Abstract
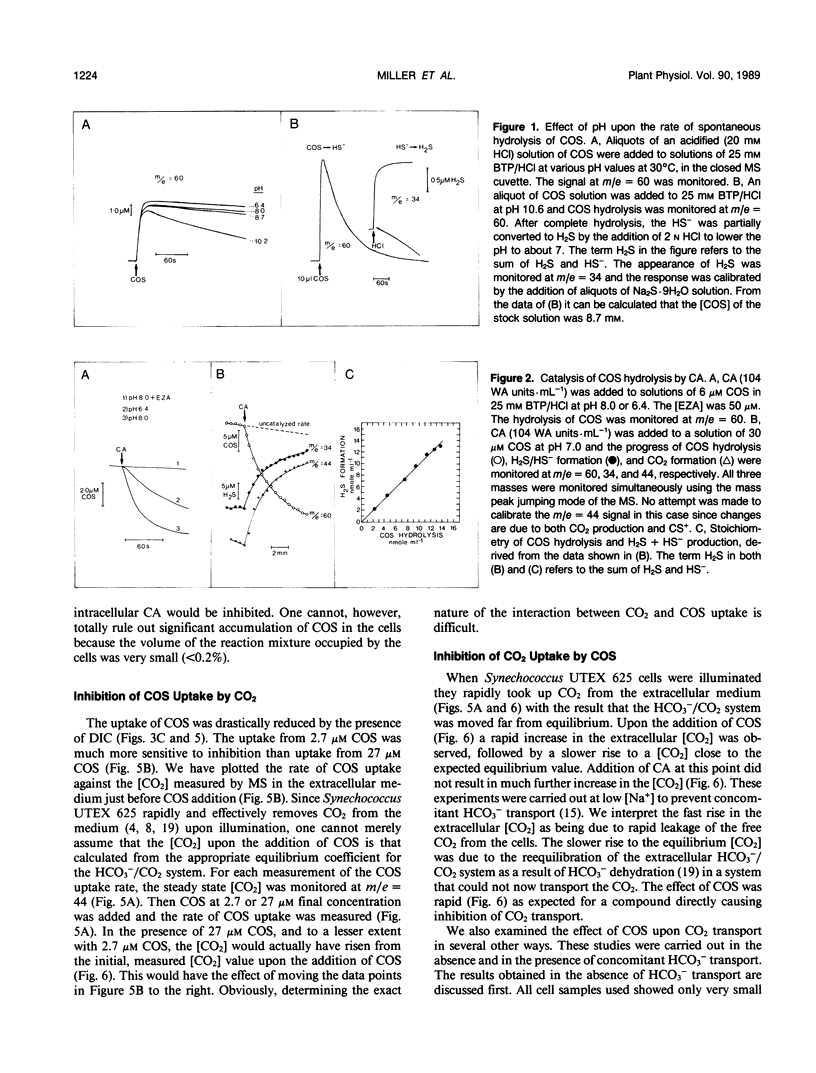
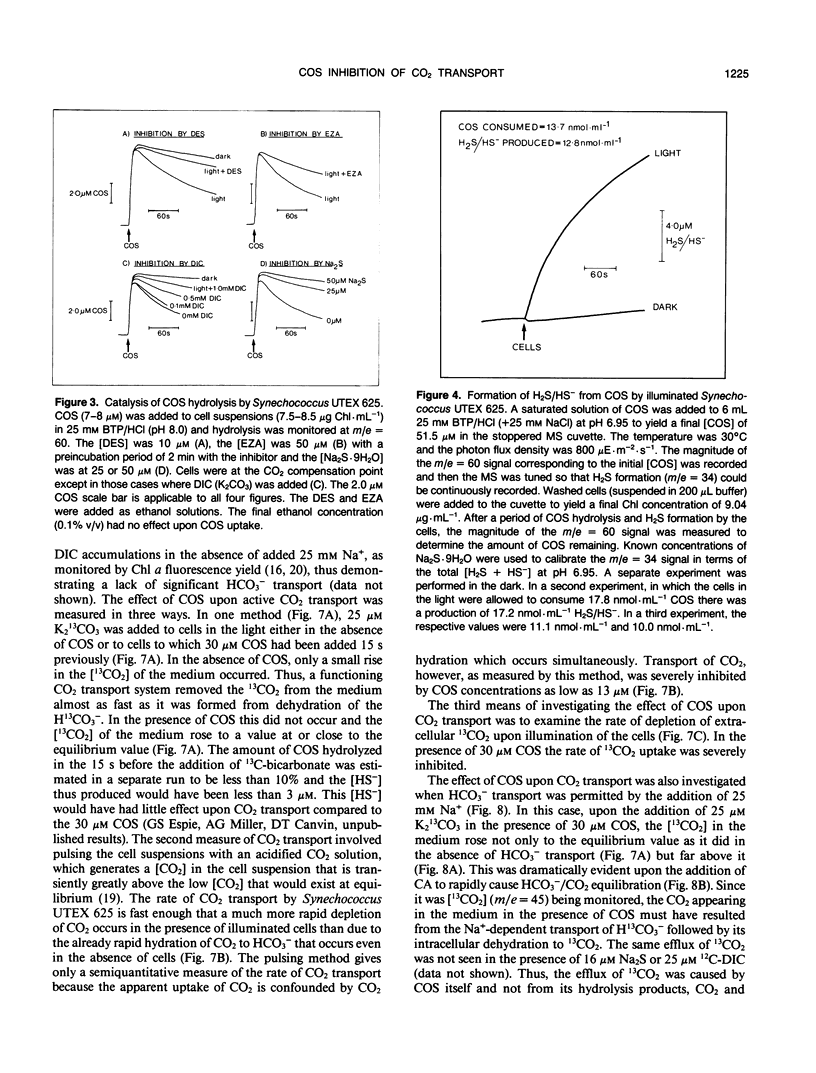
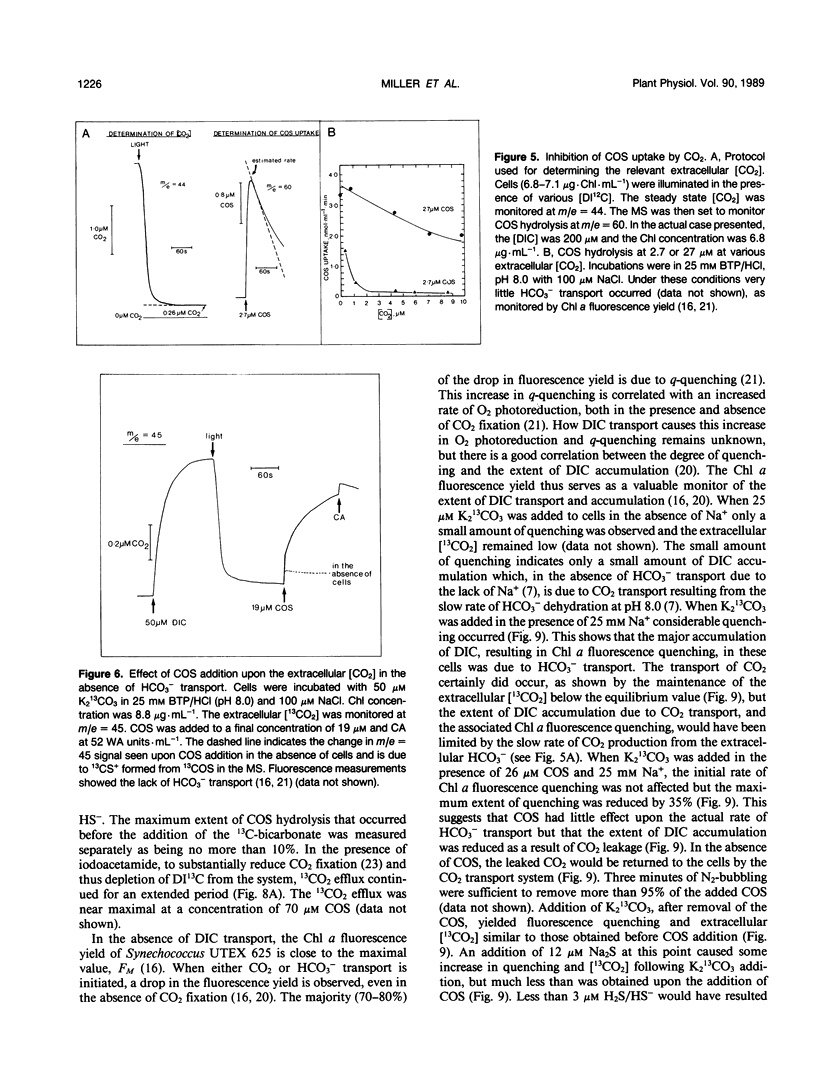
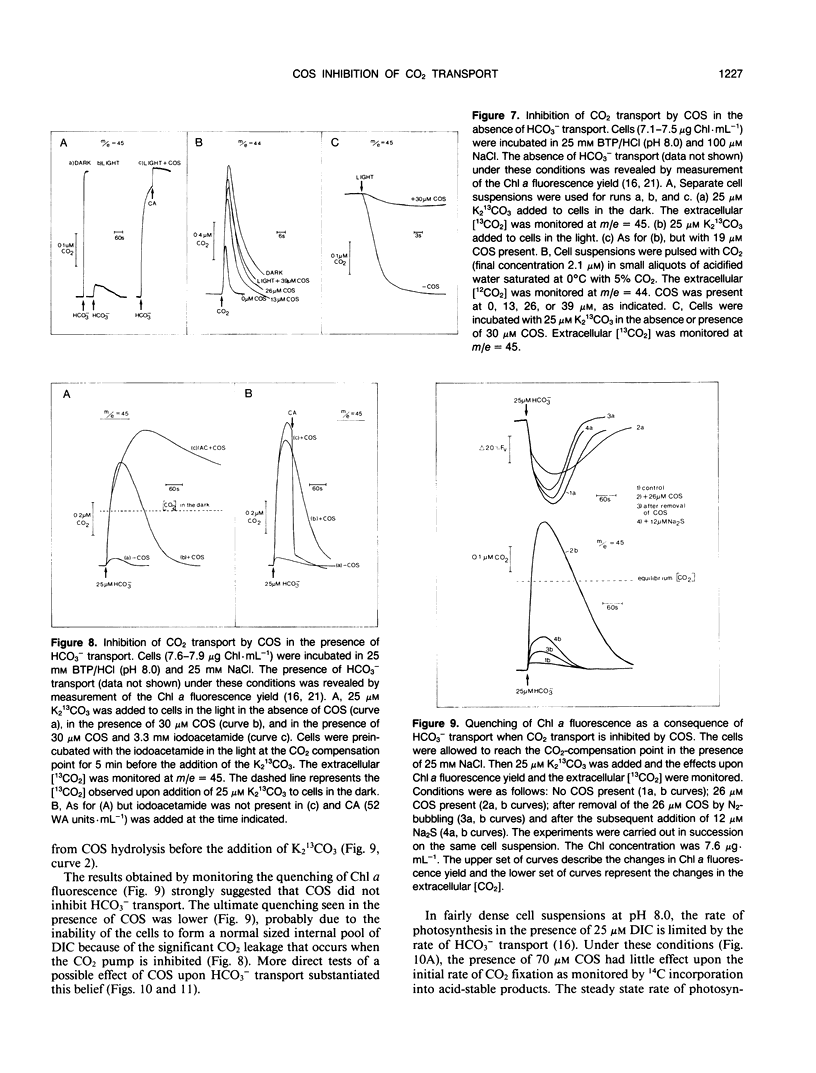
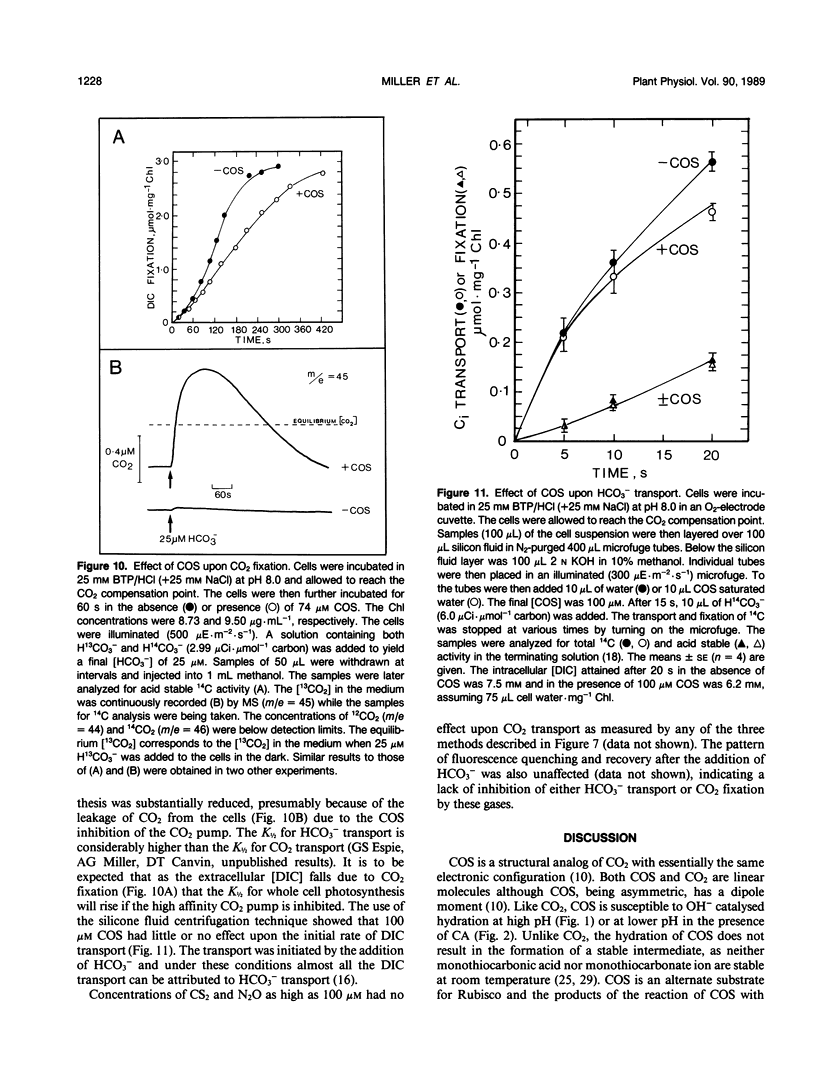
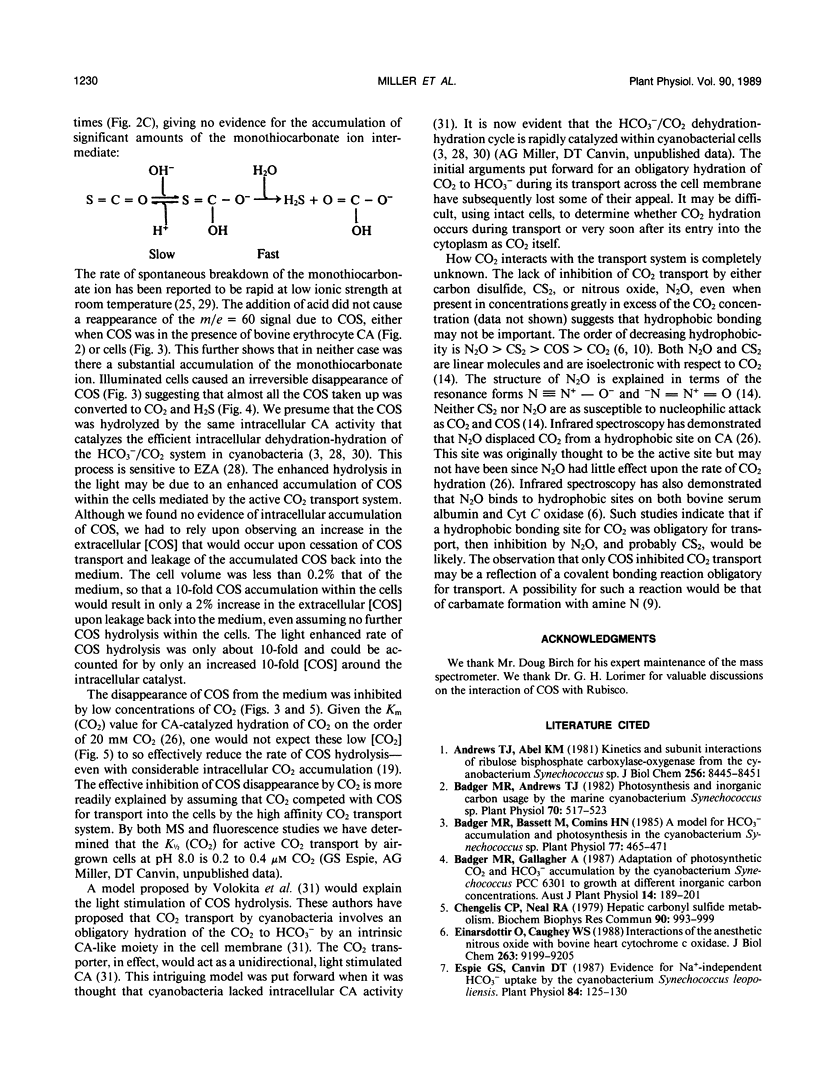
Carbon oxysulfide (carbonyl sulfide, COS) is a close structural analog of CO2. Although hydrolysis of COS (to CO2 and H2S) does occur at alkaline pH (>9), at pH 8.0 the rate of hydrolysis is slow enough to allow investigation of COS as a possible substrate and inhibitor of the active CO2 transport system of Synechococcus UTEX 625. A light-dependent uptake of COS was observed that was inhibited by CO2 and the ATPase inhibitor diethylstilbestrol. The COS taken up by the cells could not be recovered when the lights were turned off or when acid was added. It was concluded that most of the COS taken up was hydrolyzed by intracellular carbonic anhydrase. The production of H2S was observed and COS removal from the medium was inhibited by ethoxyzolamide. Bovine erythrocyte carbonic anhydrase catalysed the stoichiometric hydrolysis of COS to H2S. The active transport of CO2 was inhibited by COS