile of irinotecan was evaluated using a population PK model for irinotecan and its metabolites in pediatric patients (29). This was modified to include only compartments for irinotecan and metabolite SN-38 (29). A comprehensive description of PK methods is provided in Supplementary Data S1.
Statistical analysis
In this exploratory study, it was anticipated that ≥15 patients would be needed to define the MTD; approximately 8 patients with RMS had to be evaluable for tumor response (≥1 regorafenib dose and either ≥1 post-baseline evaluation or prior progression) and ≥6 patients with RMS were to receive the RP2D. The MTD analysis set included all patients who completed cycle 1 or discontinued treatment due to an AE or DLT. Median time from first treatment to progression or death [progression-free survival (PFS) and overall survival (OS)] was summarized using descriptive statistics only.
Data availability
The availability of the data underlying this publication will be determined according to Bayer's commitment to the EFPIA/PhRMA “Principles for responsible clinical trial data sharing”. This pertains to scope, time point, and process of data access. As such, Bayer commits to sharing upon request from qualified scientific and medical researchers’ patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the USA and EU as necessary for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the EU and US regulatory agencies on or after January 1, 2014. Interested researchers can use www.vivli.org to request access to anonymized patient-level data and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the member section of the portal. Data access will be granted to anonymized patient-level data, protocols, and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.
Results
Patients
The dose-expansion phase of the study was conducted at 10 centers in France, Italy, Spain, and the United Kingdom. Between January 2018 and February 2019, 21 patients were enrolled and treated. The median age was 10 years (range, 1.5–17.0 years; one patient was aged <2 years), 13 patients (62%) were male, and 12 had RMS and 5 had EWS (Table 1). All patients had received prior chemotherapy, including vincristine, 10 (48%) had received prior irinotecan, 16 (76%) had received >1 line of therapy, and 18 (86%) had prior radiotherapy (Table 1). As of the cutoff date for this analysis (February 1, 2021), 18 patients had discontinued treatment, 1 had completed the originally prescribed 12 cycles of treatment, and treatment was ongoing in 2 patients (both RMS). Of the 19 patients who terminated treatment, 5 were still alive at the data cutoff date.
Table 1.
Baseline demographics and disease characteristics.
| Regorafenib 72 mg/m2 with VI (CON; n = 2) | Regorafenib 72 mg/m2 with VI (SEQ; n = 6) | Regorafenib 82 mg/m2 with VI (SEQ; n = 13) | Total (N = 21) | |
|---|---|---|---|---|
| Male sex, n (%) | 2 (100) | 4 (67) | 7 (54) | 13 (62) |
| Median age, years (range) | 14 (10–17) | 12 (5–15) | 10 (1.5–16) | 10 (1.5–17) |
| Aged <2 years, n (%) | 0 | 0 | 1 (8) | 1 (5) |
| Median BSA, m2 (range) | 1.3 (0.9–1.7) | 1.3 (0.7–1.5) | 1.1 (0.5–1.9) | 1.1 (0.5–1.9) |
| Performance statusa, n (%) | ||||
| 100% | 1 (50) | 3 (50) | 7 (54) | 11 (52) |
| 90% | 0 | 3 (50) | 4 (31) | 7 (33) |
| 70%–80% | 1 (50) | 0 | 2 (15) | 3 (14) |
| Cancer type, n (%) | ||||
| RMS | 1 (50) | 1 (17) | 10 (77) | 12 (57) |
| Alveolar RMS | 0 | 0 | 8 (62) | 8 (38) |
| Embryonal RMS | 1 (50) | 0 | 2 (15) | 3 (14) |
| NOS | 0 | 1 (17) | 0 | 1 (5) |
| Ewing sarcoma | 1 (50) | 4 (67) | 0 | 5 (24) |
| Neuroblastoma | 0 | 1 (17) | 2 (15) | 3 (14) |
| Wilms tumor | 0 | 0 | 1 (8) | 1 (5) |
| Disease status, n (%) | ||||
| Relapsing | 1 (50) | 2 (33) | 10 (77) | 13 (62) |
| Refractory | 1 (50) | 3 (50) | 2 (15) | 6 (29) |
| Relapsing and refractory | 0 | 1 (17) | 1 (8) | 2 (10) |
| Tumor extent at study entry, n (%) | ||||
| Localized disease | 1 (50) | 3 (50) | 3 (23) | 7 (33) |
| Locally advanced | 0 | 1 (17) | 2 (15) | 3 (14) |
| Metastatic disease | 2 (100) | 5 (83) | 9 (69) | 16 (76) |
| Median time in weeks since initial diagnosis (range) | 222 (n = 1) | 80 (42–214; n = 6) | 81 (22–349; n = 12) | 86 (22–349; n = 19) |
| Median time in weeks since most recent progression (range) | 11 (n = 1) | 6 (2–10; n = 6) | 2 (0–13; n = 12) | 3 (0–13; n = 20) |
| Median number of lines of prior therapy | 3.5 | 2.0 | 2.0 | 2.0 |
| Prior anticancer therapies, n (%) | ||||
| Any systemic therapy | 2 (100) | 6 (100) | 13 (100) | 21 (100) |
| Vincristine | 2 (100) | 6 (100) | 13 (100) | 21 (100) |
| Irinotecan | 2 (100) | 2 (33) | 6 (46) | 10 (48) |
| >1 line of systemic treatment | 2 (100) | 5 (83) | 8 (62) | 15 (71) |
| High-dose chemotherapy/ASCR | 0 | 2 (33) | 0 | 2 (10) |
| Any radiotherapy | 2 (100) | 5 (83) | 11 (85) | 18 (86) |
| Any surgical therapeutic procedure | 2 (100) | 4 (67) | 8 (62) | 14 (67) |
Note: Percentages may not total 100% due to rounding.
Abbreviations: ASCR, autologous stem cell rescue; BSA, body surface area; CON, concomitant; NOS, not otherwise specified; RMS, rhabdomyosarcoma; SEQ, sequential; VI, vincristine and irinotecan.
aKarnofsky if aged >12 years; Lansky if aged ≤12 years.
Treatment
Among the 21 treated patients, two received concomitant regorafenib 72 mg/m2, and 19 received sequential regorafenib at 72 mg/m2 (n = 6) or 82 mg/m2 (n = 13). The median number of cycles administered was 4 (range, 1–37+); median duration of treatment, including time off drug or interruptions, was 98 days (range, 12–837+ days). Actual median daily dose was 86% (range, 70%–107%) of the median planned dose for regorafenib, 95% (range, 70%–107%) for irinotecan, and 89% (range, 41%–109%) for vincristine. Dose reductions were required in 7 (33%), 14 (67%), and 1 (5%) patient for regorafenib, irinotecan, and vincristine, respectively.
