Abstract
Objective
To investigate the joint impact of sexual orientation, gender identity, and race/ethnicity on colorectal and breast cancer screening disparities in the United States.
Methods
Utilizing sampling weighted data from the 2016 and 2018 Behavioral Risk Factor Surveillance System, we assessed differences in two metrics via chi-square statistics: 1) lifetime uptake, and 2) up-to-date colorectal and breast cancer screening by sexual orientation and gender identity, within and across racial/ethnic classifications.
Results
Within specific races/ethnicities, lifetime CRC screening was higher among gay/lesbian (within NH-White, Hispanic, and Asian/Pacific Islander) and bisexual individuals (Hispanic) compared to straight individuals, and lowest overall among transgender women and transgender nonconforming populations (p < 0.05). Asian transgender women had the lowest lifetime CRC screening (13.0%; w.n. = 1,428). Lifetime breast cancer screening was lowest among the Hispanic bisexual population (86.6%; w.n. = 26,940) and Hispanic transgender nonconforming population (71.8%; w.n. = 739); within all races, SGM individuals (except NH-White, Hispanic, and Black bisexual populations, and NH-White transgender men) had greater breast cancer screening adherence compared to straight individuals.
Conclusions
Due to small, unweighted sample sizes, results should be interpreted with caution. Heterogeneity in screening participation by SGM status within and across racial/ethnic groups were observed, revealing the need to disaggregate data to account for intersecting identities and for studies with larger sample sizes to increase estimate reliability.
Keywords: Breast cancer screening, Colorectal cancer screening, Screening disparities, Intersectionality, Sexual minority health, Gender minority health
Highlights
-
•
Lifetime CRC and breast screening are lowest among transgender women, especially among Hispanic and Asian populations.
-
•
Individuals who identified as a sexual minority often had higher lifetime screening rates than straight individuals.
-
•
Heterogeneity illustrates the need to disaggregate data to account for intersecting identities.
-
•
Larger, more representative studies necessary to improve estimate reliability.
1. Introduction
In the United States (U.S.), screening of detectable cancers, such as colorectal (CRC) and breast, remain sources of cancer burden (Centers for Disease Control and Prevention [CDC], 2012). In 2022, CRC was the fourth most prevalent type of cancer and the second leading cause of cancer death (Siegel et al., 2022). Among women, breast cancer was the number one most prevalent cancer type and the second leading cause of cancer death (Siegel et al., 2022). Studies on CRC and breast cancer screening methods have shown that mortality can be reduced by 14–65% depending on the modality (Bretthauer, 2011; Kahi et al., 2009) and age of initiation (Nelson et al., 2009; Warner, 2011), respectively. Despite the demonstrated effectiveness of cancer screening, screening rates in the US do not currently meet the goals set by the Healthy People 2030 targets of 69.3% (Office of Health Promotion and Disease Prevention [ODPHP], 2023a) and 80.3% (ODPHP, 2023b) for CRC and breast cancer, respectively.
Cancer screening inequities are disparities that are unfair, avoidable, or stemming from some form of systematic injustice (Braveman, 2006; Kawachi et al., 2002). These inequities have been documented among racial and ethnic groups. Minority groups, such as Black (Burgess et al., 2011; Sauer et al., 2018), Hispanic, and Asian/Pacific Islander (Joseph et al., 2012; CDC, 2012) have substantially lower CRC screening rates than Non-Hispanic (NH) Whites. Similar inequities by race and ethnicity in breast cancer screening exist (Sabatino et al., 2008; Smith-Bindman et al., 2006; Swan et al., 2003). For both cancer types, mortality is highest among Black women (Gerend & Pai, 2008; Marcella & Miller, 2001; Siegel et al., 2022), and racial/ethnic minority groups are more likely to be diagnosed at a late stage (Marcella & Miller, 2001; Sassi et al., 2006). These differences in outcome and survival may be associated not only with lower screening rates among racial/ethnic groups, but also with systemic barriers such as poorer access to high-quality care, delays in treatment after diagnosis, or lack of insurance (Siegel et al., 2019).
Inequities in cancer screening by sexual orientation and gender identity have also been identified (Domogauer et al., 2022). Sexual and gender minorities (SGM) include, but are not limited to, individuals who identify as lesbian, gay, bisexual, asexual, transgender, or non-binary (National Institutes of Health, 2023). Previous studies report that a greater percentage of gay/bisexual men (Heslin et al., 2008) and transgender men (Charkhchi et al., 2019; Tabaac et al., 2018) compared to heterosexual and cisgender men, respectively, received CRC screening. Additionally, sexual minority and transgender women are less likely than heterosexual and cisgender women, respectively, to have obtained a regular mammogram (Austin et al., 2013; Tabaac et al., 2018). Similar to racial and ethnic minority groups, these inequities in preventive health behavior among SGM groups may be tied to experiencing greater stressors due to discrimination and prejudice towards their identity, negatively impacting their healthcare access and outcomes (Meyer, 1995; Meyer & Frost, 2013). This elevated stress, as described by the minority stress theory, contributes to distrust towards, hindered communication with, and avoidance of future interactions with medical providers, ultimately increasing the health risks among SGM populations (Gessner et al., 2020). Other mechanisms driving screening inequities among SGM and racial and ethnic minorities include not seeking or receiving recommendations or patient education from healthcare providers (Jackson et al., 2016); for example, some providers may be unaware of breast cancer screening recommendations for transgender men (Unger, 2015). There also exist systemic barriers to screening relating to socioeconomic status, such as lack of insurance and financial concerns, or transportation or geographical barriers (e.g., living in a rural place with limited access to healthcare) (Lombardo et al., 2022; Miller et al., 2019).
According to the intersectionality theoretical framework, health behavior disparities may be increased for individuals facing multiple, intersecting types of discrimination, such as racism, sexism, homophobia, and transphobia (Damaskos et al., 2018). These individuals’ lived experiences and perceptions of mistreatment may serve as barriers to participation in protective health behaviors, such as cancer screening (Stenzel et al., 2022; Trinh et al., 2017), and lead to poorer health outcomes (Hsieh & Ruther, 2016; Trinh et al., 2017; Veenstra, 2011). Inequities in cervical cancer screening inequities by race/ethnicity and sexual and gender minority status have been examined previously (Lin et al., 2023); however, a few studies have explored the impact of this intersection on CRC and breast cancer screening. One study reported that a higher proportion of gay/bisexual men engaged in lifetime and up-to-date screening than heterosexual men for all races except Asian (Heslin et al., 2008), while lesbian women had lower odds of mammography in comparison to heterosexual women for both Black and Hispanic populations (Agénor et al., 2020).
Given the limited nature of the current literature, and the importance of considering the impact of intersecting identities when designing interventions to increase screening uptake (Ackerson & Gretebeck, 2007), more evidence is needed. Therefore, the aim of the current study is to describe variation in CRC and breast cancer screening uptake among SGM within (SGM vs. heterosexual/cisgender of the same race/ethnicity) and across (SGM of color vs. heterosexual/cisgender NH-White) racial/ethnic groups.
2. Methods
2.1. Study population
Data for this study was sourced from the Centers for Disease Control and Prevention (CDC) 2016 and 2018 Behavioral Risk Factor Surveillance System (BRFSS), which is an annual telephone survey that asks over 400,000 U.S. residents age eighteen and above about their health-related risk behaviors, chronic health conditions, and use of preventive services (CDC, 2019). We reported data from the 26 states (see Supplementary Materials for details) and territories that collected data on sexual orientation and gender identity, and from respondents within the age range of 50–75 and those who identified as NH-White, Black, Hispanic or Asian (including Pacific Islander). For breast cancer analysis specifically, we also excluded individuals who identified as cisgender male. (For details about exclusion criteria see Supplementary Materials.) The final unweighted sample size was 183,610 individuals for CRC screening (for details see Table 1a) and 103,855 individuals for breast cancer screening (for details seeTable 1b).
Table 1a.
Characteristics of individuals eligible for colorectal cancer screening by total population, sexual orientation, and gender identity (n = 183,610; w.n. = 62,857,421).
| Variables | % Overall Total Eligible Population (95% CI) n [w.n.] | Sexual Orientation |
Gender Identity |
||||||
|---|---|---|---|---|---|---|---|---|---|
| % Straight (95% CI) n [w.n.] | % Lesbian or Gay (95% CI) n [w.n.] | % Bisexual (95% CI) n [w.n.] | % Cisgender Male (95% CI) n [w.n.] | % Cisgender Female (95% CI) n [w.n.] | % Transgender Woman (95% CI) n [w.n.] | % Transgender Man (95% CI) n [w.n.] | % Transgender Nonconforming (95% CI) n [w.n.] | ||
| Gender Identity | |||||||||
| Cisgender Male | 47.0 (46.6–47.5) 79,337 [39,925,629] |
47.0 (46.5–47.5) 74,806 [37,434,735] |
62.8 (58.2–67.2) 1,655 [801,772] |
51.8 (47.4–56.3) 878 [434,784] |
|||||
| Cisgender Female | 52.6 (52.1–53.1) 103,487 [44,556,632] |
52.7 (52.2–53.2) 98,133 [41,907,979] |
36.0 (31.6–40.6) 1,129 [458,960] |
45.9 (41.6–50.4) 976 [377,481] |
|||||
| Transgender Woman | 0.2 (0.1–0.3) 286 [154,402] |
0.2 (0.1–0.2) 212 [120,640] |
0.7 (0.4–1.3) 21 [8,958] |
0.9 (0.3–2.5) 19 [7,300] |
|||||
| Transgender Man | 0.1 (0.08–0.1) 232 [90,700] |
0.1 (0.08–0.1) 193 [78,084] |
0.1 (0.04–0.3) 9 [1,316] |
0.4 (0.2–0.9) 10 [3,417] |
|||||
| Transgender Nonconforming | 0.05 (0.04–0.07) 112 [44,498] |
0.03 (0.02–0.05) 67 [25,742] |
0.04 (0.1–1.2) 10 [4,882] |
0.9 (0.4–2.0) 17 [7,623] |
|||||
| Sexual Orientation | |||||||||
| Straight | 97.4 (97.2–97.6) 173,412 [79,567,202] |
96.8 (96.5–97.0) 74,806 [37,434,735] |
98.0 (97.8–98.2) 98,133 [41,907,979] |
88.1 (78.4–93.8) 212 [120,640] |
94.2 (89.5–96.9) 193 [78,084] |