in an apparently competitive manner. When Na+-dependent HCO3− transport was allowed in the presence of COS, the extracellular [CO2] rose considerably above the equilibrium level. This CO2 appearing in the medium was derived from the dehydration of transported HCO3− and was leaked from the cells. In the presence of COS the return to the cells of this leaked CO2 was inhibited. These results showed that the Na+-dependent HCO3− transport was not inhibited by COS, whereas active CO2 transport was inhibited. When COS was removed by gassing with N2, a normal pattern of CO2 uptake was observed. The silicone fluid centrifugation method showed that COS (100 micromolar) had little effect upon the initial rate of HCO3− transport or CO2 fixation. The steady state rate of CO2 fixation was, however, inhibited about 50% in the presence of COS. This inhibition can be at least partially explained by the significant leakage of CO2 from the cells that occurred when CO2 uptake was inhibited by COS. Neither CS2 nor N2O acted like COS. It is concluded that COS is an effective and selective inhibitor of active CO2 transport.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. J., Abel K. M. Kinetics and subunit interactions of ribulose bisphosphate carboxylase-oxygenase from the cyanobacterium, Synechococcus sp. J Biol Chem. 1981 Aug 25;256(16):8445–8451. [PubMed] [Google Scholar]
- Badger M. R., Andrews T. J. Photosynthesis and Inorganic Carbon Usage by the Marine Cyanobacterium, Synechococcus sp. Plant Physiol. 1982 Aug;70(2):517–523. doi: 10.1104/pp.70.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger M. R., Bassett M., Comins H. N. A Model for HCO(3) Accumulation and Photosynthesis in the Cyanobacterium Synechococcus sp: Theoretical Predictions and Experimental Observations. Plant Physiol. 1985 Feb;77(2):465–471. doi: 10.1104/pp.77.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chengelis C. P., Neal R. A. Hepatic carbonyl sulfide metabolism. Biochem Biophys Res Commun. 1979 Oct 12;90(3):993–999. doi: 10.1016/0006-291x(79)91925-9. [DOI] [PubMed] [Google Scholar]
- Einarsdóttir O., Caughey W. S. Interactions of the anesthetic nitrous oxide with bovine heart cytochrome c oxidase. Effects on protein structure, oxidase activity, and other properties. J Biol Chem. 1988 Jul 5;263(19):9199–9205. [PubMed] [Google Scholar]