AEs
All patients experienced ≥1 treatment-emergent AE and ≥1 treatment-related AE (Supplementary Table S1). The most common (>60% of patients) AEs were diarrhea, neutropenia, anemia, abdominal pain, vomiting, and pyrexia (Table 2). The most common (>20% of patients) grade 3/4 AEs were neutropenia, anemia, leukopenia, thrombocytopenia, and increased alanine aminotransferase (ALT; Table 2). Treatment-related AEs led to dose modification of ≥1 of the three study drugs in 18 (86%) patients, and permanent discontinuation of ≥1 study drug was required for 3 (14%) patients (Supplementary Table S1). Eight (38%) patients experienced serious AEs. No treatment-related grade 5 AEs occurred (Supplementary Table S1). Neutropenia (52%), anemia (43%), and increased aspartate aminotransferase (AST; 43%) were the most common AEs considered potentially related to regorafenib (Supplementary Table S2). Grade 2 hypothyroidism was reported in 2 (10%) patients who received sequential regorafenib at 82 mg/m2, and grade 1 increased thyroid stimulating hormone was reported in 4 patients. Regorafenib-related HFSR (MedDRA term—palmar–plantar erythrodysesthesia) was reported in 3 patients (grade 1/2 in two; grade 3 in one). AEs considered potentially related to VI are reported in Supplementary Table S3.
Table 2.
Treatment-emergent AEs with an incidence ≥20% (safety analysis set).
| Regorafenib 72 mg/m2 with VI (CON; n = 2) | Regorafenib 72 mg/m2 with VI (SEQ; n = 6) | Regorafenib 82 mg/m2 with VI (SEQ; n = 13) | Total (N = 21) | |||||
|---|---|---|---|---|---|---|---|---|
| Event, n (%) | All grades | Grade ≥3 | All grades | Grade ≥3 | All grades | Grade ≥3 | All grades | Grade ≥3 |
| Diarrhea | 2 (100) | 0 | 5 (83) | 1 (17) | 12 (92) | 3 (23) | 19 (90) | 4 (19) |
| Anemia | 2 (100) | 0 | 4 (67) | 1 (17) | 9 (69) | 6 (46) | 15 (71) | 7 (33) |
| Neutropeniaa | 2 (100) | 2 (100) | 6 (100) | 6 (100) | 7 (54) | 7 (54) | 15 (71) | 15 (71) |
| Abdominal pain | 2 (100) | 2 (100) | 4 (67) | 1 (17) | 8 (62) | 1 (8) | 14 (67) | 4 (19) |
| Pyrexia | 1 (50) | 0 | 4 (67) | 0 | 8 (62) | 1 (8) | 13 (62) | 1 (5) |
| Vomiting | 2 (100) | 2 (100) | 3 (50) | 0 | 8 (62) | 2 (15) | 13 (62) | 4 (19) |
| Decreased appetite | 2 (100) | 1 (50) | 5 (83) | 0 | 5 (38) | 1 (8) | 12 (57) | 2 (10) |
| Thrombocytopeniab | 1 (50) | 1 (50) | 4 (67) | 2 (33) | 7 (54) | 4 (31) | 12 (57) | 7 (33) |
| Nausea | 1 (50) | 1 (50) | 3 (50) | 1 (17) | 6 (46) | 0 | 10 (48) | 2 (10) |
| ALT increased | 2 (100) | 2 (100) | 3 (50) | 2 (33) | 4 (31) | 1 (8) | 9 (43) | 5 (24) |
| AST increased | 2 (100) | 2 (100) | 3 (50) | 2 (33) | 4 (31) | 0 | 9 (43) | 4 (19) |
| Dry skin | 0 | 0 | 2 (33) | 0 | 6 (46) | 0 | 8 (38) | 0 |
| Hypokalemia | 2 (100) | 1 (50) | 1 (17) | 0 | 5 (38) | 3 (23) | 8 (38) | 4 (19) |
| Leukopeniac | 1 (50) | 1 (50) | 3 (50) | 3 (50) | 4 (31) | 3 (23) | 8 (38) | 7 (33) |
| Rash | 2 (100) | 0 | 3 (50) | 0 | 3 (23) | 0 | 8 (38) | 0 |
| Weight decreased | 1 (50) | 1 (50) | 2 (33) | 0 | 5 (38) | 0 | 8 (38) | 1 (5) |
| GGT increased | 2 (100) | 1 (50) | 2 (33) | 1 (17) | 3 (23) | 0 | 7 (33) | 2 (10) |
| Hypophosphatemia | 2 (100) | 1 (50) | 2 (33) | 1 (17) | 3 (23) | 1 (8) | 7 (33) | 3 (14) |
| Alopecia | 0 | 0 | 2 (33) | 0 | 4 (31) | 0 | 6 (29) | 0 |
| Fatigue | 2 (100) | 0 | 2 (33) | 0 | 2 (15) | 0 | 6 (29) | 0 |
| Headache | 1 (50) | 0 | 2 (33) | 0 | 2 (15) | 0 | 5 (24) | 0 |
| Cough | 1 (50) | 0 | 2 (33) | 0 | 2 (15) | 0 | 5 (24) | 0 |
| Pain | 0 | 0 | 1 (17) | 0 | 4 (31) | 1 (8) | 5 (24) | 1 (5) |
| Proteinuria | 1 (50) | 0 | 2 (33) | 0 | 2 (15) | 0 | 5 (24) | 0 |
| Constipation | 1 (50) | 0 | 1 (17) | 0 | 2 (15) | 0 | 4 (19) | 0 |
| TSH increased | 0 | 0 | 2 (33) | 0 | 2 (15) | 0 | 4 (19) | 0 |
| Hypocalcemia | 1 (50) | 0 | 0 | 0 | 3 (23) | 1 (8) | 4 (19) | 1 (5) |
| Epistaxis | 0 | 0 | 1 (17) | 0 | 3 (23) | 0 | 4 (19) | 0 |
| Hypertension | 0 | 0 | 2 (33) | 0 | 2 (15) | 0 | 4 (19) | 0 |
| Hyponatremia | 1 (50) | 0 | 1 (17) | 1 (17) | 2 (15) | 0 | 4 (19) | 1 (5) |
| Lethargy | 0 | 0 | 2 (33) | 0 | 2 (15) | 0 | 4 (19) | 0 |
| Myalgia | 0 | 0 | 2 (33) | 0 | 2 (15) | 0 | 4 (19) | 0 |
| Rash maculopapular | 1 (50) | 0 | 1 (17) | 1 (17) | 2 (15) | 0 | 4 (19) | 1 (5) |