67.3 (49.1–81.4) 67 [25,742] |
|||
| Lesbian or Gay | 1.6 (1.4–1.7) 2,824 [1,275,889] |
2.1 (1.9–2.3) 1,655 [801,772] |
1.1 (0.9–1.3) 1,129 [458,960] |
6.5 (3.3–12.5) 21 [8,958] |
1.6 (0.6–4.0) 9 [1,316] |
12.8 (4.2–32.9) 10 [4,882] |
|||
| Bisexual | 1.0 (0.9–1.1) 1,900 [830,605] |
1.1 (1.0–1.3) 878 [434,784] |
0.9 (0.8–1.0) 976 [377,481] |
4.1 (1.9–8.7) 19 [7,300] |
4.1 (1.9–8.7) 10 [3,417] |
19.9 (9.5–37.1) 17 [7,623] |
|||
| Age | |||||||||
| 50–59 | 66.3 (65.9–66.7) 830,605 [56,250,349] |
66.2 (65.8–66.7) 100,446 [52,728,284] |
75.2 (70.6–79.3) 1,916 [959,721] |
71.0 (66.8–74.9) 1,213 [590,212] |
67.3 (66.7–68.0) 46,865 [26,878,245] |
65.4 (64.8–66.0) 59,169 [29,127,889] |
76.4 (63.8–85.6) 179 [117,948] |
60.2 (49.5–70.1) 139 [54,624] |
67.5 (53.7–78.8) 65 [30,025] |
| 60–75 | 33.7 (33.3–34.1) 77,109 [28,590,204] |
33.7 (33.3–34.2) 72,966 [26,838,918] |
24.7 (20.7–29.4) 908 [316,167] |
28.9 (25.0–33.2) 687 [240,394] |
32.7 (32.0–33.3) 32,472 [13,047,384] |
34.6 (34.0–35.2) 44,318 [15,428,743] |
23.6 (14.4–36.2) 107 [36,454] |
39.8 (29.9–50.5) 93 [36,075] |
32.5 (21.2–46.8) 47 [14,473] |
| Race | |||||||||
| NH-White | 74.3 (73.8–74.8) 156,621 [63,057,996] |
75.7 (75.2–76.2) 149,201 [60,261,069] |
78.4 (72.4–83.4) 2,509 [1,000,215] |
70.6 (65.8–75.1) 1,605 [586,751] |
74.3 (73.5–75.1) 68,196 [29,673,071] |
74.4 (73.7–75.1) 87,808 [33,168,654] |
47.6 (31.5–64.3) 213 [73,516] |
74.0 (61.6–83.4) 190 [67,086] |
72.8 (57.9–83.8) 86 [32,375] |
| Black | 10.9 (10.6–11.2) 13,342 [9,232,404] |
10.9 (10.5–11.2) 12,245 [8,633,274] |
7.2 (5.5–9.3) 126 [91,682] |
14.3 (11.0–18.3) 134 [118,757] |
10.4 (9.9–10.9) 5,117 [4,155,023] |
11.3 (10.8–11.7) 8,145 [5,020,182] |
17.9 (8.1–35.1) 33 [27,654] |
16.7 (9.0–28.9) 22 [15,174] |
16.8 (8.0–32.1) 12 [7,489] |
| Hispanic | 10.7 (10.2–11.1) 8,241 [9,036,868] |
9.5 (9.1–9.9) 6,970 [7,568,246] |
8.5 (5.7–12.4) 134 [107,935] |
10.5 (7.6–14.2) 102 [86,830] |
11.0 (10.3–11.6) 3,506 [4,380,711] |
10.3 (9.8–10.9) 4,685 [4,593,935] |
27.4 (11.2–52.9) 26 [42,253] |
7.4 (2.7–18.5) 7 [6,703] |
5.3 (2.1–13.1) 8 [2,378] |
| Asian | 4.1 (3.9–4.4) 5,406 [3,513,285] |
3.9 (3.6–4.2) 4,996 [3,104,612] |
6.0 (2.5–13.7) 55 [76,058] |
4.6 (2.7–7.8) 59 [38,267] |
4.3 (3.9–4.7) 2,518 [1,716,824] |
4.0 (3.6–4.4) 2,849 [1,773,862] |
7.1 (2.1–21.3) 14 [10,979] |
1.9 (0.9–4.1) 13 [1,736] |
5.1 (1.6–14.7) 6 [2,257] |
| Education | |||||||||
| Elementary School or Some High School | 12.9 (11.2–12.1) 11,535 [10,911,786] |
11.6 (11.2–12.1) 9,990 [9,251,103] |
7.9 (5.5–11.3) 95 [100,921] |
13.4 (9.8–17.9) 120 [110,965] |
13.5 (12.9–14.1) 5,169 [5,381,790] |
12.2 (11.6–12.8) 6,262 [5,442,189] | 35.8 (18.7–57.5) 52 [55,250] |
16.9 (9.6–27.9) 27 [15,333] |
10.1 (4.8–19.8) 10 [4,478] |
| High School | 28.3 (27.9–28.7) 49,777 [23,991,894] |
28.7 (28.2–29.0) 47,230 [22,800,076] |
19.0 (14.9–23.8) 418 [242,310] |
23.8 (20.3–27.7) 471 [197,708] |
28.7 (28.1–29.3) 21,699 [11,456,215] |
27.9 (27.3–28.5) 27,809 [12,424,978] |
30.9 (20.0–44.5) 110 [47,777] |
33.1 (24.1–43.6) 77 [30,036] |
31.6 (19.9–46.3) 40 [14,080] |
| College | 58.7 (58.2–59.2) 121,938 [49,778,629] |
59.5 (59.0–60.0) 115,891 [47,381,044] |
73.0 (68.0–77.5) 2,307 [931,743] |
62.7 (58.1–67.2) 1,305 [521,034] |
57.6 (56.9–58.4) 52,312 [23,014,626] |
59.7 (59.0–60.4) 69,216 [26,605,133] |
33.3 (20.2–49.6) 124 [51,374] |
50.0 (39.3–60.7) 128 [45,330] |
58.3 (43.7–71.6) 62 [25,940] |
| Missing | 0.2 (0.15–0.23) 360 [158,245] |
0.2 (0.1–0.2) 301 [134,978] |
0.1 (0.02–0.2) 4 [915] |
0.1 (0.03–0.4) 4 [899] |
0.2 (0.1–0.3) 157 [72,997] |
0.2 (0.1–0.3) 200 [84,333] |
0.0 n = 0 |
0.0 n = 0 |
0.0 n = 0 |
| Marital Status | |||||||||
| Has a Partner | 64.6 (64.2–65.1) 109,895 [54,818,424] |
65.4 (64.9–65.9) 105,160 [52,030,412] |
42.7 (38.5–47.1) 1,199 [545,406] |
52.8 (48.3–57.2) 857 [438,451] |
69.1 (68.4–69.7) 51,776 [27,579,488] |
60.7 (60.0–61.3) 57,741 [27,030,421] |
59.5 (44.0–73.3) 124 [91,797] |
53.1 (42.3–63.6) 120 [48,163] |
53.1 (38.4–67.5) 50 [23,668] |
| No Partner | 35.1 (34.6–35.5) 72,950 [29,738,362] |
34.3 (33.8–34.8) 67,605 [27,288,826] |
56.9 (52.6–61.2) 1,615 [726,755] |
47.1 (42.7–51.6) 1,036 [391,199] |
30.7 (30.0–31.3) 27,301 [12,249,108] |
38.9 (38.2–39.6) 45,247 [17,340,190] |
40.4 (26.6–55.9) 161 [62,376] |
46.9 (36.4–57.7) 112 [42,536] |
46.8 (36.4–57.7) 62 [20,830] |
| Missing | 0.3 (0.3–0.4) 765 [283,768] |
0.3 (0.3–0.4) 647 [247,964] |
0.3 (0.1–0.6) 10 [3,728] |
0.1 (0.03–0.4) 7 [955] |
0.2 (0.2–0.3) 260 [97,033] |
0.4 (0.4–0.5) 499 [186,021] |
0.1 (0.02–1.1) 1 [228] |
0.0 n = 0 |
0.0 n = 0 |
| Employment Status | |||||||||
| Employed | 49.5 (49.0–50.0) 84,020 [41,973,880] |
49.9 (49.4–50.4) 79,799 [39,688,475] |
52.5 (47.9–57.1) 1,451 [669,801] |
51.5 (47.0–56.0) 899 [428,105] |
55.3 (54.6–56.0) 9,509 [5,463,526] |
44.3 (43.6–45.0) 13,851 [6,717,512] |
58.1 (42.2–72.5) 54 [22,282] |
38.8 (28.8–49.8) 60 [16,121] |
44.7 (30.8–59.5) 24 [11,144] |
| Unemployed | 14.4 (14.1–14.8) 23,527 [12,243,411] |
14.0 (13.6–14.4) 21,628 [11,139,400] |
17.4 (14.7–20.6) 448 [222,630] |
18.1 (13.9–21.8) 336 [150,055] |
13.7 (13.2–14.2) 262 [126,107] |
15.1 (14.6–15.6) 6,864 [4,209,458] |
14.4 (8.5–23.5) 5 [3,809] |
17.8 (12.1–25.3) 9 [6,604] |
25.0 (13.6–41.5) 5 [1,247] |
| Student/Homemaker | 5.1 (4.9–5.4) 7,151 [4,355,388] |
5.0 (4.7–5.3) 6,703 [3,967,671] |
1.5 (0.5–4.0) 22 [19,655] |
3.2 (2.1–4.9) 55 [26,400] |
0.3 (0.3–0.4) 28,932 [12,053,676] |
9.4 (9.0–10.0) 38,857 [13,674,354] |
2.5 (0.8–7.6) 107 [38,330] |
7.3 (2.3–20.6) 79 [31,500] |
2.8 (0.7–10.0) 36 [12,198] |
| Retired | 30.4 (30.0–30.9) 68,073 [25,829,526] |
30.7 (30.3–31.1) 64,554 [24,411,806] |
28.3 (23.7–33.4) 896 [361,607] |
26.7 (22.8–29.0) 604 [221,693] |
30.2 (29.6–30.8) 28,932 [12,053,676] |
30.7 (30.1–31.3) 38,857 [13,674,354] |
24.8 (15.2–37.8) 107 [38,330] |
34.7 (25.2–45.6) 79 [31,500] |
27.4 (16.4–42.0) 36 [12,198] |
| Missing | 0.5 (0.4–0.6) 839 [438,349] |
0.5 (0.4–0.5) 728 [359,850] |
0.2 (0.1–0.5) 7 [2,196] |
0.5 (0.2–1.5) 6 [4,353] |
0.5 (0.4–0.7) 370 [212,772] |
0.5 (0.4–0.6) 460 [222,362] |
0.2 (0.03–1.4) 1 [292] |
1.4 (0.3–6.2) 2 [1,265] |
0.0 n = 0 |
| Income as Percent of FPL | |||||||||
| <100 | 9.6 (9.3–9.9) 13,046 [8,141,002] |
8.9 (8.6–9.2) 11,678 [7,060,439] |
7.7 (5.8–10.2) 176 [97,978] |
13.3 (10.2–17.3) 195 [110,919] |
8.9 (8.4–9.4) 5,079 [3,557,122] |
10.2 (9.7–10.6) 7,868 [4,535,513] |
11.5 (5.2–23.8) 35 [17,810] |
13.8 (8.0–22.7) 33 12,507] |
13.7 (5.7–29.3) 12 [6,082] |
| 100–200 | 19.0 (18.7–19.4) 35,752 [16,159,760] |
18.8 (18.4–19.2) 33,294 [14,941,458] |
19.0 (16.0–22.4) 556 [242,120] |
25.2 (21.4–29.4) 478 [209,187] |
18.5 (18.0–19.1) 14,347 [7,405,358] |
19.4 (18.9–19.9) 21,171 [8,630,515] |
44.7 (27.3–63.5) 97 [69,069] |
26.0 (18.2–35.8) 63 [23,615] |
29.3 (16.8–45.8) 32 [13,018] |
| >200 | 71.4 (70.9–71.8) 134,812 [60,539,792] |
72.3 (71.9–72.8) 128,440 [57,565,305] |
73.3 (69.5–76.9) 2,092 [935,791] |
61.5 (56.8–70.1) 1,227 [510,500] |
72.5 (71.8–73.2) 59,911 [28,963,149] |
70.5 (69.8–71.1) 74,448 [31,390,605] |
43.7 (28.8–59.9) 154 [67,524] |
60.2 (49.5–69.9) 136 [54,577] |
57.1 (41.6–71.3) 68 [25,398] |
| Insurance Status | |||||||||
| Yes | 93.5 (93.2–93.8) 175,273 [79,326,383] |
93.8 (93.5–94.1) 165,880 [74,640,977] |
95.6 (94.1–96.7) 2,712 [1,219,496] |
91.9 (88.7–94.3) 1,789 [763,506] |
92.7 (92.2–93.1) 75,189 [36,991,481] |
94.3 (93.9–94.6) 99,356 [41,999,736] |
94.5 (89.3–97.3) 263 [145,944] |
93.0 (86.5–96.5) 215 [84,382] |
95.4 (86.2–98.6) 104 [42,439] |
| No | 6.3 (6.0–6.6) 7,961 [5,324,288] |
6.0 (5.7–6.3) 7,199 [4,758,975] |
4.4 (3.3–5.9) 112 [56,393] |
7.8 (5.5–10.9) 105 [64,518] |
7.2 (6.7–7.7) 3,991 [2,860,863] |
5.5 (5.1–5.8) 3,915 [2,441,530] |
5.4 (2.7–10.6) 22 [8,336] |
6.8 (3.3–13.3) 16 [6,168] |
4.6 (1.4–13.8) 8 [2,059] |
| Missing | 0.2 (0.2–0.3) 376 [189,882] |
0.2 (0.2–0.3) 333 [167,250] |
0.0 n = 0 | 0.3 (0.09–1.1) 6 [2,582] |
0.2 (0.1–0.2) 157 [73,284] |
0.3 (0.2–0.3) 216 [115,366] |
0.08 (0.01–0.6) 1 [122] |
0.2 (0.02–1.2) 1 [149] |
0.0 n = 0 |
| Health Care Access Hardship | |||||||||
| Yes | 9.9 (9.6–10.2) 14,489 [8,377,037] |
9.6 (9.3–10.0) 13,348 [7,657,589] |
7.3 (5.8–9.0) 219 [92,608] |
13.7 (10.8–17.2) 203 [113,593] |
9.0 (8.5–9.4) 5,570 [3,580,519] |
10.7 (10.2–11.1) 8,833 [4,753,036] |
13.5 (6.6–25.6) 37 [20,917] |
9.2 (5.1–16.1) 21 [8,389] |
11.9 (5.4–24.2) 14 [5,274] |
| No | 89.6 (89.3–90.0) 168,368 [76,048,273] |
89.9 (89.6–90.2) 159,384 [71,538,186] |
92.4 (90.5–93.9) 2,596 [1,178,363] |
84.8 (80.9–88.1) 1,689 [704,525] |
90.5 (90.0–90.9) 73,404 [36,119,455] |
88.9 (88.5–89.4) 94,270 [39,618,826] |
84.2 (71.6–91.9) 246 [130,023] |
89.4 (82.3–93.8) 209 [81,052] |
88.1 (75.8–94.6) 98 [39,224] |
| Missing | 0.5 (0.4–0.6) 753 [415,243] |
0.5 (0.4–0.5) 680 [371,428] |
0.4 (0.1–1.0) 9 [4,917] |
1.5 (0.4–5.3) 8 [12,488] |
0.6 (0.5–0.7) 363 [225,654] |
0.4 (0.4–0.5) 384 [184,770] |
2.2 (0.4–10.9) 3 [3,462] |
1.4 (0.3–6.0) 2 [1,258] |
0.0 n = 0 |
| Personal Doctor | |||||||||
| Yes | 89.6 (89.3–89.9) 167,041 [76,007,818] |
89.9 (89.6–90.2) 158,032 [71,506,907] |
93.5 (91.7–94.9) 2,620 [1,192,787] |
90.3 (87.6–92.4) 1,710 [749,753] |
87.0 (86.5–87.5) 70,323 [34,745,607] |
92.0 (91.6–92.3) 96,028 [40,975,262] |
74.9 (48.1–90.6) 243 [115,720] |
82.6 (69.3–90.8) 211 [74,880] |
86.7 (74.3–93.6) 96 [38,561] |
| No | 9.9 (9.6–10.2) 15,703 [8,396,143] |
9.6 (9.3–10.0) 14,593 [7,678,224] | 6.3 (5.0–8.1) 196 [80,845] |
9.0 (7.0–11.6) 181 [75,017] |
12.4 (11.9–13.0) 8,611 [4,968,658] |
7.5 (7.2–7.9) 7,000 [3,361,172] |
25.1 (9.4–51.9) 43 [38,682] |
11.9 (5.8–23.1) 18 [10,823] |
13.3 (6.4–25.7) 16 [5,937] |
| Missing | 0.5 (0.5–0.6) 866 [436,592] |
0.5 (0.4–0.5) 787 [382,071] |
0.2 (0.08–0.4) 8 [2,257] |
0.7 (0.2–2.3) 9 [5,835] |
0.5 (0.5–0.6) 403 [211,363] |
0.5 (0.4–0.6) 459 [220,198] |
0.0 n = 0 |
5.5 (1.2–21.2) 3 [4,996] |
0.0 n = 0 |
| Checkup in the Last 2 Years | |||||||||
| Yes | 90.5 (90.3–90.8) 167,477 [76,818,767] |
90.6 (90.4–90.9) 158,245 [72,121,104] |
91.8 (89.4–93.7) 2,596 [1,171,302] |
89.7 (87.0–92.0) 1,723 [745,634] |
88.8 (88.3–89.2) 71,138 [35,439,326] |
92.1 (91.8–92.5) 95,632 [41,057,052] |
90.3 (77.9–96.1) 256 [139,474] |
87.5 (79.5–92.7) 205 [79,389] |
89.0 (77.1–95.1) 99 [39,586] |
| No | 8.6 (8.4–8.9) 14,594 [7,314,607] |
8.6 (8.3–8.9) 13,778 [6,838,588] |
7.1 (5.3–9.3) 205 [89,965] |
9.5 (7.3–12.1) 168 [78,540] |
10.5 (10.0–10.9) 7,582 [4,178,387] |
7.0 (6.7–7.3) 6,947 [3,107,615] |
9.1 (3.5–21.8) 26 [14,103] |
9.6 (5.0–17.4) 20 [8,664] |
8.4 (3.5–18.8) 12 [3,741] |
| Missing | 0.8 (0.8–0.9) 1,539 [707,179] |
0.8 (0.7–0.8) 1,389 [607,510] |
1.1 (0.6–2.1) 23 [14,621] |
0.8 (0.3–2.3) 9 [6,431] |
0.8 (0.7–0.9) 617 [307,915] |
0.9 (0.8–1.0) 908 [391,965] |
0.5 (0.1–2.2) 4 [824] |
2.9 (1.1–7.3) 7 [2,646] |
2.6 (0.4–16.4) 1 [1,172] |
Table 1b.