- Espie G. S., Canvin D. T. Evidence for Na-Independent HCO(3) Uptake by the Cyanobacterium Synechococcus leopoliensis. Plant Physiol. 1987 May;84(1):125–130. doi: 10.1104/pp.84.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espie G. S., Miller A. G., Birch D. G., Canvin D. T. Simultaneous Transport of CO(2) and HCO(3) by the Cyanobacterium Synechococcus UTEX 625. Plant Physiol. 1988 Jul;87(3):551–554. doi: 10.1104/pp.87.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G. H., Pierce J. Carbonyl sulfide: an alternate substrate for but not an activator of ribulose-1,5-bisphosphate carboxylase. J Biol Chem. 1989 Feb 15;264(5):2764–2772. [PubMed] [Google Scholar]
- Miller A. G., Canvin D. T. Na-Stimulation of Photosynthesis in the Cyanobacterium Synechococcus UTEX 625 Grown on High Levels of Inorganic Carbon. Plant Physiol. 1987 May;84(1):118–124. doi: 10.1104/pp.84.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Colman B. Active transport and accumulation of bicarbonate by a unicellular cyanobacterium. J Bacteriol. 1980 Sep;143(3):1253–1259. doi: 10.1128/jb.143.3.1253-1259.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Espie G. S., Canvin D. T. Active Transport of CO(2) by the Cyanobacterium Synechococcus UTEX 625 : Measurement by Mass Spectrometry. Plant Physiol. 1988 Mar;86(3):677–683. doi: 10.1104/pp.86.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Espie G. S., Canvin D. T. Active Transport of Inorganic Carbon Increases the Rate of O(2) Photoreduction by the Cyanobacterium Synechococcus UTEX 625. Plant Physiol. 1988 Sep;88(1):6–9. doi: 10.1104/pp.88.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Espie G. S., Canvin D. T. Chlorophyll a Fluorescence Yield as a Monitor of Both Active CO(2) and HCO(3) Transport by the Cyanobacterium Synechococcus UTEX 625. Plant Physiol. 1988 Mar;86(3):655–658. doi: 10.1104/pp.86.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Turpin D. H., Canvin D. T. Growth and Photosynthesis of the Cyanobacterium Synechococcus leopoliensis in HCO(3)-Limited Chemostats. Plant Physiol. 1984 Aug;75(4):1064–1070. doi: 10.1104/pp.75.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Togasaki R. K. Carbonyl sulfide: an inhibitor of inorganic carbon transport in cyanobacteria. Plant Physiol. 