| Abdominal distension | 0 | 0 | 1 (17) | 0 | 2 (15) | 0 | 3 (14) | 0 |
| Asthenia | 0 | 0 | 1 (17) | 0 | 2 (15) | 1 (8) | 3 (14) | 1 (5) |
| Blood bilirubin increased | 1 (50) | 0 | 1 (17) | 0 | 1 (8) | 0 | 3 (14) | 0 |
| Blood creatinine increased | 0 | 0 | 0 | 0 | 3 (23) | 1 (8) | 3 (14) | 1 (5) |
| Dyspepsia | 1 (50) | 0 | 1 (17) | 0 | 1 (8) | 0 | 3 (14) | 0 |
| Febrile neutropenia | 1 (50) | 1 (50) | 1 (17) | 1 (17) | 1 (8) | 1 (8) | 3 (14) | 3 (14) |
| Leukocyturia | 0 | 0 | 2 (33) | 0 | 1 (8) | 0 | 3 (14) | 0 |
| Lymphopeniad | 1 (50) | 0 | 1 (17) | 0 | 1 (8) | 1 (8) | 3 (14) | 1 (5) |
| Pain in extremity | 0 | 0 | 2 (33) | 0 | 1 (8) | 0 | 3 (14) | 0 |
| Pain in jaw | 1 (50) | 0 | 1 (17) | 0 | 1 (8) | 0 | 3 (14) | 0 |
| HFSR | 0 | 0 | 3 (50) | 1 (17) | 0 | 0 | 3 (14) | 1 (5) |
| Pruritus | 1 (50) | 0 | 1 (17) | 0 | 1 (8) | 0 | 3 (14) | 0 |
| Stomatitis | 0 | 0 | 2 (33) | 0 | 1 (8) | 0 | 3 (14) | 0 |
| Tachycardia | 0 | 0 | 0 | 0 | 3 (23) | 0 | 3 (14) | 0 |
| Toothache | 0 | 0 | 2 (33) | 0 | 1 (8) | 0 | 3 (14) | 0 |
| URT infection | 1 (50) | 0 | 1 (17) | 0 | 1 (8) | 0 | 3 (14) | 0 |
| Acute kidney injury | 0 | 0 | 0 | 0 | 2 (15) | 1 (8) | 2 (10) | 1 (5) |
| Bone pain | 0 | 0 | 1 (17) | 0 | 1 (8) | 0 | 2 (10) | 0 |
| Clostridium difficile infection | 0 | 0 | 2 (33) | 1 (17) | 0 | 0 | 2 (10) | 1 (5) |
| Dermatitis diaper | 0 | 0 | 0 | 0 | 2 (15) | 0 | 2 (10) | 0 |
| Dizziness | 0 | 0 | 2 (33) | 0 | 0 | 0 | 2 (10) | 0 |
| Dysgeusia | 1 (50) | 0 | 0 | 0 | 1 (8) | 0 | 2 (10) | 0 |
| Ear pain | 1 (50) | 0 | 0 | 0 | 1 (8) | 0 | 2 (10) | 0 |
| Erythema | 0 | 0 | 0 | 0 | 2 (15) | 0 | 2 (10) | 0 |
| Glycosuria | 1 (50) | 0 | 0 | 0 | 1 (8) | 0 | 2 (10) | 0 |
| Hyperglycemia | 1 (50) | 0 | 1 (17) | 0 | 0 | 0 | 2 (10) | 0 |
| Hypoalbuminemia | 0 | 0 | 1 (17) | 0 | 1 (8) | 0 | 2 (10) | 0 |
| Hypomagnesemia | 0 | 0 | 0 | 0 | 2 (15) | 0 | 2 (10) | 0 |
| Hypotension | 0 | 0 | 0 | 0 | 2 (15) | 2 (15) | 2 (10) | 2 (10) |
| Hypothyroidism | 0 | 0 | 0 | 0 | 2 (15) | 0 | 2 (10) | 0 |
| Insomnia | 1 (50) | 0 | 1 (17) | 0 | 0 | 0 | 2 (10) | 0 |
| Leukocytosis | 1 (50) | 0 | 0 | 0 | 1 (8) | 0 | 2 (10) | 0 |
| Lipase increased | 0 | 0 | 1 (17) | 1 (17) | 1 (8) | 1 (8) | 2 (10) | 2 (10) |
| Muscle spasms | 0 | 0 | 0 | 0 | 2 (15) | 0 | 2 (10) | 0 |
| Nasopharyngitis | 0 | 0 | 1 (17) | 0 | 1 (8) | 0 | 2 (10) | 0 |
| Neck pain | 0 | 0 | 2 (33) | 1 (17) | 0 | 0 | 2 (10) | 1 (5) |
| Oral pain | 0 | 0 | 1 (17) | 0 | 1 (8) | 0 | 2 (10) | 0 |
| Pain of skin | 0 | 0 | 1 (17) | 1 (17) | 1 (8) | 0 | 2 (10) | 1 (5) |
| Peripheral sensory neuropathy | 1 (50) | 1 (50) | 1 (17) | 0 | 0 | 0 | 2 (10) | 1 (5) |
| Prothrombin time ratio increased | 0 | 0 | 2 (33) | 0 | 0 | 0 | 2 (10) | 0 |
| Rhinorrhea | 0 | 0 | 0 | 0 | 2 (15) | 0 | 2 (10) | 0 |
| Skin hyperpigmentation | 0 | 0 | 2 (33) | 0 | 0 | 0 | 2 (10) | 0 |
| Vascular access complication | 1 (50) | 0 | 1 (17) | 0 | 0 | 0 | 2 (10) | 0 |
Abbreviations: AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; HFSR, hand–foot skin reaction; TSH, thyroid stimulating hormone; URT, upper respiratory tract; VI, vincristine and irinotecan; WBC, white blood cell.
aIncluding neutrophil count decreased.
bIncluding platelet count decreased.
cIncluding WBC count decreased.
dIncluding lymphocyte count decreased.
DLTs
Four patients experienced DLTs during cycle 1 (Supplementary Table S4). Both patients in cohort 1a experienced grade 3 DLTs at regorafenib 72 mg/m2. One patient experienced peripheral sensory neuropathy, ALT/AST increases, hepatic pain, and drug-induced liver injury (all grade 3), and one patient experienced vomiting, abdominal pain, and febrile bone marrow aplasia (all grade 3); consequently, the concomitant schedule was discontinued. Two patients in cohort 1b experienced DLTs (Supplementary Table S4); one had grade 3 maculopapular rash and increased AST (at 72 mg/m2) and the other experienced grade 3 thrombocytopenia (at 82 mg/m2). The regorafenib 82 mg/m2 group was expanded and 6 additional patients experienced no DLTs. The MTD and RP2D of regorafenib in the sequential combination regimen were determined to be 82 mg/m2 once daily.