Characteristics of individuals eligible for breast cancer screening by total population, sexual orientation, and gender identity. (n = 103,855; w.n. = 44,730,594).
| Variables | % Overall Total Eligible Population (95% CI) n [w.n.] | Sexual Orientation |
Gender Identity |
|||||
|---|---|---|---|---|---|---|---|---|
| % Straight (95% CI) n [w.n.] | % Lesbian or Gay (95% CI) n [w.n.] | % Bisexual (95% CI) n [w.n.] | % Cisgender Female (95% CI) n [w.n.] | % Transgender Woman (95% CI) n [w.n.] | % Transgender Man (95% CI) n [w.n.] | % Transgender Nonconforming (95% CI) n [w.n.] | ||
| Gender Identity | ||||||||
| Cisgender Female | 99.8 (99.7–99.8) 103,282 [44,449,368] |
99.8 (99.7–99.8) 97,937 [41,817,861] |
99.1 (98.3–99.5) 1,125 [455,599] |
99.2 (97.8–99.7) 972 [376,907] |
||||
| Transgender Woman | 0.06 (0.04–0.09) 77 [27,777] |
0.06 (0.04–0.09) 57 [23,707] |
0.005 (0.002–1.2) 6 [2,186] |
0.06 (0.03–0.2) 5 [246] |
||||
| Transgender Man | 0.1 (0.1–0.2) 56 [22,382] |
0.1 (0.1–0.2) 37 [17,515] |
0.07 (0.02–0.3) 4 [322] |
0.003 (0.008–1.1) 3 [1,116] |
||||
| Transgender Nonconforming | 0.05 (0.03–0.08) 56 [22,382] |
0.04 (0.03–0.07) 37 [17,515] |
0.4 (0.1–0.9) 5 [1,665] |
0.5 (0.09–2.3) 5 [1,727] |
||||
| Sexual Orientation | ||||||||
| Straight | 98.0 (97.8–98.2) 98,440 [42,082,276] |
98.0 (97.8–98.2) 97,937 [41,817,861] |
90.7 (79.6–96.1) 57 [23,707] |
97.3 (92.4–99.1) 125 [51,325] |
83.8 (64.0–93.8) 37 [17,515] |
|||
| Lesbian or Gay | 0.1 (0.09–1.3) 1,143 [460,168] |
1.1 (0.9–1.3) 1,125 [455,599] |
8.4 (3.3–19.7) 6 [2,186] |
0.6 (0.2–2.2) 4 [322] |
8.0 (3.0–20.0) 5 [1,665] |
|||
| Bisexual | 0.9 (0.8–1.0) 989 [381,124] |
0.9 (0.8–1.0) 972 [376,907] |
0.9 (0.3–2.6) 5 [246] |
2.1 (0.6–7.5) 3 [1,116] |
8.3 (1.6–32.6) 5 [1,727] |
|||
| Age | ||||||||
| 50–59 | 65.4 (64.8–66.0) 59,386 [29,240,474] |
65.4 (64.8–66.0) 56,237 [27,533,589] |
72.9 (62.4–81.3) 797 [335,405] |
71.5 (66.0–76.5) 644 [272,677] |
65.4 (64.8–66.0) 59,089 [29,086,646] |
74.3 (59.2–85.2) 46 [20,628] |
58.6 (45.0–71.0) 87 [33,812] |
63.7 (43.7–79.9) 31 [14,253] |
| 60–75 | 34.6 (34.0–35.2) 44,469 [15,490,120] |
34.6 (34.0–35.2) 42,203 [14,548,687] |
27.1 (18.7–37.6) 346 [124,763] |
28.5 (23.5–34.0) 345 [108,447] |
34.6 (34.0–35.2) 44,193 [15,362,722] |
25.7 (14.8–40.8) 31 [7,149] |
41.3 (29.0–55.0) 62 [23,864] |
36.3 (20.1–56.3) 25 [8,129] |
| Race | ||||||||
| NH-White | 74.4 (73.7–75.1) 88,098 [33,287,778] |
75.9 (75.2–76.6) 84,306 [31,947,628] |
79.2 (67.2–87.6) 1,023 [364,256] |
78.5 (72.9–83.3) 845 [299,327] |
74.5 (73.8–75.2) 87,688 [33,133,075] |
64.3 (43.0–81.1) 56 [17,865] |
78.8 (63.1–89.0) 122 [45,456] |
85.3 (70.8–93.3) 41 [19,094] |
| Black | 11.3 (10.8–11.7) 8,180 [5,047,195] |
11.3 (10.8–11.7) 7,521 [4,745,060] |
6.8 (4.3–10.5) 51 [31,121] |
11.2 (7.6–16.1) 69 [42,570] |
11.3 (10.8–11.7) 8,109 [5,011,856] |
15.1 (6.3–31.7) 11 [4,183] |
11.3 (4.4–28.9) 14 [6,497] |
9.5 (3.6–22.7) 7 [2,130] |
| Hispanic | 10.3 (9.8–10.9) 4,708 [4,609,827] |
9.2 (8.6–9.7) 3,961 [3,851,420] |
6.6 (3.5–12.1) 46 [30,246] |
8.2 (5.4–12.2) 53 [31,116] |
10.2 (9.7–10.8) 4,643 [4,534,120] | 19.0 (6.0–46.2) 6 [5,269] |
7.6 (2.0–25.0) 5 [4,381] |
4.6 (1.2–16.5) 5 [1,029] |
| Asian | 4.0 (3.6–4.4) 2,869 [1,785,796] |
3.7 (3.3–4.1) 2,652 [1,538,168] |
7.5 (1.7–27.3) 23 [34,545] |
2.1 (0.9–5.2) 22 [8,112] |
4.0 (3.6–4.4) 2,842 [1,770,318] |
1.7 (0.4–6.4) 4 [459] |
2.3 (0.9–5.9) 8 [1,342] |
0.6 (0.2–2.1) 3 [129] |
| Education | ||||||||
| Elementary School or Some High School | 12.2 (11.6–12.8) 6,294 [5,447,703] |
10.9 (10.3–11.5) 5,394 [4,576,653] |
11.7 (6.6–19.7) 41 [53,679] |
5.6 (3.8–8.4) 53 [21,490] |
12.1 (11.5–12.6) 6,183 [5,356,431] |
15.6 (6.5–32.9) 10 [4,331] |
16.8 (8.4–30.8) 18 [9,673] |
6.2 (1.7–19.9) 4 [1,383] |
| High School | 27.9 (27.3–28.5) 27,928 [12,476,603] |
28.2 (27.7–28.8) 26,512 [11,882,980] |
14.2 (10.8–18.6) 175 [65,495] |
23.6 (18.7–29.2) 224 [89,808] | 27.9 (27.3–28.5) 27,729 [12,393,462] |
30.6 (16.6–49.4) 22 [8,492] |
33.1 (22.2–46.2) 51 [19,081] |
25.8 (12.8–45.3) 17 [5,783] |
| College | 59.7 (59.1–60.4) 69,435 [26,721,532] |
60.7 (60.0–61.4) 66,369 [25,544,267] |
74.0 (66.6–80.3) 926 [340,740] |
70.8 (65.1–75.9) 710 [269,695] |
59.9 (59.2–60.6) 69,176 [26,615,089] |
53.8 (35.2–71.4) 45 [14,954] |
50.1 (36.7–63.6) 80 [28,922] |
68.0 (48.3–82.8) 35 [15,216] |
| Missing | 0.2 (0.1–0.3) 198 [84,757] |
0.2 (0.1–0.3) 165 [78,376] |
0.06 (0.008–0.2) 1 [254] |
0.03 (0.007–0.5) 2 [130] |
0.2 (0.1–0.3) 194 [84,386] |
0.0 n = 0 |
0.0 n = 0 |
0.0 n = 0 |
| Marital Status | ||||||||
| Has a Partner | 60.7 (60.0–61.3) 57,918 [27,142,125] |
61.3 (60.7–62.0) 55,438 [25,818,559] |
45.9 (38.5–53.5) 565 [211,274] |
51.1 (45.2–57.0) 460 [194,862] |
60.8 (60.1–61.4) 57,662 [27,008,742] |
48.2 (30.1–66.8) 32 [13,390] |
48.9 (35.5–62.5) 67 [28,224] |
64.4 (44.8–80.2) 24 [14,422] |
| No Partner | 38.9 (38.3–39.5) 45,435 [17,400,842] |
38.3 (37.6–38.9) 42,570 [16,098,115] |
53.4 (45.7–60.9) 571 [245,820] |
48.2 (43.8–52.6) 528 [186,233] |
38.8 (38.2–39.5) 45,121 [17,254,568] |
51.8 (33.2–69.9) 45 [14,387] |
51.1 (37.5–64.5) 82 [29,452] |
35.6 (19.8–55.2) 32 [7,960] |
| Missing | 0.4 (0.4–0.5) 502 [187,628] |
0.4 (0.3–0.5) 432 [165,602] |
0.7 (0.3–1.6) 7 [3,074] |
0.008 (0.001–0.06) 1 [27] |
0.4 (0.4–0.5) 499 [186,058] |
0.0 n = 0 |
0.0 n = 0 |
0.0 n = 0 |
| Employment Status | ||||||||
| Employed | 44.3 (43.7–45.0) 43,597 [19,816,593] |
44.8 (44.2–45.5) 41,568 [18,872,962] |
46.3 (38.8–54.0) 575 [213,228] |
51.7 (45.8–57.6) 458 [197,037] |
44.4 (43.7–45.0) 43,436 [19,721,400] |
43.0 (25.6–62.3) 27 [11,947] |
33.0 (21.4–47.1) 42 [19,028] |
36.5 (19.3–58.0) 16 [8,175] |
| Unemployed | 15.1 (14.6–15.6) 13,942 [6,740,523] | 14.6 (14.1–15.1) 12,877 [6,146,528] |
18.8 (13.8–25.1) 185 [86,407] |
17.6 (13.5–22.6) 186 [67,049] |
15.0 (14.5–15.5) 13,795 [6,676,043] |
30.4 (14.8–52.3) 19 [8,448] |
17.9 (11.0–27.7) 44 [10,331] |
21.0 (9.5–40.3) 16 [4,696] |
| Student/Homemaker | 9.4 (8.9–9.9) 6,877 [4,218,190] |
9.1 (8.7–9.7) 6,461 [3,849,739] |
4.2 (1.6–10.5) 18 [19,495] |
6.8 (4.4–10.3) 51 [25,786] |
9.4 (8.9–9.9) 6,831 [4,186,726] |
3.3 (0.9–10.9) 3 [913] |
11.4 (3.7–30.1) 9 [6,604] |
1.2 (0.2–7.9) 2 [278] |
| Retired | 30.7 (30.1–31.3) 38,979 [13,732,361] |
31.0 (30.4–31.6) 37,130 [13,046,552] |
30.3 (21.9–40.3) 360 [139,357] |
23.7 (19.1–29.1) 293 [90,459] |
30.7 (30.1–31.3) 38,764 [13,645,961] |
23.3 (12.7–38.8) 28 [6,470] |
35.5 (23.8–49.1) 52 [20,448] |
0.02 (0.01–0.04) 22 [9,234] |
| Missing | 0.5 (0.4–0.6) 460 [222,927] |
0.4 (0.3–0.5) 404 [166,495] |
0.4 (0.09–1.4) 5 [1,681] |
0.2 (0.03–1.5) 1 [793] |
0.5 (0.4–0.6) 456 [219,239] |
0.0 n = 0 |
0.003 (0.0006–0.01) 2 [1,265] |
0.0 n = 0 |
| Income as Percent of FPL | ||||||||
| <100 | 10.2 (9.7–10.6) 7,909 [4,547,644] |
9.4 (9.0–9.9) 7,116 [3,975,757] |
7.2 (4.1–12.4) 72 [33,138] |
13.8 (10.2–18.4) 116 [52,648] |
10.1 (9.7–10.6) 7,830 [4,505,826] |
2.2 (0.8–5.6) 7 [609] |
11.2 (6.1–19.7) 23 [6,481] |
12.0 (3.9–31.3) 9 [2,691] |
| 100–200 | 19.4 (18.9–19.9) 21,273 [8,672,300] |
19.2 (18.7–19.7) 19,985 [8,083,570] |
21.2 (15.7–28.0) 207 [97,384] |
21.5 (16.9-26.9) 236 [81,780] |
19.4 (18.8–19.9) 21,112 [8,601,069] |
27.7 (14.3–46.8) 20 [7,699] |
24.2 (15.4–35.8) 42 [13,948] |
25.7 (11.2–48.8) 15 [5,760] |
| >200 | 70.4 (69.8–71.1) 74,673 [31,510,650] |
71.3 (70.7–71.9) 71,339 [30,022,948] |
71.6 (64.2–78.0) 864 [329,646] |
64.7 (58.8–70.2) 637 [246,697] |
70.5 (69.9–71.1) 74,340 [31,342,473] |
70.1 (51.3–83.9) 50 [19,469] |
64.6 (52.0–75.4) 84 [37,247] |
62.2 (40.7–79.8) 32 [13,931] |
| Insurance Status | ||||||||
| Yes | 94.3 (93.9–94.6) 99,711 [42,165,920] |
94.6 (94.2–94.9) 94,716 [39,807,479] |
97.0 (95.1–98.2) 1,102 [446,412] |
92.2 (88.3–94.9) 939 [351,463] |
94.3 (93.9–94.6) 99,190 [41,913,472] |
95.8 (87.2–98.7) 73 [26,622] |
90.7 (80.7–95.7) 136 [52,290] |
99.5 (97.6–99.9) 54 [22,273] |
| No | 5.5 (5.1–5.9) 3,930 [2,451,170] |
5.2 (4.8–5.5) 3,542 [2,175,998] |
3.0 (1.8–4.9) 41 [13,756] |
7.3 (4.7–11.2) 45 [27,866] |
5.5 (5.1–5.8) 3,881 [2,422,728] |
3.7 (1.0–12.7) 3 [1,034] |
9.1 (4.1–19.1) 12 [5,237] |
0.5 (0.1–2.4) 2 [109] |
| Missing | 0.3 (0.2–0.3) 214 [113,504] |
0.2 (0.2–0.3) 182 [98,798] |
0.0 n = 0 | 0.5 (0.1–2.3) 5 [1,796] |
0.3 (0.04–1.8) 211 [113,168] |
0.4 (0.1–3.1) 1 [122] |
0.3 (0.04–1.8) 1 [149] |
0.0 n = 0 |
| Health Care Access Hardship | ||||||||
| Yes | 10.7 (10.2–11.1) 8,874 [4,768,206] |
10.4 (9.9–10.8) 8,203 [4,363,388] |
7.5 (5.2–10.6) 102 [34,322] |
14.2 (10.2–19.5) 122 [54,157] | 10.6 (10.2–11.1) 8,781 [4,712,611] |
4.5 (1.9–10.1) 8 [1,240] |
13.0 (6.9–23.3) 17 [7,510] |
11.3 (3.4–31.5) 9 [2,535] |
| No | 88.9 (88.5–89.4) 94,596 [39,774,302] |
89.2 (88.8–89.7) 89,886 [37,547,973] |
92.4 (89.3–94.7) 1,039 [425,239] |
84.7 (79.2–88.9) 863 [322,648] |
89.0 (88.5–89.4) 94,122 [39,553,578] |
84.2 (57.9–95.4) 67 [23,399] |
86.3 (76.1–92.6) 131 [49,791] |
88.7 (68.5–96.6) 47 [19,847] |
| Missing | 0.4 (0.4–0.5) 385 [188,086] |
0.4 (0.3–0.5) 351 [170,915] |
0.1 (0.03–0.6) 2 [607] |