1988 Nov;88(3):800–804. doi: 10.1104/pp.88.3.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocker Y., Sarkanen S. Carbonic anhydrase: structure catalytic versatility, and inhibition. Adv Enzymol Relat Areas Mol Biol. 1978;47:149–274. doi: 10.1002/9780470122921.ch3. [DOI] [PubMed] [Google Scholar]
- Spiller H., Wynns G. C., Tu C. Role of Photosynthetic Reactions in the Activity of Carbonic Anhydrase in Synechococcus sp. (UTEX 2380) in the Light : Inhibitor Studies Using the O-Exchange in C/O-Labeled Bicarbonate. Plant Physiol. 1988 Apr;86(4):1185–1192. doi: 10.1104/pp.86.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C., Spiller H., Wynns G. C., Silverman D. N. Carbonic Anhydrase and the Uptake of Inorganic Carbon by Synechococcus sp. (UTEX-2380). Plant Physiol. 1987 Sep;85(1):72–77. doi: 10.1104/pp.85.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volokita M., Zenvirth D., Kaplan A., Reinhold L. Nature of the Inorganic Carbon Species Actively Taken Up by the Cyanobacterium Anabaena variabilis. Plant Physiol. 1984 Nov;76(3):599–602. doi: 10.1104/pp.76.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]