Antitumor activity
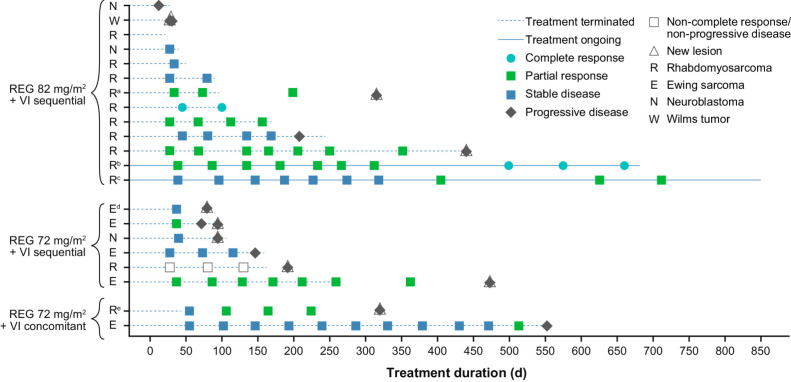
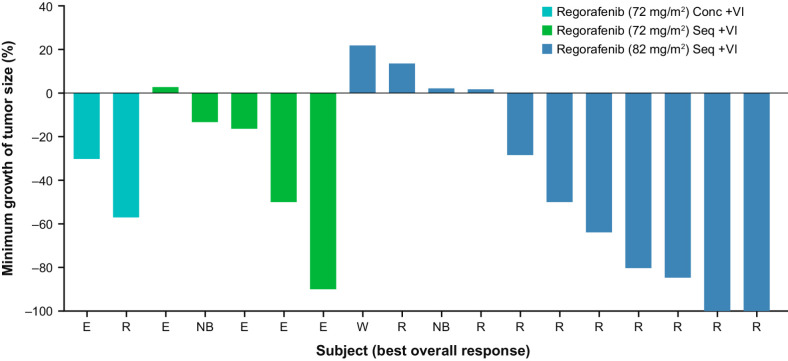
All 21 patients were evaluable for tumor response; 2 had complete responses (CR), 8 had PRs, 7 had SD, and 1 (with non-measurable disease) had non-CR/non-progressive disease (PD), that is, persistence of ≥1 non-target lesion. The overall response rate (ORR; CR + PR) was 48% (10/21) and disease control rate (DCR; CR + PR + SD + non-CR/non-PD) was 86% (18/21; Table 3). A total of 7 of 12 patients with RMS achieved a CR (n = 2) or PR (n = 5; Figs. 1 and 2); responses were seen in 5 of 8 patients with alveolar histology and 2 of 3 with embryonal histology (one patient had RMS not otherwise specified), in 3 of 4 patients with refractory disease and 4 of 7 with relapsing disease (status unknown in one patient), and in 3 of 6 patients who received prior irinotecan and 4 of 6 who did not. A total of 2 of 5 patients with EWS achieved a PR (Figs. 1 and 2). In 10 patients with RMS treated with regorafenib 82 mg/m2 and VI sequentially, the ORR was 60% [95% confidence interval (CI), 26.2–87.8] and DCR was 90% (95% CI, 55.5–99.7; Table 3). Two patients achieved PRs after 14 cycles of treatment (Fig. 1). In addition to the 2 CRs reported, an additional patient with metastatic RMS refractory to frontline therapy achieved and sustained a CR after the data cutoff date. Among the 10 responding patients, median duration of response (DOR; time from initial confirmed response to progression or death) was 13.5 months [95% CI, 1.3 to not estimable (NE)] and median time to response was 6.3 weeks (95% CI, 5.3–15.4). In patients (n = 6) treated with sequential regorafenib 82 mg/m2 plus VI, median DOR was 13.5 months (95% CI, 9.3–NE). Median PFS was 7.0 months (95% CI, 2.9–14.8) and median OS was 8.7 months (95% CI, 5.5–16.3).
Table 3.
Tumor response (efficacy analysis set; cutoff value, February 1, 2021).
| Regorafenib 72 mg/m2 concomitant with VI | Regorafenib 72 mg/m2 sequential with VI | Regorafenib 82 mg/m2 sequential with VI | Total | |||||
|---|---|---|---|---|---|---|---|---|
| Best overall response (RECIST v1.1) | All (n = 2) | RMS (n = 1) | All (n = 6) | RMS (n = 1) | All (n = 13) | RMS (n = 10) | All (N = 21) | RMS (n = 12) |
| CR, n (%; 95% CI) | 0 | 0 | 0 | 0 | 2 (15.4; 1.9–45.4) | 2 (20.0; 2.5–55.6) | 2 (9.5; 1.2–30.4) | 2 (16.7; 2.1–48.4) |
| PR, n (%; 95% CI) | 2 (100; 15.8–100) | 1 (100; 2.5–100) | 2 (33.3; 4.3–77.7) | 0 | 4 (30.8; 9.1–61.4) | 4 (40.0; 12.2–73.8) | 8 (38.1; 18.1–61.6) | 5 (41.7; 15.2–72.3) |
| SD, n (%; 95% CI) | 0 | 0 | 3 (50.0; 11.8–88.2) | 0 | 4 (30.8; 9.1–61.4) | 3 (30.0; 6.7–65.2) | 7 (33.3; 14.6–57.0) | 3 (25.0; 5.5–57.2) |
| Non-CR/non-PD, n (%; 95% CI) | 0 | 0 | 1 (16.7; 0.4–64.1) | 1 (100; 2.5–100) | 0 | 0 | 1 (4.8; 0.1–23.8) | 1 (8.3; 0.2–38.5) |
| DCRa, n (%; 95% CI) | 2 (100; 15.8–100) | 1 (100; 2.5–100) | 6 (100; 54.1–100) | 1 (100; 2.5–100) | 10 (76.9; 46.2–95.0) | 9 (90.0; 55.5–99.7) | 18 (85.7; 63.7–97.0) | 11 (91.7; 61.5–99.8) |
| PD, n (%; 95% CI) | 0 | 0 | 0 | 0 | 2 (15.4; 1.9–45.4) | 0 | 2 (9.5; 1.2–30.4) | 0 |
| Not assessedb, n (%; 95% CI) | 0 | 0 | 0 | 0 | 1 (7.7; 0.2–36.0) | 1 (10.0; 0.3–44.5) | 1 (4.8; 0.1–23.8) | 1 (8.3; 0.2–38.5) |
| Overall response ratec, n (%; 95% CI) | 2 (100; 15.8–100) | 1 (100; 2.5–100) | 2 (33.3; 4.3–77.7) | 0 | 6 (46.2; 19.2–74.9) | 6 (60.0; 26.2–87.8) | 10 (47.6; 25.7–70.2) | 7 (58.3; 27.7–84.8) |
Abbreviations: CI, confidence interval; CR, complete response; DCR, disease control rate; PD, progressive disease; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; RMS, rhabdomyosarcoma; SD, stable disease; VI, vincristine and irinotecan.
aCR + PR + SD + non-CR/non-PD.
bOne patient was not evaluable for radiologic response due to early clinical progression.
cCR + PR.
Figure 1.
Time on treatment and overall response. Five partial responses and one complete response were confirmed in the study treatment period; duration of 42 days was required for overall response of stable disease. aPatient received radiotherapy in the follow-up period after treatment discontinuation (days 136 to 175). bPatient received radiotherapy on study days 526 to 537. cPatient received radiotherapy after recording of first partial response. dPatient received radiotherapy in the follow-up period after treatment discontinuation. ePatient continued treatment with regorafenib plus VI off study. REG, regorafenib; VI, vincristine and irinotecan.
Figure 2.