1.1 (0.2–5.2) 4 [4,319] |
0.4 (0.4–0.5) 379 [183,179] |
11.3 (2.0–44.2) 2 [3,139] |
0.7 (0.09–4.5) 1 [376] |
0.0 n = 0 |
| Personal Doctor | ||||||||
| Yes | 91.9 (91.6–92.3) 96,373 [41,129,262] |
92.2 (91.8–92.6) 91,525 [38,806,938] |
95.3 (93.2–96.8) 1,069 [438,693] | 90.9 (86.9–93.8) 905 [346,400] |
92.0 (91.6–92.4) 95,854 [40,894,246] |
81.5 (60.3–92.7) 65 [22,638] |
84.8 (64.5–94.5) 140 [48,886] |
82.4 (59.8–93.6) 50 [18,443] |
| No | 7.6 (7.2–7.9) 7,022 [3,375,298] |
7.3 (7.0–7.7) 6,503 [3,084,599] |
4.5 (3.1–6.6) 72 [20,867] |
7.8 (5.3–11.4) 79 [29,832] |
7.5 (7.2–7.9) 6971 [3,333,531] |
18.5 (7.3–39.7) 12 [5,139] |
8.1 (2.3–25.1) 7 [4,675] |
17.6 (6.4–40.2) 6 [3,939] |
| Missing | 0.5 (0.4–0.6) 460 [226,034] |
0.5 (0.4–0.5) 412 [190,738] |
0.1 (0.03–0.6) 2 [607] |
1.3 (0.3–5.0) 5 [4,892] |
0.5 (0.4–0.6) 457 [221,591] |
0.0 n = 0 |
7.1 (1.2–32.0) 2 [4,114] |
0.0 n = 0 |
| Checkup in the Last 2 Years | ||||||||
| Yes | 92.2 (91.8–92.5) 95,969 [41,221,401] |
92.2 (91.8–92.5) 91,014 [38,790,548] |
92.0 (88.2–94.6) 1,050 [423,270] |
92.4 (88.7–95.0) 914 [352,223] |
92.2 (91.8–92.5) 95,450 [40,963,215] |
94.9 (88.8–97.8) 69 [26,360] |
90.8 (81.1–95.8) 133 [52,369] |
87.4 (67.6–95.9) 50 [19,571] |
| No | 7.0 (6.7–7.3) 6,975 [3,129,787] |
7.1 (6.7–7.4) 6,609 [2,972,844] |
6.0 (3.9–9.2) 81 [27,557] |
6.2 (4.1–9.3) 69 [23,703] |
7.0 (6.7–7.3) 6,934 [3,110,404] |
4.6 (1.9–10.6) 7 [1,275] |
6.1 (2.2–16.3) 10 [3,543] |
12.6 (4.1–32.4) 6 [2,811] |
| Missing | 0.8 (0.7–1.0) 911 [379,406] |
0.8 (0.7–0.9) 817 [318,884] |
2.0 (0.8–4.8) 12 [9,341] |
1.4 (0.4–5.1) 6 [5,198] |
0.8 (0.7–1.0) 898 [375,749] |
0.5 (0.07–3.7) 1 [142] |
3.1 (1.1–8.3) 6 [1,764] |
0.0 n = 0 |
2.2. Primary outcomes
Our primary outcomes were two-fold: 1) lifetime cancer screening (“Never screened” vs. "Ever screened”), and 2) up-to-date screening among those who had screened at least once in their lifetimes (“Not recently screened” vs. “Screened According to Guidelines”). We used questions from the BRFSS questionnaire to ascertain screening status for the following testing methods: blood stool test (fecal occult blood test), colonoscopy or sigmoidoscopy, and mammogram. The questions are posed in the following way: “Have you ever had a <testing method>?” (to which participants could respond with “Yes” or “No”). To determine screening adherence, the question “How long has it been since you had your last < testing method>?” (to which participants could respond with “Within the past year,” “Within the past 2 years,” “Within the past 3 years,” “Within the past 5 years,” or “5 or more years ago”) was used (CDC, 2015; 2018).
Up-to-date (adherent) screening was defined in accordance to the United States Preventive Services Task Force (USPSTF) guidelines at the time of collection, 2016 and 2018. For colorectal cancer, respondents aged 50 to 75 were considered adherent to recommended screening guidelines if they reported completing at least one of the following three tests: 1) High-sensitivity fecal occult blood test (FOBT) or blood stool test within the past year; 2) Flexible sigmoidoscopy in the previous 5 years with FOBT in the last three years; or 3) Colonoscopy screening in the previous 10 years (Bibbins-Domingo et al., 2016). For breast cancer, USPSTF guidelines define biannual screening mammography for women aged 50 to 75 is considered adherent (Siu, 2016). However, it is unclear how these guidelines apply to transgender, nonbinary, nonconforming, or intersex individuals who are were either assigned female sex at birth or have breasts (Caughey et al., 2021). For example, transgender men who have not completed a bilateral mastectomy, or have only undergone breast reduction surgery, may be at risk for breast cancer due to residual breast tissue (Fehl et al., 2019). Additionally, transgender women may be at risk of developing breast cancer due to the use of gender-affirming hormone therapy to increase estrogen levels while suppressing endogenous testosterone (von Vaupel‐Klein & Walsh, 2021). Therefore, we will use the term “individuals” to refer to all those who are at risk for breast cancer, as well as use the term “recent” or “up-to-date” in lieu of “adherent” to describe screening behavior since not all eligible individuals are included in the recommendations. When referencing previous literature, we will use the language of the cited article.
We evaluated screening according to sexual and gender minority (SGM) and racial/ethnic minority status. The BRFSS included three categories for sexual orientation: straight, lesbian or gay, and bisexual for both CRC and breast cancer analysis. For CRC, gender identity categories included cisgender male, cisgender female, male-to-female (MTF) transgender, female-to-male (FTM) transgender, and transgender nonconforming. For breast cancer, the four gender identity categories were cisgender female, MTF transgender, FTM transgender, and transgender nonconforming. We included both MTF, FTM, and nonconforming transgender populations because the BRFSS does not survey if participants have had hormone therapy or gender-affirming surgery, and thus these populations may be eligible for breast cancer screening. From here on, we will call MTF transgender individuals “transgender women” and FTM transgender individuals “transgender men.” Racial and ethnic classifications included NH-White, Black, Hispanic, and Asian (which included Pacific Islander). Health care access hardship is defined as having a time in the past 12 months when the respondent needed to see a doctor but could not due to cost.
2.3. Statistical analysis
We calculated weighted and unweighted percentages of the prevalence of each screening outcome within each of the demographic categories. In this paper we report the weighted proportions; 95% confidence intervals and weighted and unweighted population sizes are included in supplementary tables. To adjust for population size we used sampling weights and stratum indicators provided by the BRFSS (CDC, 2019). For descriptive statistics and comparison of differences in screening adherence by SGM identity within each race/ethnic category, Chi-square analyses were conducted. For participants with missing data on socio-demographic variables (including marital status, educational attainment, household income relative to the federal poverty level, and health care access), missing indicators were used (Table 1a, Table 1b) (Gelberg et al., 2000). All analyses were conducted in Stata version 17 (StataCorp, College Station, TX). Because the goal of the presented paper was to describe screening behaviors according to racial/ethnic and SGM categories, no additional models were run.
3. Results
3.1. Total population characteristics
The sociodemographic characteristics of the participants included in the analytic dataset, separated by cancer screening type, are shown in Table 1a, Table 1b. Of the total population of individuals eligible for CRC screening (n = 183,610), 97.4% identified as straight, 1.6% as lesbian or gay, and 1.0% as bisexual, while 47.0% identified as men, 52.6% as women, 0.2% as transgender women, 0.1% as transgender men, and 0.05% as nonbinary. Of the total population of breast cancer screening-eligible individuals (n = 103,855), 98.0% identified as straight, 0.01% as lesbian or gay, and 0.9% as bisexual, while 47.0% identified as men, 99.8% as women, 0.06% as transgender women, 0.1% as transgender men, and 0.05% as nonbinary. The majority of individuals in both cancer screening groups received at least a college degree, were employed, reported an annual family income of over 200% of the federal poverty level (FPL), and had health insurance and regular access to a primary care physician. There were no significant differences in employment, insurance, or healthcare access status between SGM and non-SGM groups (overlapping confidence intervals).
3.2. Screening behaviors by SGM and racial/ethnic categories separately
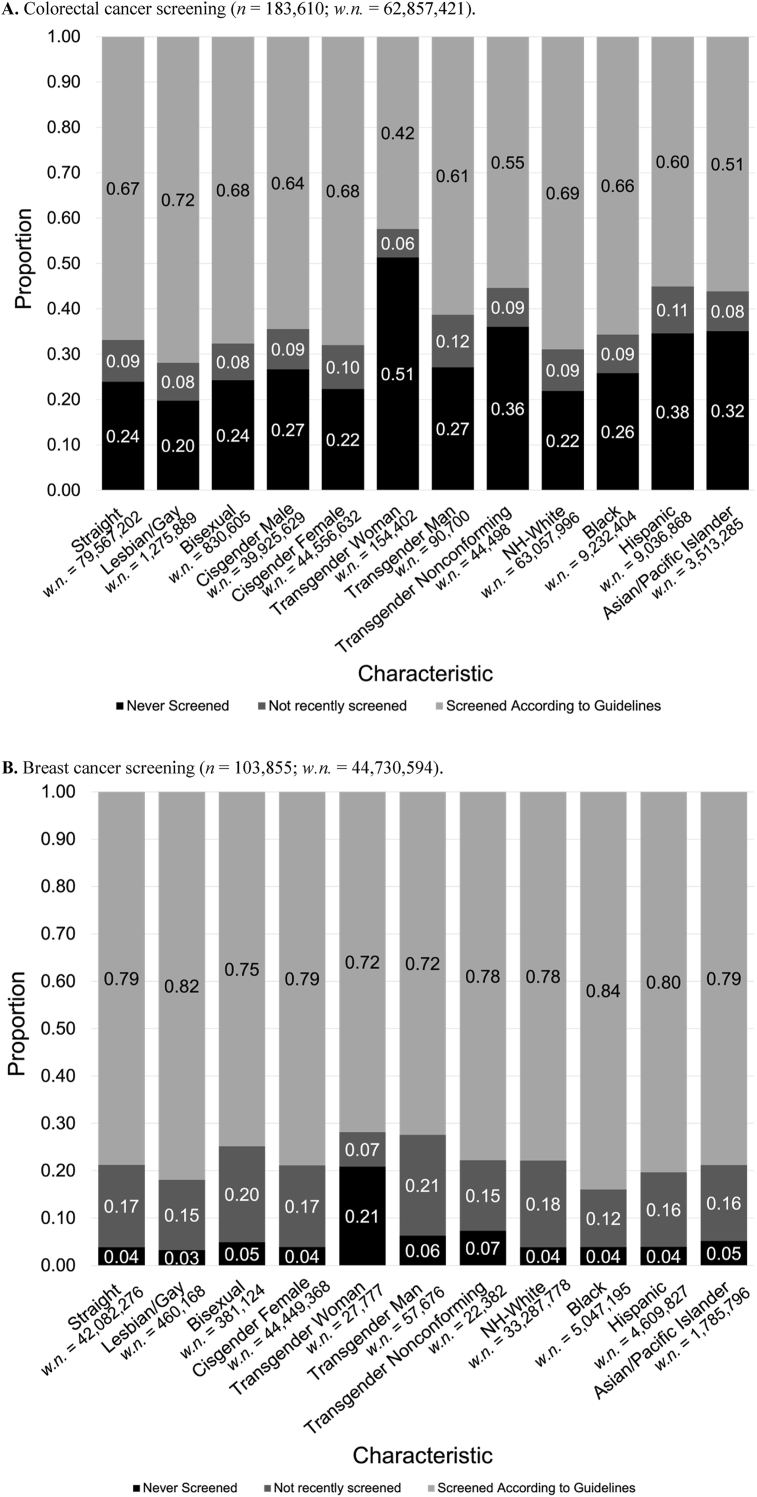
Variation in CRC screening status was analyzed according to SGM and racial/ethnic minority status separately (Fig. 1a). The majority of individuals in each category had screened according to guidelines, except for transgender women, who had both the lowest up-to-date and lowest lifetime screening prevalence (42.4% and 48.7%, respectively). Lesbian/gay individuals (71.9%) and cisgender women (68.0%) had the highest prevalence of up-to-date screening among all sexual orientation and gender identity categories, respectively. Among all races/ethnicities, the NH-White population had the highest proportion of lifetime screening and up-to-date screening (78.1%, 68.9%), while Hispanic (62.1%, 60.4%) and Asian populations (68.3%, 50.8%) had the lowest lifetime screening and up-to-date screening. All transgender categories (transgender men, transgender women, transgender nonconforming) had lower lifetime screening and up-to-date screening compared to the cisgender populations.
