Abstract
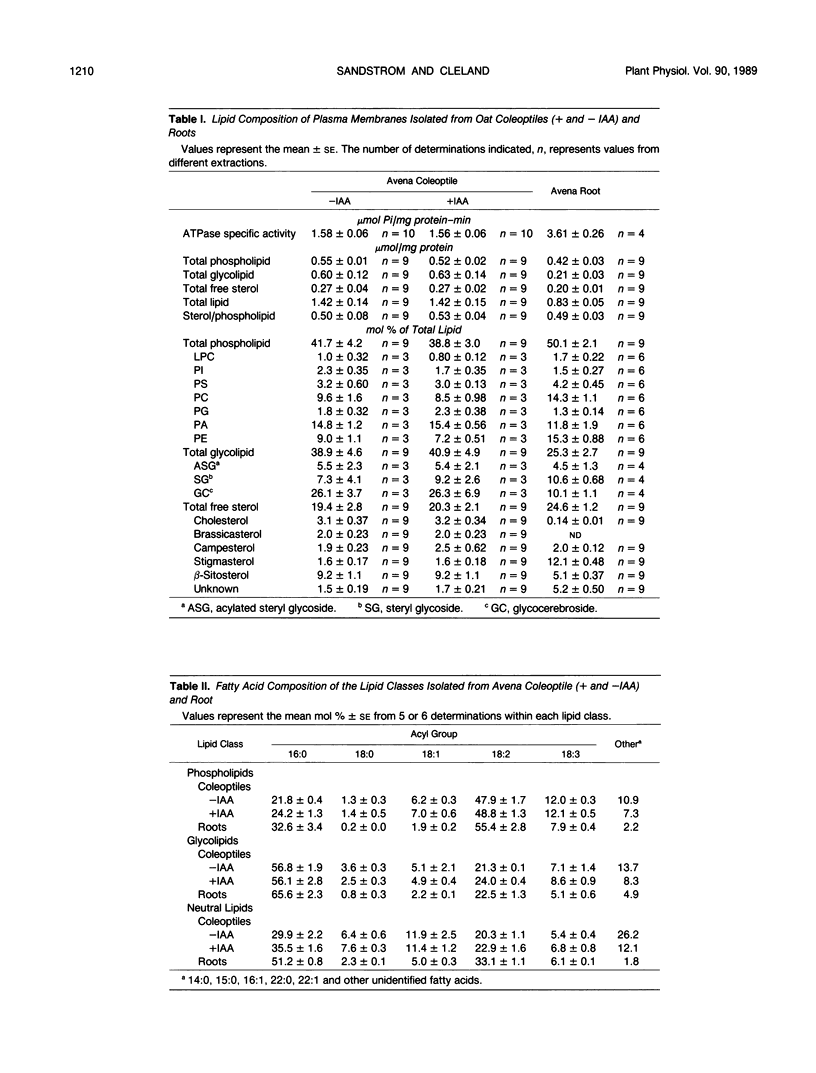
The total lipid composition of plasma membranes (PM), isolated by the phase partitioning method from two different oat (Avena sativa L.) tissues, the root and coleoptile, was compared. In general, the PM lipid composition was not conserved between these two organs of the oat seedling. Oat roots contained 50 mole percent phospholipid, 25 mole percent glycolipid, and 25 mole percent free sterol, whereas comparable amounts in the coleoptile were 42, 39, and 19 mole percent, respectively. Individual lipid components within each lipid class also showed large variations between the two tissues. Maximum specific ATPase activity in the root PM was more than double the activity in the coleoptile. Treatment of coleoptile with auxin for 1 hour resulted in no detectable changes in PM lipids or extractable ATPase activity. Differences in the PM lipid composition between the two tissues that may define the limits of ATPase activity are discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bladocha M., Benveniste P. Manipulation by tridemorph, a systemic fungicide, of the sterol composition of maize leaves and roots. Plant Physiol. 1983 Apr;71(4):756–762. doi: 10.1104/pp.71.4.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R. E. Kinetics of Hormone-induced H Excretion. Plant Physiol. 1976 Aug;58(2):210–213. doi: 10.1104/pp.58.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R. Auxin-induced hydrogen ion excretion from Avena coleoptiles. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3092–3093. doi: 10.1073/pnas.70.11.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettlinger C., Lehle L. Auxin induces rapid changes in phosphatidylinositol metabolites. Nature. 1988 Jan 14;331(6152):176–178. doi: 10.1038/331176a0. [DOI] [PubMed] [Google Scholar]
- Galliard T. Techniques for overcoming problems of lipolytic enzymes and lipoxygenases in the preparation of plant organelles. Methods Enzymol. 1974;31:520–528. doi: 10.1016/0076-6879(74)31056-7. [DOI] [PubMed] [Google Scholar]
- Kasamo K., Nouchi I. The Role of Phospholipids in Plasma Membrane ATPase Activity in Vigna radiata L. (Mung Bean) Roots and Hypocotyls. Plant Physiol. 1987 Feb;83(2):323–328. doi: 10.1104/pp.83.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch D. V., Steponkus P. L. Plasma Membrane Lipid Alterations Associated with Cold Acclimation of Winter Rye Seedlings (Secale cereale L. cv Puma). Plant Physiol. 1987 Apr;83(4):761–767. doi: 10.1104/pp.83.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester C. P., Kjellbom P., Andersson B., Larsson C. Lipid composition of plasma membranes isolated from light-grown barley (Hordeum vulgare) leaves: identification of cerebroside as a major component. Arch Biochem Biophys. 1987 Jun;255(2):385–391. doi: 10.1016/0003-9861(87)90406-1. [DOI] [PubMed] [Google Scholar]