Best overall response depicted by the percentage of change in target lesion size relative to baseline and by dose level in 18 patients with measurable disease. Of the 21 patients treated, one patient was not evaluated for radiologic response due to early clinical progression, one patient had non-measurable but evaluable disease, and one patient had a different imaging modality post-baseline for determining progression. Conc, concomitant; E, Ewing sarcoma; N, neuroblastoma; R, rhabdomyosarcoma; Seq, sequential; VI, vincristine and irinotecan; W, Wilms tumor.
Despite the discontinuation of the concomitant regorafenib schedule, the 2 patients in cohort 1a eventually achieved PRs on regorafenib: one patient (with EWS) after prolonged SD with dose reduction, and the second (with RMS) after study treatment discontinuation (due to investigator decision), who continued off-study treatment with vincristine, irinotecan, and regorafenib (off-study data not shown).
Case vignettes of 2 patients who achieved CR are provided in Supplementary Data S2 and Supplementary Fig. S2.
PK
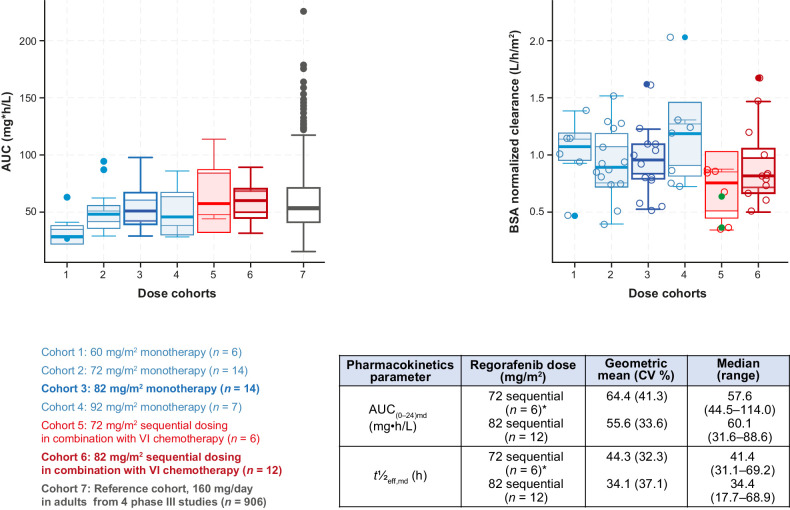
PK data were available for 20 patients (Fig. 3). Median exposure to regorafenib 82 mg/m2 plus VI in cohort 1b (sequential; n = 12), as indicated by the area under the concentration–time curve (AUC), was similar to that achieved in adults treated with regorafenib 160 mg/day in phase III studies (Fig. 3), and to that achieved in pediatric patients treated with single-agent regorafenib 82 mg/m2. The effective half-life in the expansion phase ranged from 17.7 to 69.2 hours (median, 38.9 hours; Fig. 3), suggesting that steady-state exposure would be achieved after 3.0–11.5 days (median, 6.5 days). The results indicated that all patients achieved steady-state exposure in cycle 1 following nominal dosing. Body surface area-normalized clearance of regorafenib was consistent in pediatric patients receiving sequential regorafenib 72 or 82 mg/m2 plus VI (Fig. 3). The distribution of individual clearance values for irinotecan and its active metabolite do not suggest that the PK of irinotecan was altered by concomitant or sequential administration of regorafenib, and the estimated clearances in this study are similar to literature values (ref. 29; Supplementary Figs. S3 and S4; see Supplementary Fig. S4 for methodology of comparing median clearances with published data).
Figure 3.
BSA-normalized exposure AUC (left) and clearance of regorafenib (right) and pharmacokinetics parameters of regorafenib administered with VI (bottom right table). The closed green circles denote concomitant dosing—lower BSA-normalized clearance in one of the two patients in the concomitant dosing cohort is not likely to be due to a drug–drug interaction, as irinotecan, SN-38, and vincristine do not inhibit CYP3A4. Boxplots show the median (solid line at center of box) and its approximate 95% confidence interval (gray area), the interquartile range (box), 1.5× interquartile range (whiskers), and outliers (closed circles). *An additional two patients received concomitant regorafenib 72 mg/m2 (AUC(0–24)md range, 62.8–114.0 mg•h/L and t½eff,md range, 50.3–64.3 hours). Box plots for different doses of regorafenib monotherapy (blue boxes) are shown for comparison. AUC(0–24)md, area under the plasma concentration–time curve over a dosing interval of 24 hours after multiple dosing; BSA, body surface area; CV, coefficient of variation; t½eff,md, effective half-life after multiple dosing; VI, vincristine and irinotecan.
Discussion
Regorafenib monotherapy prolonged PFS in adult patients with advanced non-adipocytic soft tissue sarcomas (30), and showed preliminary antitumor activity in the dose-escalation part of this phase I study (24). These results, along with preclinical antitumor activity when combined with irinotecan (13), prompted us to further explore regorafenib in combination with VI in the present study. The main goal was to define the RP2D to take the combination forward to phase II studies in patients in relapse in the FaR-RMS study [NCT04625907 (ref. 31); EudraCT number 2018–000515–24], a European pediatric Soft Tissue Sarcoma Study Group (EpSSG; ref. 32) trial for patients with newly diagnosed and relapsed RMS.
Regorafenib was tolerable at 72 and 82 mg/m2 with appropriate dosing modifications where required, and when given sequentially with VI in pediatric patients. Enrollment in the concomitant group was stopped early after the first 2 patients experienced DLTs at 72 mg/m2, although both patients eventually achieved PRs. In contrast, only 1 of 6 patients experienced a DLT in the sequential schedule. The 4 DLTs encountered during the present study were all different. One incident of thrombocytopenia and one of rash were also reported as DLTs in the single-agent dose-escalation phase of the study (24). A lack of a consistent pattern of DLTs across the two phases suggests that the overall pattern of AEs may provide a more reliable indicator of the safety profile. Nonetheless, elevated aminotransferase levels occurred in 2 patients with DLTs, and approximately 20%–25% of patients had grade 3 transaminitis. Similarly, grade 3 thrombocytopenia was observed in 14% of patients, and was a DLT in one. This information will be useful in managing AEs in patients on treatment with regorafenib in combination with VI, and suggests the importance of monitoring liver function and for potential hematologic toxicity that could help decision-making when considering dose modifications of regorafenib or VI, respectively. Decisions about which drug to dose modify were made based on expected toxicities that were in turn based on known safety profiles of individual drugs. For example, dose modification of irinotecan occurred first for myelosuppression, whereas dose modification of regorafenib occurred first for skin toxicities, hypertension, and elevated transaminases. Peripheral neuropathy led to vincristine dose modifications, and gastrointestinal toxicities led to modifications of both regorafenib and irinotecan. Other non-hematologic toxicities generally led to dose modification of regorafenib first, unless the investigator could clearly relate it to another drug.