Fig. 1.
(A) Colorectal and (B) breast cancer screening behaviors by SGM and racial/ethnic categories separately. Weighted n's (w.n.) are reported.
For all groups assessed for breast cancer screening (except for transgender women), less than 10% of individuals had never been screened in their lifetime, while the majority (>90%) of individuals were up-to-date with screening (Fig. 1b). Similar to CRC, transgender women had the lowest proportions of lifetime screening (79.1%) and up-to-date screening (71.8%) (Fig. 1b).
3.3. CRC screening behaviors at the intersection of SGM status and race/ethnicity
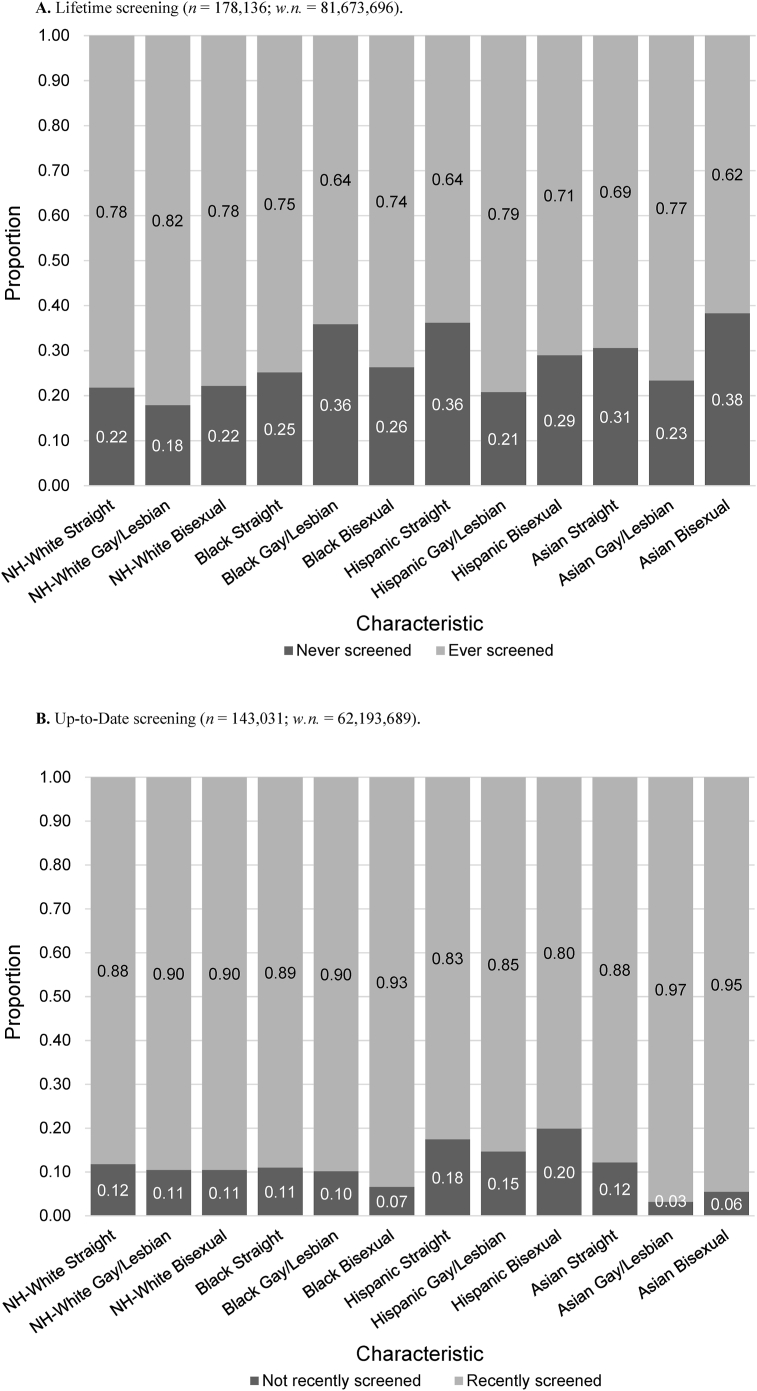
NH-White, Hispanic, or Asian gay/lesbian individuals and Hispanic bisexual indivon Vaupel-Klein & Walsh, 2021viduals had a greater proportion of lifetime CRC screening compared to straight individuals (Fig. 2a, Suppl. Table S1a). For the Black population only, lifetime CRC screening was lower for both categories of SGM individuals (gay/lesbian 64.1%; bisexual 73.7%) compared to those who reported they were straight (74.8%). Across all races, the gay/lesbian NH-White population had the highest (82.1%) while the bisexual Asian population had the lowest (61.7%) lifetime screening. Within all races, gay/lesbian individuals who had screened at least once in their life had higher proportions of up-to-date screening than their straight counterparts (Fig. 2b, Suppl. Table S1a); higher proportions of up-to-date screening were found among NH-White, Black, and Asian bisexual individuals as well. However, these differences were not statistically significant.
Fig. 2.
Colorectal cancer screening behavior, (A) Lifetime and (B) Up-to-date, according to sexual orientation and race (Asian includes Pacific Islander). Complete n's, weighted n's (w.n.), and 95% confidence intervals are found in Suppl. Tables S1a and S1b.
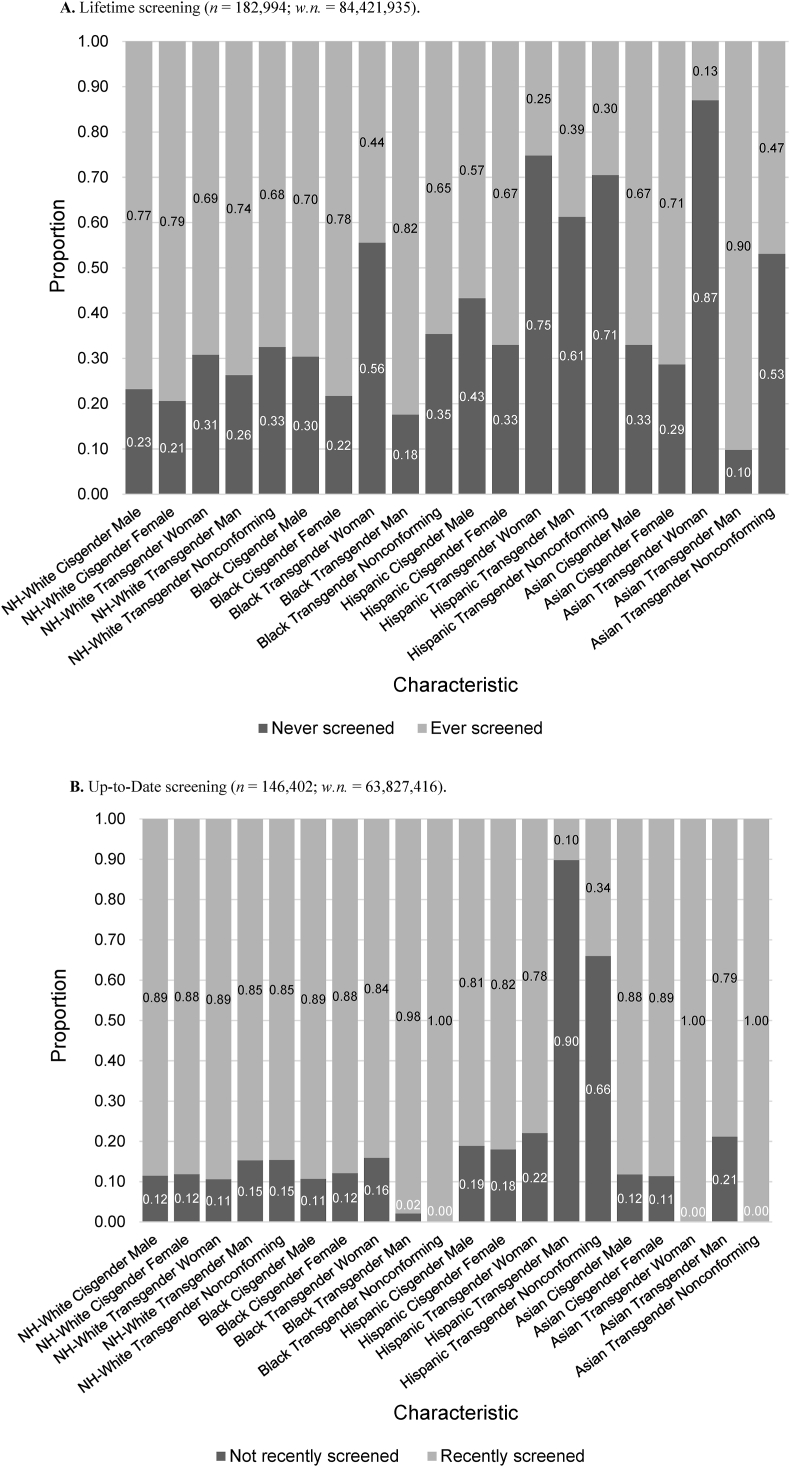
Lifetime CRC screening proportions differed significantly by gender identity within and across races (p < 0.05) (Fig. 3a, Suppl. Table S1b). Within each race, lifetime screening was lower among transgender women and nonconforming individuals compared to other gender categories, with the lowest screening proportion among Asian transgender women (13.0%). Meanwhile, individuals who identified as cisgender generally had the highest proportion of lifetime screening within each race, except for Black (82.4%) and Asian (90.2%) transgender men, who had the highest lifetime screening prevalence across all races. For individuals who had screened at least once in their lifetime, up-to-date screening did not differ significantly by gender identity within each race, but it was overall lowest among Hispanic transgender men (10.2%) (Fig. 3b, Suppl. Table S1b).
Fig. 3.
Colorectal cancer screening behavior, (A) Lifetime and (B) Up-to-date, according to gender identity and race (Asian includes Pacific Islander). Complete n's, weighted n's (w.n.), and 95% confidence intervals are found in Suppl. Tables S2a and S2b.
3.4. Breast cancer screening behaviors at the intersection of SGM status and race/ethnicity
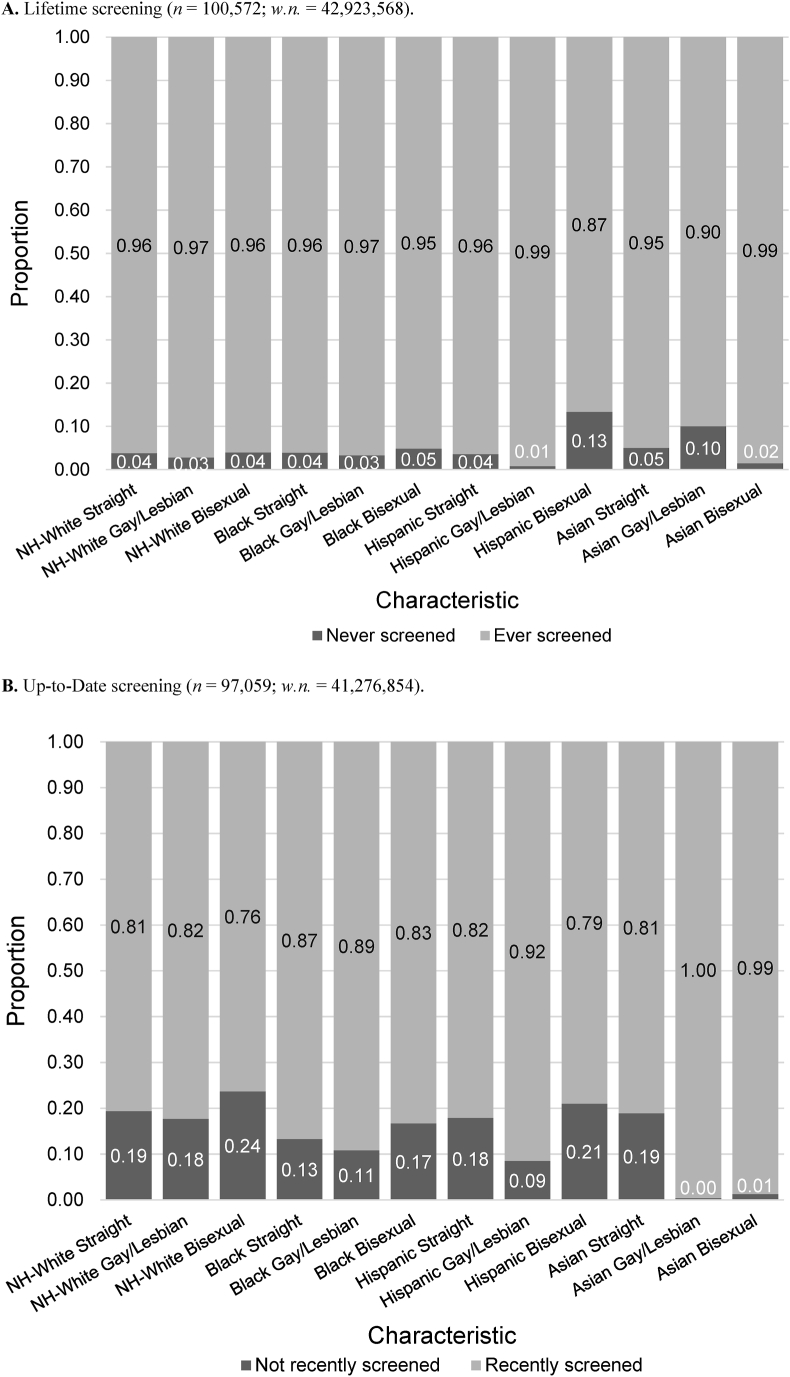
Among NH-White, Black, and Asian populations, there were no significant differences in lifetime screening by sexual orientation (Fig. 4a, Suppl. Table S2a). However, within the Hispanic population, gay/lesbian individuals had significantly higher, while bisexual individuals had significantly lower, lifetime screening prevalence than straight individuals (p = 0.0325). Across all racial/ethnic populations, Hispanic bisexual (86.6%) and Asian gay/lesbian individuals (90.0%) had the lowest proportion of lifetime screening, while Hispanic gay/lesbian (99.2%) and Asian bisexual (98.5%) individuals had the highest proportion. There were also no statistically significant differences in up-to-date screening within NH-White, Black, and Hispanic races by sexual orientation, although a greater proportion of gay/lesbian individuals had recently screened than straight individuals and bisexual individuals (Fig. 4b, Suppl. Table S2a). Within the population of Asians who had screened at least once in their lifetime, both sexual minority populations had significantly greater recent screening prevalence than the straight group (p < 0.0001).