- Roughan P. G., Batt R. D. Quantitative analysis of sulfolipid (sulfoquinovosyl diglyceride) and galactolipids (monogalactosyl and digalactosyl diglycerides) in plant tissues. Anal Biochem. 1968 Jan;22(1):74–88. doi: 10.1016/0003-2697(68)90261-3. [DOI] [PubMed] [Google Scholar]
- Sandstrom R. P., Deboer A. H., Lomax T. L., Cleland R. E. Latency of Plasma Membrane H-ATPase in Vesicles Isolated by Aqueous Phase Partitioning : Increased substrate Accessibility or Enzyme Activation. Plant Physiol. 1987 Nov;85(3):693–698. doi: 10.1104/pp.85.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R. Purification of the proton pumping ATPase from plant plasma membranes. Biochem Biophys Res Commun. 1984 Jun 15;121(2):735–740. doi: 10.1016/0006-291x(84)90243-2. [DOI] [PubMed] [Google Scholar]
- Uemura M., Yoshida S. Involvement of Plasma Membrane Alterations in Cold Acclimation of Winter Rye Seedlings (Secale cereale L. cv Puma). Plant Physiol. 1984 Jul;75(3):818–826. doi: 10.1104/pp.75.3.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman C. E., Travis R. L. Phospholipid composition of a plasma membrane-enriched fraction from developing soybean roots. Plant Physiol. 1985 Oct;79(2):494–498. doi: 10.1104/pp.79.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright L. C., McMurchie E. J., Pomeroy M. K., Raison J. K. Thermal behavior and lipid composition of cauliflower plasma membranes in relation to ATPase activity and chilling sensitivity. Plant Physiol. 1982 Jun;69(6):1356–1360. doi: 10.1104/pp.69.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Uemura M. Lipid Composition of Plasma Membranes and Tonoplasts Isolated from Etiolated Seedlings of Mung Bean (Vigna radiata L.). Plant Physiol. 1986 Nov;82(3):807–812. doi: 10.1104/pp.82.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Uemura M. Protein and Lipid Compositions of Isolated Plasma Membranes from Orchard Grass (Dactylis glomerata L.) and Changes during Cold Acclimation. Plant Physiol. 1984 May;75(1):31–37. doi: 10.1104/pp.75.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatkis A., Zak B. Study of a new cholesterol reagent. Anal Biochem. 1969 Apr 11;29(1):143–148. doi: 10.1016/0003-2697(69)90017-7. [DOI] [PubMed] [Google Scholar]