The safety profile of regorafenib-based regimens in pediatric patients (data were insufficient to evaluate the safety profile in infants, with only one patient aged <2 years) was consistent with that in adults, but with some differences in incidence and severity possibly attributable to the extent of previous treatment and fewer comorbidities. The most common AEs in adults were HFSR, fatigue, diarrhea, and hypertension (30, 33–40). A similar overall safety profile was seen in the dose-escalation part in pediatric patients treated at 82 mg/m2 but with lower frequencies of some of the most common regorafenib-related AEs following treatment with regorafenib plus VI, including increased bilirubin (14% vs. 57%), increased AST (43% vs. 50%), and HFSR (14% vs. 29%; ref. 24), and no new unexpected toxicities were reported. Hypothyroidism, a known regorafenib-related AE, occurred with a lower incidence in the phase I monotherapy study compared with its reported incidence in adults (24); in this study, a similarly low rate was seen, occurring in just 2 patients.
All patients in the regorafenib with VI combination group experienced ≥1 AE considered to be related to treatment. Most AEs were toxicities known to be related to regorafenib or VI, most of which were non-serious. Administration of regorafenib in combination with VI results in a higher incidence of neutropenia; however, whether this is caused by regorafenib, irinotecan, or a combined effect remains unclear. Despite this observed increase, most toxicities observed in the study were manageable with dose modifications. Although 86% of patients required a dose reduction, toxicity was highly variable across patients and variably related to each component of the combination. This required an individualized approach to dose modifications. Furthermore, high median percentages of planned doses given (86%–95% for the three components) suggest that patients were achieving substantial drug exposure over their full-treatment course.
The PK profile of regorafenib in children was similar to that observed in adults, including high inter-subject variability. Exposure to regorafenib was consistent after single-agent regorafenib in the dose-escalation part of the study and after regorafenib with VI. The geometric mean AUC after treatment with regorafenib 82 mg/m2 in the dose-escalation phase was similar to that observed with sequential regorafenib and VI in the dose-expansion phase, and with that estimated after 160 mg/day dosing in a 70 kg patient according to a population PK model based on data from 906 patients (28). Although limited, these data suggest that there is no clinically significant PK drug–drug interaction when regorafenib is administered with VI in either schedule. Mechanistically, a drug–drug interaction would not be expected because neither vincristine nor irinotecan are potent inhibitors of CYP3A4 (41).
Clinical activity was observed in patients with EWS and RMS. Two patients with RMS had CRs; both patients had presented with relapsing disease at study entry, and one had received prior VI. Responses were seen in patients with alveolar and embryonal RMS, including patients who were refractory to prior treatment. Notably, two patients achieved late PRs after 14 cycles of treatment. Despite some limitations (not powered as a phase II trial and response was not a primary endpoint), the ORR across all dose levels and regimens was 48% in the present study, indicating consistent activity in pediatric patients, reaching 60% in patients with RMS treated with regorafenib 82 mg/m2 and VI sequentially. Durable responses were seen in some patients; median DOR was 13.5 months.
The VI combination has provided response rates of 26%–37% in patients with relapsed RMS (42, 43), and response rates of 25%–68% when combined with temozolomide (VIT) in patients with RMS or EWS (44–50). Although a randomized comparison of VI versus regorafenib plus VI would be the ideal way to demonstrate the contribution of regorafenib, such a study would not be considered ethical in RMS now that VIT has been recently defined as the standard of care at relapse for RMS in Europe (43). Therefore, the EpSSG decided that VI combined with regorafenib would be compared with VIT in the FaR-RMS trial (31) in patients with RMS at relapse.
In conclusion, the regorafenib RP2D was 82 mg/m2/day to be administered sequentially, which provides exposure similar to the recommended dose in adults (160 mg/day). The results demonstrate that regorafenib can be combined with standard dose VI in pediatric patients with relapsed/refractory RMS, EWS, and other solid tumors, with manageable safety, and the combination exhibits antitumor activity in this patient population.
Supplementary Material
Supplementary methods
Supplementary results
Table showing treatment-emergent adverse events
Table of AEs potentially related to regorafenib
Table of AEs potentially related to VI
Table of dose-limiting toxicities
Table showing the representativeness of study participants
Chart showing treatment schema.
Patient scans showing progressive reduction of tumor size in a patient with metastatic alveolar rhabdomyosarcoma.
Distribution of individual clearance of irinotecan and its active metabolite SN-38.
Distribution of the median clearance of irinotecan and its active metabolite (SN-38) derived from the published variability distribution of the clearances versus the estimated median clearance of irinotecan and SN-38 in this study.
Acknowledgments
The authors thank the patients, their families, and participating study centers. This study was sponsored by Bayer. Editorial assistance in the preparation of this article was provided by OPEN Health Communications (London, UK), with financial support from Bayer.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This article is featured in Selected Articles from This Issue, p. 4315
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
M. Casanova reports personal fees from Bayer during the conduct of the study, as well as personal fees from Pfizer, AstraZeneca, and Servier outside the submitted work. F. Bautista reports personal fees from Bayer outside the submitted work. L.V. Marshall reports other support from Bayer during the conduct of the study, as well as personal fees from Bayer, DayOne Biopharmaceuticals, Illumina, Merck, and Eisai outside the submitted work. A. Cañete Nieto reports grants, personal fees, and non-financial support from Ricordati, as well as grants and personal fees from Norgine outside the submitted work. B.A. Ploeger reports personal fees from Bayer AG during the conduct of the study. B.J. Brennan reports personal fees from Bayer during the conduct of the study, as well as personal fees from Bayer outside the submitted work; in addition, B.J. Brennan is owner of Roche stock as part of past employment. U. Mueller reports personal fees from Bayer AG during the conduct of the study, as well as personal fees from Bayer AG outside the submitted work. H. Zebger-Gong reports other support from employment with Bayer and Daiichi Sankyo, as well as stock with Bayer outside the submitted work. J.W. Chung reports personal fees from Bayer Healthcare Pharma during the conduct of the study. B. Geoerger reports personal fees from Bayer during the conduct of the study. No disclosures were reported by the other authors.
Authors' Contributions
M. Casanova: Investigation, writing–original draft, writing–review and editing. F. Bautista: Investigation, writing–review and editing. Q. Campbell-Hewson: Investigation, writing–review and editing. G. Makin: Investigation, writing–review and editing. L.V. Marshall: Investigation, writing–review and editing. A.C. Verschuur: Investigation, writing–review and editing. A. Cañete Nieto: Investigation, writing–review and editing. N. Corradini: Investigation, writing–review and editing. B.A. Ploeger: Data curation, formal analysis, writing–review and editing. B.J. Brennan: Data curation, formal analysis, writing–review and editing. U. Mueller: Data curation, formal analysis, writing–review and editing. H. Zebger-Gong: Data curation, formal analysis, writing–review and editing. J.W. Chung: Data curation, formal analysis, writing–review and editing. B. Geoerger: Investigation, writing–original draft, writing–review and editing.