Fig. 4.
Breast cancer screening behavior, (A) Lifetime and (B) Up-to-date, according to sexual orientation and race (Asian includes Pacific Islander). Complete n's, weighted n's (w.n.), and 95% confidence intervals are found in Suppl. Tables S3a and S3b.
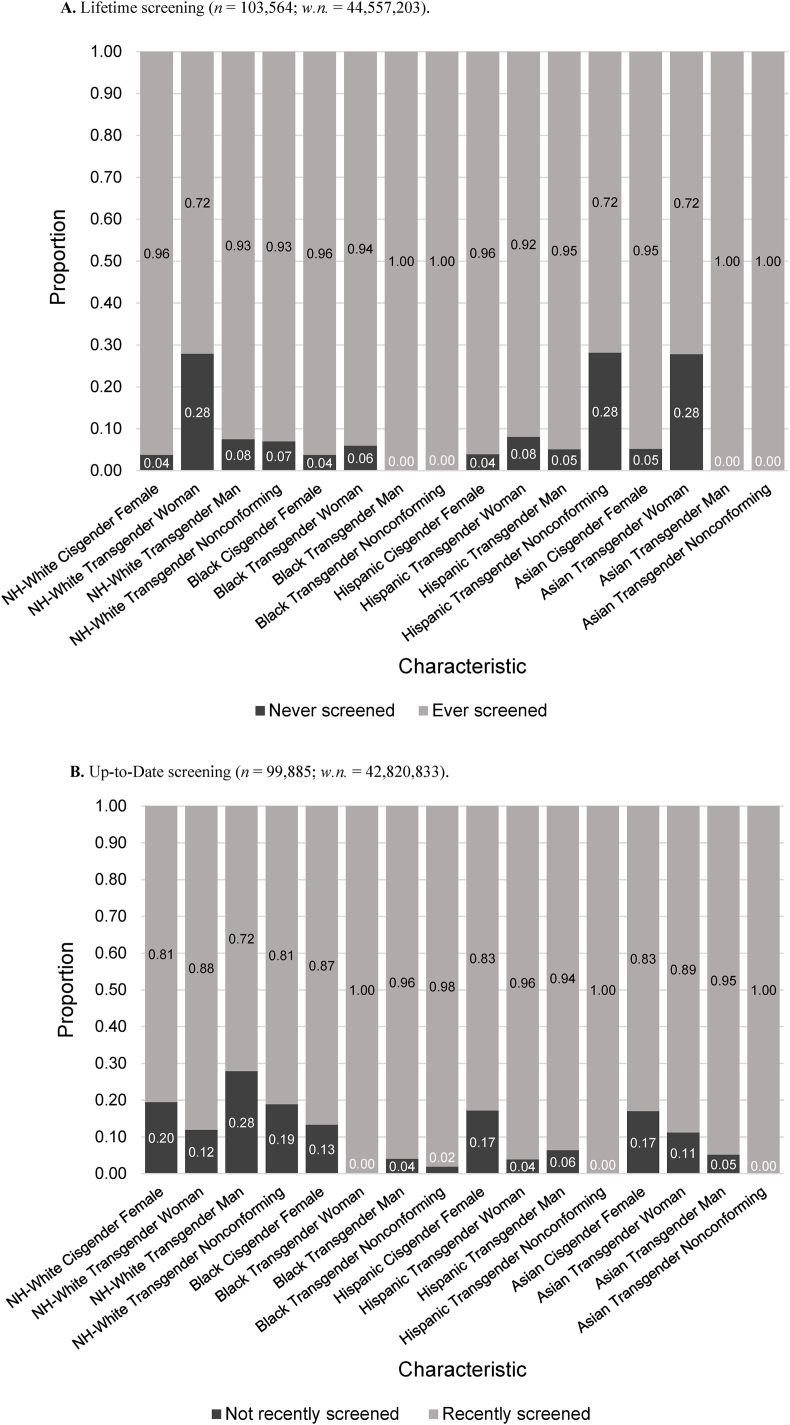
Lifetime breast cancer screening only differed significantly by gender identity within the NH-White population (p < 0.0001), with NH-White, transgender women having the lowest proportion of lifetime screening (72.1%) (Fig. 5a, Suppl. Table S2b). In all the other racial/ethnic groups, transgender women had lower lifetime screening prevalence than cisgender women, but the difference was not statistically significant. For categories with n > 20 across all races, NH-White (96.2%) and Black (96.2%) cisgender women had the highest proportion of lifetime screening. For individuals who had screened at least once in their lifetime, the majority were up-to-date with screening; within the Asian population, transgender individuals had significantly greater up-to-date screening than cisgender individuals (p < 0.0002) (Fig. 5b, Suppl. Table S2b). It is important to note that sample size was extremely limited for this analysis.
Fig. 5.
Breast cancer screening behavior, (A) Lifetime and (B) Up-to-date, according to gender identity and race (Asian includes Pacific Islander). Complete n's, weighted n's (w.n.), and 95% confidence intervals are found in Suppl. Tables S4a and S4b.
3.5. Screening behaviors for population that did not report sexual orientation or gender identity
We analyzed the screening behavior within racial/ethnic groups among the population without SGM responses, to determine if there were any systematic differences compared to the SGM-reporting population (Suppl. Tables S4 and S6). While a higher proportion of the non-SGM-reporting population identified as Hispanic or Asian compared to the SGM-reporting population (non-overlapping confidence intervals), screening behavior by race/ethnicity was overall similar (differences ±2%/overlapping confidence interval) regardless of SGM disclosure.
4. Discussion and conclusion
In the U.S., screening for detectable cancers like colorectal and breast cancers, which rank among the most prevalent and leading causes of death (Siegel et al., 2022), continues to be a significant challenge contributing to the burden of cancer. Inequalities in cancer screening among racial and ethnic categories (Burgess et al., 2011; Joseph et al., 2012; Sauer et al., 2018) and by sexual orientation and gender identity (Domogauer et al., 2022) have also been documented. However, little is known about the intersections of sexual orientation and gender identity with racial and ethnic categories. Due to the limited scope of existing literature and the crucial significance of accounting for the impact of intersecting identities when developing interventions to enhance screening uptake, there is a clear need for further evidence in this area. To address this using BRFSS data, we assessed variation in colorectal and breast cancer screening behavior in the US, using an intersectional analysis capturing the dimensions of sexual orientation, gender identity, and race/ethnicity. Our goal was to quantify the disparities found among these populations. We found that within and across all races, transgender women had among the lowest lifetime participation in CRC and breast cancer screening, while gay/lesbian individuals had among the highest prevalence of up-to-date screening for both cancer types. However, due to small unweighted sample sizes, some differences were not statistically significant and need to be further studied and interpreted in the context of findings from other research. Below, we discuss our findings in detail.
In the current study, we observed that within specific races/ethnicities, the point estimate for lifetime CRC screening was higher among gay/lesbian (NH-White, Hispanic, Asian) and bisexual individuals (Hispanic) compared to straight individuals. Likewise, prior work examining intersectional identities reported in all racial/ethnic groups, gay/bisexual men had a greater percentage of lifetime CRC screening and up-to-date screening compared with heterosexual men (Heslin et al., 2008). Similar to our findings, prior work has also found gay/bisexual men had greater CRC screening adherence than straight individuals (Charkhchi et al., 2019; Heslin et al., 2008).
We further found significant differences in lifetime CRC screening by gender identity: transgender women and nonconforming individuals had the lowest lifetime CRC screening proportions within each race, while across all races, Black and Asian transgender men had the highest lifetime CRC screening. However, we did not find statistically significant differences in up-to-date screening between groups, which contrasted the results of previous BRFSS studies (Charkhchi et al., 2019; Tabaac et al., 2018) that reported lowest screening adherence among transgender women. Our findings on lifetime screening emphasize the importance of disaggregating levels of outcomes when analyzing vulnerable populations (Datta et al., 2022), especially considering how individuals who have never been screened are at higher risk of being diagnosed with invasive cancer than individuals who have been screened previously (Peterson et al., 2016).
For breast cancer, we found that differences in lifetime screening within race/ethnicity were significant within the Hispanic population only; Hispanic gay/lesbian individuals had the highest proportion, while the Hispanic bisexual population had the lowest proportion, of lifetime screening, which were both true across all races as well. Further, within all racial/ethnic groups, we found up-to-date screening to be greater for gay/lesbian individuals (with significant difference only found for Asian population) and lower for bisexual individuals (except Asian bisexual, which had higher screening adherence) in comparison to straight individuals. This was consistent with previous studies examining differences in screening behavior based on sexual orientation only (Austin et al., 2013; Bazzi et al., 2015; Charkhchi et al., 2019). However, according to a past study on intersectionality in breast cancer screening using NHIS data, Latina lesbian women had the lowest prevalence, while Black lesbian women had the highest prevalence, of completing a mammogram within the past year (Agénor et al., 2020). They excluded the Asian population due to small sample size (Agénor et al., 2020).
Across all races, all transgender groups had significantly greater up-to-date breast cancer screening prevalence than the cisgender population, but this result may be skewed due to small sample size. The large confidence intervals for the proportions suggest that these findings should be interpreted with caution. Within all racial/ethnic groups, lifetime screening was lowest among transgender women, but the difference was only significant among the NH-White population. Transgender women, who were assigned male sex at birth, may have increased risk for breast cancer from hormone therapy or gender-affirming therapy and therefore should be screened, yet may not partake in mammography due to lack of knowledge or recommendations (de Blok et al., 2019). Confusion about screening engagement may stem from the sex-specific nature of official recommendation statements; for example, USPSTF guidelines for breast cancer screening still use “women,” which may cause misunderstandings among some members of the transgender or nonbinary community (Domogauer et al., 2022). To minimize ambiguity, organ-specific guidelines as opposed to sex- or gender-specific guidelines should be detailed where possible (Caughey et al., 2021).
The small sample size for racial/ethnic minority groups in this study, especially for breast cancer screening, is indicative of these populations' underrepresentation in research. Some barriers to participation may include language unfamiliarity, past negative experiences, and insufficient recruitment efforts (Huang & Coker, 2010; Liu et al., 2019). Additionally, for many of these racial/ethnic minority groups, identifying as SGM is culturally unacceptable and stigmatized, and leads to suppression of or reluctance to disclose these identities (Ching et al., 2018; Choudhury et al., 2009; Kennamer et al., 2000). In fact, Black, Asian, and Hispanic populations are more likely to respond “not sure or don't know” compared with NH-White Americans, and the latter two also more likely to refuse to answer, to questions about sexual orientation according to prior work (Fredriksen-Goldsen & Muraco, 2010; Kim & Fredriksen-Goldsen, 2013) and this study (Tables S2 and S4). Another consideration for the limited sample size of this study is that CRC and breast cancer are more common in older adults, a population that may not have a significant openly SGM-identifying population due to historical and social contexts (Fredriksen-Goldsen & Muraco, 2010). Individuals over the age of 60 today lived in a period when same-sex relationships were stigmatized and criminalized, and many spent the majority of their adulthood hiding their sexual orientation or gender identity (Fredriksen-Goldsen & Muraco, 2010), which may be reflected in their survey responses. Here, 20.4% and 18.7% of individuals in the 60–75 age group did not respond to the sexual orientation or gender identity questions, respectively, in the BRFSS. The Institute of Medicine acknowledges this gap, describing older SGM adults as a population whose needs are understudied (Garofalo, 2011). Additionally, populations with higher participation rates in both the BRFSS survey and overall research are more likely to be college-educated and have higher income and access to health care resources (Tables S3 and S5), which are all positively associated to higher screening rates (Centers for Disease Control and Prevention, 2012). Therefore, the sample used for these analyses are not fully representative of the general U.S. population or SGM groups, who report generally both lower financial (low standard of living, inability to afford basic necessities, and high financial worry) and community (safety and security) well-being than their heterosexual or cisgender counterparts (Ceres et al., 2018). Future studies therefore need to be conducted with larger sample sizes and more diverse participant demographics in order to assess the true preventive health behaviors of older SGM populations.
There are many potential reasons behind non-participation or non-adherence to cancer screening guidelines, including not understanding screening is necessary, fear of pain/discomfort (for CRC screening specifically), and lack of recommendation by a healthcare provider (Jones, Devers, Kuzel, & Woolf, 2010-; Jones, Woolf, et al., 2010-; Kawar, 2013). This last reason is closely tied with medical mistrust, which is especially prevalent among minority groups. Medical mistrust develops in response to both the systemic and individual-level oppression and discrimination (e.g., racism, sexism, homophobia, transphobia, etc.) perceived by patients in a healthcare setting, and leads to greater hesitancy towards and reduced engagement with medical establishment (Brenick et al., 2017; Jaiswal & Halkitis, 2019). Patients who are fearful or distrusting of the healthcare system are more likely to avoid, less likely to be informed about, or less likely to be recommended to access and utilize screening services (Huang & Coker, 2010). Past studies have shown greater rates of medical mistrust among Black and Hispanic individuals due to history of mistreatment, racism, and discrimination by healthcare providers (Shelton et al., 2011). Similarly for SGM patients, traumatic or non-affirming experiences with the medical system and societal stigma regarding their identity increase their likelihood to avoid or delay care (Quinn et al., 2015). The older age of the CRC and breast cancer screening-eligible population further contributes to screening non-adherence, as their medical mistrust may stem from experiencing the cumulative impact of a lifetime of discrimination and the chronic burden of suppressing their identity (Flatt et al., 2021).
Additionally, SGM groups may face barriers to cancer screening due to lower socioeconomic status (SES). Compared to the general population and non-SGM population, SGM individuals are more likely to live in poverty (Kates et al., 2018; Schneebaum & Badgett, 2019; Taylor, 2013), lack health insurance (Buchmueller & Carpenter, 2010; Hsieh & Ruther, 2016; Ponce et al., 2010), or have plans with inadequate coverage (Blosnich, 2017; Nguyen et al., 2018). Difficulty affording and accessing proper healthcare translates to SGM individuals neither having nor routinely visiting a primary care provider, or avoiding/delaying medical care due to cost (Barrett & Wholihan, 2016), at greater rates than non-SGM individuals (Blosnich, 2017; Blosnich et al., 2014; Buchmueller & Carpenter, 2010; Hsieh & Ruther, 2016; Ward et al., 2014). Similar patterns are found for racial/ethnic minority groups, who have higher poverty rates compared to NH-White populations (McKinnon, 2002; Williams, 2008), and are less likely to have health coverage (Jeudin et al., 2014) or consistent medical care (Williams, 2008). SGM individuals who are also low SES experience even greater levels of societal marginalization and oppression, increasing barriers to care and worsening health outcomes as stated by the intersectionality framework. Therefore, interventions to increase CRC and breast cancer screening uptake must target all an individual's overlapping identities.