References
- 1. US Food and Drug Administration. Stivarga (regorafenib) prescribing information. Bayer HealthCare Pharmaceuticals, Whippany, NJ, USA. 2020. Available from: https://labeling.bayerhealthcare.com/html/products/pi/Stivarga_PI.pdf. [Google Scholar]
- 2. European Medicines Agency. Stivarga (regorafenib) summary of product characteristics. Bayer AG, Leverkusen, Germany. 2023. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/stivarga#product-information-section. [Google Scholar]
- 3. Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schutz G, et al. Regorafenib (BAY 73–4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 2011;129:245–55. [DOI] [PubMed] [Google Scholar]
- 4. Abou-Elkacem L, Arns S, Brix G, Gremse F, Zopf D, Kiessling F, et al. Regorafenib inhibits growth, angiogenesis, and metastasis in a highly aggressive, orthotopic colon cancer model. Mol Cancer Ther 2013;12:1322–31. [DOI] [PubMed] [Google Scholar]
- 5. Chan AS, Leung SY, Wong MP, Yuen ST, Cheung N, Fan YW, et al. Expression of vascular endothelial growth factor and its receptors in the anaplastic progression of astrocytoma, oligodendroglioma, and ependymoma. Am J Surg Pathol 1998;22:816–26. [DOI] [PubMed] [Google Scholar]
- 6. Eggert A, Ikegaki N, Kwiatkowski J, Zhao H, Brodeur GM, Himelstein BP. High-level expression of angiogenic factors is associated with advanced tumor stage in human neuroblastomas. Clin Cancer Res 2000;6:1900–8. [PubMed] [Google Scholar]
- 7. Komuro H, Kaneko S, Kaneko M, Nakanishi Y. Expression of angiogenic factors and tumor progression in human neuroblastoma. J Cancer Res Clin Oncol 2001;127:739–43. [DOI] [PubMed] [Google Scholar]
- 8. Fakhari M, Pullirsch D, Paya K, Abraham D, Hofbauer R, Aharinejad S. Upregulation of vascular endothelial growth factor receptors is associated with advanced neuroblastoma. J Pediatr Surg 2002;37:582–7. [DOI] [PubMed] [Google Scholar]
- 9. Miyagami M, Katayama Y. Angiogenesis of glioma: evaluation of ultrastructural characteristics of microvessels and tubular bodies (Weibel–Palade) in endothelial cells and immunohistochemical findings with VEGF and p53 protein. Med Mol Morphol 2005;38:36–42. [DOI] [PubMed] [Google Scholar]
- 10. Crose LE, Etheridge KT, Chen C, Belyea B, Talbot LJ, Bentley RC, et al. FGFR4 blockade exerts distinct antitumorigenic effects in human embryonal versus alveolar rhabdomyosarcoma. Clin Cancer Res 2012;18:3780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schiavetti A, McDowell HP, Conti L, Altavista P, Antenucci A, Pizer B, et al. Vascular endothelial growth factor serum levels in children with newly diagnosed rhabdomyosarcoma. Pediatr Blood Cancer 2012;59:627–30. [DOI] [PubMed] [Google Scholar]
- 12. Paugh BS, Zhu X, Qu C, Endersby R, Diaz AK, Zhang J, et al. Novel oncogenic PDGFRA mutations in pediatric high-grade gliomas. Cancer Res 2013;73:6219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Daudigeos-Dubus E, Le Dret L, Lanvers-Kaminsky C, Bawa O, Opolon P, Vievard A, et al. Regorafenib: antitumor activity upon mono and combination therapy in preclinical pediatric malignancy models. PLoS One 2015;10:e0142612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pastore G, Peris-Bonet R, Carli M, Martinez-Garcia C, Sanchez de Toledo J, Steliarova-Foucher E. Childhood soft tissue sarcomas incidence and survival in European children (1978–1997): report from the automated childhood cancer information system project. Eur J Cancer 2006;42:2136–49. [DOI] [PubMed] [Google Scholar]
- 15. Malempati S, Hawkins DS. Rhabdomyosarcoma: review of the Children's Oncology Group (COG) Soft-Tissue Sarcoma Committee experience and rationale for current COG studies. Pediatr Blood Cancer 2012;59:5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chisholm JC, Marandet J, Rey A, Scopinaro M, de Toledo JS, Merks JH, et al. Prognostic factors after relapse in nonmetastatic rhabdomyosarcoma: a nomogram to better define patients who can be salvaged with further therapy. J Clin Oncol 2011;29:1319–25. [DOI] [PubMed] [Google Scholar]
- 17. Goldstein M, Meller I, Orr-Urtreger A. FGFR1 over-expression in primary rhabdomyosarcoma tumors is associated with hypomethylation of a 5′ CpG island and abnormal expression of the AKT1, NOG, and BMP4 genes. Genes Chromosomes Cancer 2007;46:1028–38. [DOI] [PubMed] [Google Scholar]
- 18. Taniguchi E, Nishijo K, McCleish AT, Michalek JE, Grayson MH, Infante AJ, et al. PDGFR-A is a therapeutic target in alveolar rhabdomyosarcoma. Oncogene 2008;27:6550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Missiaglia E, Selfe J, Hamdi M, Williamson D, Schaaf G, Fang C, et al. Genomic imbalances in rhabdomyosarcoma cell lines affect expression of genes frequently altered in primary tumors: an approach to identify candidate genes involved in tumor development. Genes Chromosomes Cancer 2009;48:455–67. [DOI] [PubMed] [Google Scholar]
- 20. Taylor JG VI, Cheuk AT, Tsang PS, Chung JY, Song YK, Desai K, et al. Identification of FGFR4-activating mutations in human rhabdomyosarcomas that promote metastasis in xenotransplanted models. J Clin Invest 2009;119:3395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ehnman M, Missiaglia E, Folestad E, Selfe J, Strell C, Thway K, et al. Distinct effects of ligand-induced PDGFRα and PDGFRβ signaling in the human rhabdomyosarcoma tumor cell and stroma cell compartments. Cancer Res 2013;73:2139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sastre J, Argiles G, Benavides M, Feliu J, Garcia-Alfonso P, Garcia-Carbonero R, et al. Clinical management of regorafenib in the treatment of patients with advanced colorectal cancer. Clin Transl Oncol 2014;16:942–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grothey A. Management of patients with relapsed/refractory metastatic colorectal cancer. Clin Adv Hematol Oncol 2019;17 Suppl 7:14–6. [PubMed] [Google Scholar]
- 24. Geoerger B, Morland B, Jimenez I, Frappaz D, Pearson ADJ, Vassal G, et al. Phase 1 dose-escalation and pharmacokinetic study of regorafenib in paediatric patients with recurrent or refractory solid malignancies. Eur J Cancer 2021;153:142–52. [DOI] [PubMed] [Google Scholar]
- 25. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 26. Lewington V, Lambert B, Poetschger U, Sever ZB, Giammarile F, McEwan AJB, et al. 123I-mIBG scintigraphy in neuroblastoma: development of a SIOPEN semi-quantitative reporting, method by an international panel. Eur J Nucl Med Mol Imaging 2017;44:234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Park JR, Bagatell R, Cohn SL, Pearson AD, Villablanca JG, Berthold F, et al. Revisions to the international neuroblastoma response criteria: a consensus statement from the National Cancer Institute clinical trials planning meeting. J Clin Oncol 2017;35:2580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Keunecke A, Hoefman S, Drenth HJ, Zisowsky J, Cleton A, Ploeger BA. Population pharmacokinetics of regorafenib in solid tumours: exposure in clinical practice considering enterohepatic circulation and food intake. Br J Clin Pharmacol 2020;86:2362–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thompson PA, Gupta M, Rosner GL, Yu A, Barrett J, Bomgaars L, et al. Pharmacokinetics of irinotecan and its metabolites in pediatric cancer patients: a report from the Children's Oncology Group. Cancer Chemother Pharmacol 2008;62:1027–37. [DOI] [PubMed] [Google Scholar]
- 30. Mir O, Brodowicz T, Italiano A, Wallet J, Blay JY, Bertucci F, et al. Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 2016;17:1732–42. [DOI] [PubMed] [Google Scholar]
- 31. FaR-RMS: an overarching study for children and adults with frontline and relapsed rhabdomyosarcoma (FaR-RMS). 2020. Available from:https://clinicaltrials.gov/ct2/show/NCT04625907.
- 32. The European Paediatric Soft Tissue Sarcoma Study Group. Available from: https://www.epssgassociation.it/en/.
- 33. Demetri GD, Reichardt P, Kang YK, Blay JY, Rutkowski P, Gelderblom H, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303–12. [DOI] [PubMed] [Google Scholar]
- 35. Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2015;16:619–29. [DOI] [PubMed] [Google Scholar]
- 36. Pavlakis N, Sjoquist KM, Martin AJ, Tsobanis E, Yip S, Kang YK, et al. Regorafenib for the treatment of advanced gastric cancer (INTEGRATE): a multinational placebo-controlled phase II trial. J Clin Oncol 2016;34:2728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56–66. [DOI] [PubMed] [Google Scholar]
- 38. Duffaud F, Mir O, Boudou-Rouquette P, Piperno-Neumann S, Penel N, Bompas E, et al. Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: a non-comparative, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol 2019;20:120–33. [DOI] [PubMed] [Google Scholar]
- 39. Lombardi G, De Salvo GL, Brandes AA, Eoli M, Ruda R, Faedi M, et al. Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol 2019;20:110–9. [DOI] [PubMed] [Google Scholar]
- 40. Sun W, Patel A, Normolle D, Patel K, Ohr J, Lee JJ, et al. A phase 2 trial of regorafenib as a single agent in patients with chemotherapy-refractory, advanced, and metastatic biliary tract adenocarcinoma. Cancer 2019;125:902–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. US Food and Drug Administration. Drug development and drug interactions table of substrates, inhibitors and inducers. 2023. Available from:https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactions-table-substrates-inhibitors-and-inducers#table1-2.
- 42. Mascarenhas L, Lyden ER, Breitfeld PP, Walterhouse DO, Donaldson SS, Paidas CN, et al. Randomized phase II window trial of two schedules of irinotecan with vincristine in patients with first relapse or progression of rhabdomyosarcoma: a report from the Children's Oncology Group. J Clin Oncol 2010;28:4658–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Defachelles AS, Bogart E, Casanova M, Merks JHM, Bisogno G, Calareso G, et al. Randomized phase II trial of vincristine–irinotecan with or without temozolomide, in children and adults with relapsed or refractory rhabdomyosarcoma: a European Paediatric Soft-Tissue Sarcoma Study Group and Innovative Therapies for Children with Cancer trial. J Clin Oncol 2021;39:2979–90. [DOI] [PubMed] [Google Scholar]
- 44. Wagner LM, McAllister N, Goldsby RE, Rausen AR, McNall-Knapp RY, McCarville MB, et al. Temozolomide and intravenous irinotecan for treatment of advanced Ewing sarcoma. Pediatr Blood Cancer 2007;48:132–9. [DOI] [PubMed] [Google Scholar]
- 45. Casey DA, Wexler LH, Merchant MS, Chou AJ, Merola PR, Price AP, et al. Irinotecan and temozolomide for Ewing sarcoma: the Memorial Sloan-Kettering experience. Pediatr Blood Cancer 2009;53:1029–34. [DOI] [PubMed] [Google Scholar]
- 46. Mixon BA, Eckrich MJ, Lowas S, Engel ME. Vincristine, irinotecan, and temozolomide for treatment of relapsed alveolar rhabdomyosarcoma. J Pediatr Hematol Oncol 2013;35:e163–6. [DOI] [PubMed] [Google Scholar]
- 47. Raciborska A, Bilska K, Drabko K, Chaber R, Pogorzala M, Wyrobek E, et al. Vincristine, irinotecan, and temozolomide in patients with relapsed and refractory Ewing sarcoma. Pediatr Blood Cancer 2013;60:1621–5. [DOI] [PubMed] [Google Scholar]
- 48. Kurucu N, Sari N, Ilhan IE. Irinotecan and temozolamide treatment for relapsed Ewing sarcoma: a single-center experience and review of the literature. Pediatr Hematol Oncol 2015;32:50–9. [DOI] [PubMed] [Google Scholar]
- 49. Winter S, Fasola S, Brisse H, Mosseri V, Orbach D. Relapse after localized rhabdomyosarcoma: evaluation of the efficacy of second-line chemotherapy. Pediatr Blood Cancer 2015;62:1935–41. [DOI] [PubMed] [Google Scholar]
- 50. Buyukkapu Bay S, Kebudi R, Gorgun O, Zulfikar B, Darendeliler E, Cakir FB. Vincristine, irinotecan, and temozolomide treatment for refractory/relapsed pediatric solid tumors: a single center experience. J Oncol Pharm Pract 2019;25:1343–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary methods
Supplementary results
Table showing treatment-emergent adverse events
Table of AEs potentially related to regorafenib
Table of AEs potentially related to VI
Table of dose-limiting toxicities
Table showing the representativeness of study participants
Chart showing treatment schema.
Patient scans showing progressive reduction of tumor size in a patient with metastatic alveolar rhabdomyosarcoma.
Distribution of individual clearance of irinotecan and its active metabolite SN-38.
Distribution of the median clearance of irinotecan and its active metabolite (SN-38) derived from the published variability distribution of the clearances versus the estimated median clearance of irinotecan and SN-38 in this study.
Data Availability Statement
The availability of the data underlying this publication will be determined according to Bayer's commitment to the EFPIA/PhRMA “Principles for responsible clinical trial data sharing”. This pertains to scope, time point, and process of data access. As such, Bayer commits to sharing upon request from qualified scientific and medical researchers’ patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the USA and EU as necessary for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the EU and US regulatory agencies on or after January 1, 2014. Interested researchers can use www.vivli.org to request access to anonymized patient-level data and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the member section of the portal. Data access will be granted to anonymized patient-level data, protocols, and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.