There were several limitations to this study. First, the BRFSS does not specify whether the various colorectal tests were done for routine screening or diagnosis (confirmation of CRC upon presentation of symptoms), so CRC screening uptake may not be accurately reported (El-Serag et al., 2006; Haque et al., 2005; Joseph et al., 2018). Furthermore, patients may overstate how recent screenings were performed (Tabaac et al., 2018) due to recall or social desirability bias (Joseph et al., 2018). Third, as aforementioned, despite combining datasets from two different years, small sample sizes of intersecting populations and variability in the data contributed to wide confidence intervals for reported estimates, and therefore results should be used to guide future confirmatory studies instead of making strong conclusions (Hackshaw, 2008). Additionally, the data was limited to the 26 U.S. states and territories that asked questions about gender and sexual identity, which may not be generalizable to the rest of the SGM population across America (Gonzalez, 2018). However, it is unclear if the states included showed any patterns towards attitudes of LGBTQ + acceptance,.
Additionally, while one advantage of the BRFSS questionnaire structure is its straightforwardness in administering to large populations, the broad categorization of socio-demographic categories, such as for race and ethnicity, may also create the potential to overlook the heterogeneity in income or education level, cultural beliefs and barriers, and more within the sub-populations. Furthermore, the BRFSS combined gay and lesbian into one category when surveying sexual orientation, which may obscure important differences in life experiences, psychosocial stressors, and screening behavior between the two distinct population groups (Harper et al., 2004; Garofalo, 2011). Previous research has found that gay men are more likely, while lesbian women are less likely, than heterosexuals to have a primary care provider and health insurance (Lunn et al., 2017), which may impact patterns in health access and behavior. Another limitation to conducting secondary analysis with variables already established by the BRFSS is missing the opportunity to ask survey questions that are inherently intersectional (i.e., do not separate or hierarchize levels of identity), or ones that assess constructs such as societal norms, prejudice, and discrimination instead of solely demographic variables (Bowleg, 2008). Quantitative and qualitative research highlighting the mutually constitutive nature of intersectional identities will provide better understanding of individual-level and systematic barriers to care, and their interventions.
Finally, we were unable to analyze screening behaviors at the intersection of sexual orientation and gender identity because the population of respondents with overlapping SGM identities was too small. Small sample size also limited our ability to explore the intersecting effects of SES and SGM status on screening behavior. Considering the compounding effects that multiple minority identities have on an individual's health outcomes (Hsieh & Ruther, 2016; Trinh et al., 2017; Veenstra, 2011), it becomes all the more important to understand the experiences of, and find tailored interventions, for individuals who have multiple, intersecting minority identities. While all individuals occupy multiple social identities, future work to establish more conclusive findings should focus on expanding datasets to specifically include more individuals in marginalized groups, placing special consideration on remediating or addressing the historical and cultural contexts that influence their study participation rates.
Our findings demonstrate that colorectal and breast cancer screening for eligible individuals differs according to SGM status within and across race/ethnicity, especially for those who are members of intersecting minority subpopulations. For both types of cancer and across all races, lifetime screening prevalence was lowest among transgender women and transgender nonconforming individuals; this difference compared to lifetime screening prevalence among cisgender populations was the largest among Hispanic and Asian populations. However, for some racial/ethnic groups, sexual minority individuals were found to have higher prevalence of lifetime screening for both cancer types than heterosexual individuals. These findings highlight the intersecting and potentially bidirectional ways in which sexual orientation and gender identity, and racial and ethnic identity, as markers of systems of oppression (such as racism, heterosexism, sexism), may impact preventive health behaviors. However, since confidence intervals are wide, there is a need to conduct more research on representative sample of SGM. While the current dataset provides useful information, it is inadequate to provide a full idea of the state of screening uptake in these vulnerable groups due to their limits in representation. Future research should collect data via mixed methods in order to provide a more complete understanding of who is not benefiting from preventive screening and the reasons why. In this way more informed interventions can be developed to target populations in need.
Financial disclosure statement
This work was supported by Cedars-Sinai Cancer. The funding body played no role in the design, data collection, analysis, and interpretation of the data.
Conflict of interest disclosure statement
All authors of this article declare they have no conflicts of interest.
Ethical statement
Ethical approval was not required as analysis was conducted on an existing data source (Behavioral Risk Factor Surveillance System) available to the public.
Author statement
Emmeline Lin: data curation, formal analysis, methodology, interpretation, writing (original draft), and writing (review and editing). Patrycja Sleboda: data curation, formal analysis, methodology, interpretation, supervision, validation, writing (original draft), writing (review and editing). Bobbie J. Rimel: interpretation, writing (review and editing). Geetanjali D. Datta: conceptualization, formal analysis, funding acquisition, methodology, interpretation, project administration, supervision, writing (original draft), writing (review and editing).
All authors reviewed the final version of the manuscript.
Declaration of competing interest
All authors of this article declare they have no relevant conflicts of interest.
Acknowledgments
This work was supported by Cedars-Sinai Cancer. The funding body played no role in the design, data collection, analysis, and interpretation of the data.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ssmph.2023.101540.
Appendix A. Supplementary data
The following is the supplementary data to this article:
Data availability
BRFSS data are publically available
References
- Ackerson K., Gretebeck K. Factors influencing cancer screening practices of underserved women. Journal of the American Academy of Nurse Practitioners. 2007;19(11):591. doi: 10.1111/j.1745-7599.2007.00268.x. [DOI] [PubMed] [Google Scholar]
- Agénor M., Pérez A.E., Tabaac A.R., Bond K.T., Charlton B.M., Bowen D.J., Austin S.B. Sexual orientation identity disparities in mammography among white, Black, and Latina U.S. Women. LGBT Health. 2020;7(6):312–320. doi: 10.1089/lgbt.2020.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin S.B., Pazaris M.J., Nichols L.P., Bowen D., Wei E.K., Spiegelman D. An examination of sexual orientation group patterns in mammographic and colorectal screening in a cohort of U.S. women. Cancer Causes & Control. 2013;24(3):539–547. doi: 10.1007/s10552-012-9991-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett N., Wholihan D. Providing palliative care to LGBTQ patients. Nursing Clinics. 2016;51(3):501–511. doi: 10.1016/j.cnur.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Bazzi A.R., Whorms D.S., King D.S., Potter J. Adherence to mammography screening guidelines among transgender persons and sexual minority women. American Journal of Public Health. 2015;105(11):2356–2358. doi: 10.2105/AJPH.2015.302851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibbins-Domingo K., Grossman D.C., Curry S.J., Davidson K.W., Epling J.W., García F.A., Krist A.H. Screening for colorectal cancer: US preventive services Task Force recommendation statement. JAMA. 2016;315(23):2564–2575. doi: 10.1001/jama.2016.5989. [DOI] [PubMed] [Google Scholar]
- de Blok C.J., Wiepjes C.M., Nota N.M., van Engelen K., Adank M.A., Dreijerink K.M.…den Heijer M. Breast cancer risk in transgender people receiving hormone treatment: Nationwide cohort study in The Netherlands. BMJ. 2019;365 doi: 10.1136/bmj.l1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blosnich J.R. Sexual orientation differences in satisfaction with healthcare: Findings from the behavioral risk factor surveillance system, 2014. LGBT Health. 2017;4(3):227–231. doi: 10.1089/lgbt.2016.0127. [DOI] [PubMed] [Google Scholar]
- Blosnich J.R., Farmer G.W., Lee J.G., Silenzio V.M., Bowen D.J. Health inequalities among sexual minority adults: Evidence from ten US states, 2010. American Journal of Preventive Medicine. 2014;46(4):337–349. doi: 10.1089/lgbt.2016.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowleg L. When Black+ lesbian+ woman≠ Black lesbian woman: The methodological challenges of qualitative and quantitative intersectionality research. Sex Roles. 2008;59:312–325. doi: 10.1007/s11199-008-9400-z. [DOI] [Google Scholar]
- Braveman P. Health disparities and health equity: Concepts and measurement. Annual Review of Public Health. 2006;27:167–194. doi: 10.1146/annurev.publhealth.27.021405.102103. [DOI] [PubMed] [Google Scholar]
- Brenick A., Romano K., Kegler C., Eaton L.A. Understanding the influence of stigma and medical mistrust on engagement in routine healthcare among Black women who have sex with women. LGBT Health. 2017;4(1):4–10. doi: 10.1089/lgbt.2016.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretthauer M. Colorectal cancer screening. Journal of Internal Medicine. 2011;270(2):87–98. doi: 10.1111/j.1365-2796.2011.02399.x. [DOI] [PubMed] [Google Scholar]
- Buchmueller T., Carpenter C.S. Disparities in health insurance coverage, access, and outcomes for individuals in same-sex versus different-sex relationships, 2000–2007. American Journal of Public Health. 2010;100(3):489–495. doi: 10.2105/AJPH.2009.160804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess D.J., Van Ryn M., Grill J., Noorbaloochi S., Griffin J.M., Ricards J., Partin M.R. Presence and correlates of racial disparities in adherence to colorectal cancer screening guidelines. Journal of General Internal Medicine. 2011;26:251–258. doi: 10.1007/s11606-010-1575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey A.B., Krist A.H., Wolff T.A., Barry M.J., Henderson J.T., Owens D.K.…Mangione C.M. USPSTF approach to addressing sex and gender when making recommendations for clinical preventive services. JAMA. 2021;326(19):1953–1961. doi: 10.1001/jama.2021.15731. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Cancer screening-United States, 2010. MMWR. Morbidity and mortality weekly report. 2012;61(3):41–45. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention 2016 behavioral risk factor surveillance system questionnaire. BRFSS Questionnaires. 2015 https://www.cdc.gov/brfss/questionnaires/pdf-ques/2016_BRFSS_Questionnaire_FINAL.pdf [Google Scholar]
- Centers for Disease Control and Prevention . 2018. 2018 BRFSS questionnaire. BRFSS questionnaires.https://www.cdc.gov/brfss/questionnaires/pdf-ques/2018_BRFSS_English_Questionnaire-508.pdf [Google Scholar]
- Centers for Disease Control and Prevention . 2019. Behavioral risk factor surveillance system: Complex sampling weights and preparing 2018 BRFSS module data for analysis.https://www.cdc.gov/brfss/annual_data/2018/pdf/Complex-Smple-Weights-Prep-Module-Data-Analysis-2018-508.pdf Accessed April 15, 2023. [Google Scholar]
- Ceres M., Quinn G.P., Loscalzo M., Rice D. Cancer screening considerations and cancer screening uptake for lesbian, gay, bisexual, and transgender persons. Seminars in Oncology Nursing. 2018;34(1):37–51. doi: 10.1016/j.soncn.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charkhchi P., Schabath M.B., Carlos R.C. Modifiers of cancer screening prevention among sexual and gender minorities in the behavioral risk factor surveillance system. Journal of the American College of Radiology. 2019;16(4):607–620. doi: 10.1016/j.jacr.2019.02.042. Part B) [DOI] [PubMed] [Google Scholar]
- Ching T.H.W., Lee S.Y., Chen J., So R.P., Williams M.T. A model of intersectional stress and trauma in asian American sexual and gender minorities. Psychology of violence. 2018;8(6):657–668. doi: 10.1037/vio0000204. [DOI] [Google Scholar]
- Choudhury P.P., Badhan N.S., Chand J., Chhugani S., Choksey R., Husainy S., Wat E.C. Community alienation and its impact on help-seeking behavior among LGBTIQ south Asians in southern California. Journal of Gay & Lesbian Social Services. 2009;21(2–3):247–266. doi: 10.1080/10538720902772196. [DOI] [Google Scholar]
- Damaskos P., Amaya B., Gordon R., Walters C.B. Intersectionality and the LGBT cancer patient. Seminars in Oncology Nursing. 2018;34(1):30–36. doi: 10.1016/j.soncn.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta G.D., Pana M.P., Mayrand M.-H., Glenn B. Racial/ethnic inequalities in cervical cancer screening in the United States: An outcome reclassification to better inform interventions and benchmarks. Preventive Medicine. 2022;159 doi: 10.1016/j.ypmed.2022.107055. 107055-107055. [DOI] [PubMed] [Google Scholar]
- Domogauer J., Cantor T., Quinn G., Stasenko M. Disparities in cancer screenings for sexual and gender minorities. Current Problems in Cancer. 2022;46(5) doi: 10.1016/j.currproblcancer.2022.100858. [DOI] [PubMed] [Google Scholar]
- El-Serag H.B., Petersen L., Hampel H., Richardson P., Cooper G. The use of screening colonoscopy for patients cared for by the Department of Veterans Affairs. Archives of Internal Medicine. 2006;166(20):2202–2208. doi: 10.1001/archinte.166.20.2202. [DOI] [PubMed] [Google Scholar]
- Fehl A., Ferrari S., Wecht Z., Rosenzweig M. Breast cancer in the transgender population. Journal of the Advanced Practitioner in Oncology. 2019;10(4):387. doi: 10.6004/jadpro.2019.10.4.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatt J.D., Cicero E.C., Kittle K.R., Brennan-Ing M. Recommendations for advancing research with sexual and gender minority older adults. The Journals of Gerontology: Serie Bibliographique. 2021;77(1):1–9. doi: 10.1093/geronb/gbab127. [DOI] [PubMed] [Google Scholar]
- Fredriksen-Goldsen K.I., Muraco A. Aging and sexual orientation: A 25-year review of the literature. Research on Aging. 2010;32(3):372–413. doi: 10.1177/0164027509360355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo R. The National Academies Press; 2011. The health of lesbian, gay, bisexual, and transgender people: Building a foundation for better understanding. [PubMed] [Google Scholar]
- Gelberg L., Andersen R.M., Leake B.D. The Behavioral Model for Vulnerable Populations: Application to medical care use and outcomes for homeless people. Health Services Research. 2000;34(6):1273–1302. [PMC free article] [PubMed] [Google Scholar]
- Gerend M.A., Pai M. Social determinants of black-white disparities in breast cancer mortality: A review. Cancer Epidemiology Biomarkers & Prevention. 2008;17(11):2913–2923. doi: 10.1158/1055-9965.EPI-07-0633. [DOI] [PubMed] [Google Scholar]
- Gessner M., Bishop M.D., Martos A., Wilson B.D., Russell S.T. Sexual minority people's perspectives of sexual health care: Understanding minority stress in sexual health settings. Sexuality Research and Social Policy. 2020;17:607–618. doi: 10.1007/s13178-019-00418-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez B.D. Promise of mobile health technology to reduce disparities in patients with cancer and survivors. JCO clinical cancer informatics. 2018;2018(2):1–9. doi: 10.1200/CCI.17.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackshaw A. Small studies: Strengths and limitations. European Respiratory Journal. 2008;32(5):1141–1143. doi: 10.1183/09031936.00136408. [DOI] [PubMed] [Google Scholar]
- Haque R., Chiu V., Mehta K.R., Geiger A.M. An automated data algorithm to distinguish screening and diagnostic colorectal cancer endoscopy exams. JNCI Monographs. 2005;2005(35):116–118. doi: 10.1093/jncimonographs/lgi049. [DOI] [PubMed] [Google Scholar]
- Harper G.W., Jernewall N., Zea M.C. Giving voice to emerging science and theory for lesbian, gay, and bisexual people of color. Cultural Diversity and Ethnic Minority Psychology. 2004;10(3):187. doi: 10.1037/1099-9809.10.3.187. [DOI] [PubMed] [Google Scholar]
- Heslin K.C., Gore J.L., King W.D., Fox S.A. Sexual orientation and testing for prostate and colorectal cancers among men in California. Medical Care. 2008;46(12):1240. doi: 10.1097/MLR.0b013e31817d697f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh N., Ruther M. Sexual minority health and health risk factors: Intersection effects of gender, race, and sexual identity. American Journal of Preventive Medicine. 2016;50(6):746–755. doi: 10.1016/j.amepre.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.-h., Coker A.D. Examining issues affecting African American participation in research studies. Journal of Black Studies. 2010;40(4):619–636. doi: 10.1177/002193470831774. [DOI] [Google Scholar]
- Jackson C.S., Oman M., Patel A.M., Vega K.J. Health disparities in colorectal cancer among racial and ethnic minorities in the United States. Journal of Gastrointestinal Oncology. 2016;7(Suppl 1):S32. doi: 10.3978/j.issn.2078-6891.2015.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal J., Halkitis P.N. Towards a more inclusive and dynamic understanding of medical mistrust informed by science. Behavioral Medicine. 2019;45(2):79–85. doi: 10.1080/08964289.2019.1619511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeudin P., Liveright E., Del Carmen M.G., Perkins R.B. Race, ethnicity, and income factors impacting human papillomavirus vaccination rates. Clinical Therapeutics. 2014;36(1):24–37. doi: 10.1016/j.clinthera.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Jones R.M., Devers K.J., Kuzel A.J., Woolf S.H. Patient-reported barriers to colorectal cancer screening: A mixed-methods analysis. American Journal of Preventive Medicine. 2010-a;38(5):508–516. doi: 10.1016/j.clinthera.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R.M., Woolf S.H., Cunningham T.D., Johnson R.E., Krist A.H., Rothemich S.F., Vernon S.W. The relative importance of patient-reported barriers to colorectal cancer screening. American Journal of Preventive Medicine. 2010-b;38(5):499–507. doi: 10.1016/j.amepre.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph D.A., King J.B., Miller J.W., Richardson L.C., Centers for Disease Control and Prevention, f. D. C. a. P Prevalence of colorectal cancer screening among adults--Behavioral Risk Factor Surveillance System, United States, 2010. MMWR. Morbidity and mortality weekly report. 2012;61:51–56. [PubMed] [Google Scholar]
- Joseph D.A., King J.B., Richards T.B., Thomas C.C., Richardson L.C. Peer reviewed: Use of colorectal cancer screening tests by state. Preventing Chronic Disease. 2018;15 doi: 10.5888/pcd15.170535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahi C.J., Imperiale T.F., Juliar B.E., Rex D.K. Effect of screening colonoscopy on colorectal cancer incidence and mortality. Clinical Gastroenterology and Hepatology. 2009;7(7):770–775. doi: 10.1016/j.cgh.2008.12.030. [DOI] [PubMed] [Google Scholar]
- Kates J., Ranji U., Beamesderfer A., Salganicoff A., Dawson L. Kaiser Family Foundation; 2018. Health and access to care and coverage for lesbian, gay, bisexual, and transgender (LGBT) individuals in the US.https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-and-access-to-care-and-coverage-for-lesbian-gay-bisexual-and-transgender-individuals-in-the-u-s [Google Scholar]
- Kawachi I., Subramanian S., Almeida-Filho N. A glossary for health inequalities. Journal of Epidemiology & Community Health. 2002;56(9):647–652. doi: 10.1136/jech.56.9.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawar L.N. Barriers to breast cancer screening participation among Jordanian and Palestinian American women. European Journal of Oncology Nursing. 2013;17(1):88–94. doi: 10.1016/j.ejon.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Kennamer J.D., Honnold J., Bradford J., Hendricks M. Differences in disclosure of sexuality among African American and White gay/bisexual men: Implications for HIV/AIDS prevention. AIDS Education and Prevention. 2000;12(6):519. [PubMed] [Google Scholar]
- Kim H.-J., Fredriksen-Goldsen K.I. Nonresponse to a question on self-identified sexual orientation in a public health survey and its relationship to race and ethnicity. American Journal of Public Health. 2013;103(1):67–69. doi: 10.2105/AJPH.2012.300835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E., Sleboda P., Rimel B.J., Chen J.T., Hernandez D.V., Datta G.D. Sexual orientation and gender identity inequities in cervical cancer screening by race and ethnicity. Cancer Causes & Control. 2023:1–19. doi: 10.1007/s10552-023-01771-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Elliott A., Strelnick H., Aguilar-Gaxiola S., Cottler L.B. Asian Americans are less willing than other racial groups to participate in health research. Journal of clinical and translational science. 2019;3(2–3):90–96. doi: 10.1017/cts.2019.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo J., Ko K., Shimada A., Nelson N., Wright C., Chen J., Simone N.L. Perceptions of and barriers to cancer screening by the sexual and gender minority community: A glimpse into the health care disparity. Cancer Causes & Control. 2022;33(4):559–582. doi: 10.1007/s10552-021-01549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn M.R., Cui W., Zack M.M., Thompson W.W., Blank M.B., Yehia B.R. Sociodemographic characteristics and health outcomes among lesbian, gay, and bisexual US adults using healthy people 2020 leading health indicators. LGBT Health. 2017;4(4):283–294. doi: 10.1089/lgbt.2016.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcella S., Miller J.E. Racial differences in colorectal cancer mortality: The importance of stage and socioeconomic status. Journal of Clinical Epidemiology. 2001;54(4):359–366. doi: 10.1016/s0895-4356(00)00316-4. [DOI] [PubMed] [Google Scholar]
- McKinnon J. 2002. The black population in the United States: March 2002. Current population reports. [Google Scholar]
- Meyer I.H. Minority stress and mental health in gay men. Journal of Health and Social Behavior. 1995:38–56. doi: 10.2307/2137286. [DOI] [PubMed] [Google Scholar]
- Meyer I.H., Frost D.M. 2013. Minority stress and the health of sexual minorities. [Google Scholar]
- Miller B.C., Bowers J.M., Payne J.B., Moyer A. Barriers to mammography screening among racial and ethnic minority women. Social Science & Medicine. 2019;239 doi: 10.1016/j.socscimed.2019.112494. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health . 2023. About SGMRO. Sexual and gender minority research office.https://dpcpsi.nih.gov/sgmro [Google Scholar]
- Nelson H.D., Tyne K., Naik A., Bougatsos C., Chan B.K., Humphrey L. Screening for breast cancer: An update for the US preventive services Task Force. Annals of Internal Medicine. 2009;151(10) doi: 10.7326/0003-4819-151-10-200911170-00009. 727-W242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen K.H., Trivedi A.N., Shireman T.I. Lesbian, gay, and bisexual adults report continued problems affording care despite coverage gains. Health Affairs. 2018;37(8):1306–1312. doi: 10.1377/hlthaff.2018.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of Disease Prevention and Health Promotion Increase the proportion of adults who get screened for colorectal cancer — C-07 Data. Healthy People 2030. 2023-a https://health.gov/healthypeople/objectives-and-data/browse-objectives/cancer/increase-proportion-adults-who-get-screened-colorectal-cancer-c-07/data [Google Scholar]
- Office of Disease Prevention and Health Promotion Increase the proportion of females who get screened for breast cancer — C-05. Healthy People 2030. 2023-b https://health.gov/healthypeople/objectives-and-data/browse-objectives/cancer/increase-proportion-females-who-get-screened-breast-cancer-c-05 [Google Scholar]
- Peterson E.B., Ostroff J.S., DuHamel K.N., D'Agostino T.A., Hernández M., Canzona M.R., Bylund C.L. Impact of provider-patient communication on cancer screening adherence: A systematic review. Preventive Medicine. 2016;93:96–105. doi: 10.1016/j.ypmed.2016.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce N.A., Cochran S.D., Pizer J.C., Mays V.M. The effects of unequal access to health insurance for same-sex couples in California. Health Affairs. 2010;29(8):1539–1548. doi: 10.1377/hlthaff.2009.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn G.P., Sanchez J.A., Sutton S.K., Vadaparampil S.T., Nguyen G.T., Green B.L.…Schabath M.B. Cancer and lesbian, gay, bisexual, transgender/transsexual, and queer/questioning (LGBTQ) populations. CA: A Cancer Journal for Clinicians. 2015;65(5):384–400. doi: 10.3322/caac.21288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatino S.A., Coates R.J., Uhler R.J., Breen N., Tangka F., Shaw K.M. Disparities in mammography use among US women aged 40-64 years, by race, ethnicity, income, and health insurance status, 1993 and 2005. Medical Care. 2008:692–700. doi: 10.1097/MLR.0b013e31817893b1. [DOI] [PubMed] [Google Scholar]
- Sassi F., Luft H.S., Guadagnoli E. Reducing racial/ethnic disparities in female breast cancer: Screening rates and stage at diagnosis. American Journal of Public Health. 2006;96(12):2165–2172. doi: 10.2105/AJPH.2005.071761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer A.G., Liu B., Siegel R.L., Jemal A., Fedewa S.A. Comparing cancer screening estimates: Behavioral risk factor surveillance system and national health interview survey. Preventive Medicine. 2018;106:94–100. doi: 10.1016/j.ypmed.2017.10.019. [DOI] [PubMed] [Google Scholar]
- Schneebaum A., Badgett M.L. Poverty in US lesbian and gay couple households. Feminist Economics. 2019;25(1):1–30. doi: 10.1080/13545701.2018.1441533. [DOI] [Google Scholar]
- Shelton R.C., Goldman R.E., Emmons K.M., Sorensen G., Allen J.D. An investigation into the social context of low-income, urban Black and Latina women: Implications for adherence to recommended health behaviors. Health Education & Behavior. 2011;38(5):471–481. doi: 10.1177/1090198110382502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA: A Cancer Journal for Clinicians. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA: a cancer journal for clinicians. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- Siu A.L. Screening for breast cancer: US preventive services Task Force recommendation statement. Annals of Internal Medicine. 2016;164(4):279–296. doi: 10.7326/M15-2886. [DOI] [PubMed] [Google Scholar]
- Smith-Bindman R., Miglioretti D.L., Lurie N., Abraham L., Ballard Barbash R., Strzelczyk J.…Kerlikowske K. Does utilization of screening mammography explain racial and ethnic differences in breast cancer? Annals of Internal Medicine. 2006;144(8):541–553. doi: 10.7326/0003-4819-144-8-200604180-00004. [DOI] [PubMed] [Google Scholar]
- Stenzel A.E., Bustamante G., Sarkin C.A., Harripersaud K., Jewett P., Teoh D., Vogel R.I. The intersection of sexual orientation with race and ethnicity in cervical cancer screening. Cancer. 2022;128(14):2753–2759. doi: 10.1002/cncr.34213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan J., Breen N., Coates R.J., Rimer B.K., Lee N.C. Progress in cancer screening practices in the United States: Results from the 2000 national health interview survey. Cancer. 2003;97(6):1528–1540. doi: 10.1002/cncr.11208. [DOI] [PubMed] [Google Scholar]
- Tabaac A.R., Sutter M.E., Wall C.S., Baker K.E. Gender identity disparities in cancer screening behaviors. American Journal of Preventive Medicine. 2018;54(3):385–393. doi: 10.1016/j.amepre.2017.11.009. [DOI] [PubMed] [Google Scholar]
- Taylor P. Pew Research Center; 2013. A survey of LGBT Americans: Attitudes, experiences and values in changing times.https://www.pewresearch.org/social-trends/2013/06/13/a-survey-of-lgbt-americans/ [Google Scholar]
- Trinh M.-H., Agénor M., Austin S.B., Jackson C.L. Health and healthcare disparities among U.S. Women and men at the intersection of sexual orientation and race/ethnicity: A nationally representative cross-sectional study. BMC Public Health. 2017;17(1) doi: 10.1186/s12889-017-4937-9. 964-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger C.A. Care of the transgender patient: A survey of gynecologists' current knowledge and practice. Journal of Women's Health. 2015;24(2):114–118. doi: 10.1089/jwh.2014.4918. [DOI] [PubMed] [Google Scholar]
- von Vaupel‐Klein A.M., Walsh R.J. Considerations in genetic counseling of transgender patients: Cultural competencies and altered disease risk profiles. Journal of Genetic Counseling. 2021;30(1):98–109. doi: 10.1002/jgc4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra G. Race, gender, class, and sexual orientation: Intersecting axes of inequality and self-rated health in Canada. International Journal for Equity in Health. 2011;10(1) doi: 10.1186/1475-9276-10-3. 3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward B.W., Dahlhamer J.M., Galinsky A.M., Joestl S.S. Sexual orientation and health among US adults: National health interview survey, 2013. National health statistics reports. 2014;(77):1–10. [PubMed] [Google Scholar]
- Warner E. Breast-cancer screening. New England Journal of Medicine. 2011;365(11):1025–1032. doi: 10.1056/NEJMcp1101540. [DOI] [PubMed] [Google Scholar]
- Williams D.R. Racial/ethnic variations in women's health: The social embeddedness of health. American Journal of Public Health. 2008;98(Supplement 1):S38–S47. doi: 10.2105/AJPH.98.Supplement_1.S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
BRFSS data are publically available