Abstract
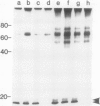
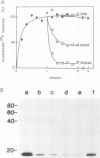
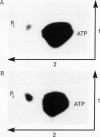
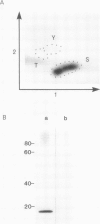
Plasma membrane vesicles from pea (Pisum sativum L.) seedlings contain an autophosphorylating calcium-activated protein kinase of relative molecular weight 18,000 (phosphoprotein 18, pp 18). Pulse chase analysis revealed that pp 18 autophosphorylation exhibited very rapid turnover. pp 18 was the only detectable plasma membrane protein to have this property. pp 18 has been highly purified by affinity chromatography and the final preparation contains peptides of relative molecular weight 67,000, 48,000, and 18,000. Highly purified pp 18 still showed rapid cycling of autophosphorylated phosphate attached to pp 18. Turnover of pp 18 autophosphorylation was accelerated by ADP, but this effect did not appear to be the back reaction of the kinase since inorganic phosphate accumulation is increased by ADP. A rapid cycling of phosphorylation is an ideal control point in signal transduction.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bearden J. C., Jr Quantitation of submicrogram quantities of protein by an improved protein-dye binding assay. Biochim Biophys Acta. 1978 Apr 26;533(2):525–529. doi: 10.1016/0005-2795(78)90398-7. [DOI] [PubMed] [Google Scholar]
- Blowers D. P., Trewavas A. J. Autophosphorylation of plasma membrane bound calcium calmodulin dependent protein kinase from pea seedlings and modification of catalytic activity by autophosphorylation. Biochem Biophys Res Commun. 1987 Mar 13;143(2):691–696. doi: 10.1016/0006-291x(87)91409-4. [DOI] [PubMed] [Google Scholar]
- Burnell J. N., Hatch M. D. Regulation of C4 photosynthesis: identification of a catalytically important histidine residue and its role in the regulation of pyruvate,Pi dikinase. Arch Biochem Biophys. 1984 May 15;231(1):175–182. doi: 10.1016/0003-9861(84)90375-8. [DOI] [PubMed] [Google Scholar]
- Cheng Y. S., Chen L. B. Detection of phosphotyrosine-containing 34,000-dalton protein in the framework of cells transformed with Rous sarcoma virus. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2388–2392. doi: 10.1073/pnas.78.4.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie G. Pseudosubstrates turn off protein kinases. Nature. 1988 Oct 13;335(6191):592–593. doi: 10.1038/335592a0. [DOI] [PubMed] [Google Scholar]
- Hunter T. A thousand and one protein kinases. Cell. 1987 Sep 11;50(6):823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- LaPorte D. C., Koshland D. E., Jr A protein with kinase and phosphatase activities involved in regulation of tricarboxylic acid cycle. Nature. 1982 Dec 2;300(5891):458–460. doi: 10.1038/300458a0. [DOI] [PubMed] [Google Scholar]
- Nimmo G. A., Borthwick A. C., Holms W. H., Nimmo H. G. Partial purification and properties of isocitrate dehydrogenase kinase/phosphatase from Escherichia coli ML308. Eur J Biochem. 1984 Jun 1;141(2):401–408. doi: 10.1111/j.1432-1033.1984.tb08205.x. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr Assays of protein kinase. Methods Enzymol. 1983;99:3–6. doi: 10.1016/0076-6879(83)99034-1. [DOI] [PubMed] [Google Scholar]
- Roux S. J., Serlin B. S. Cellular mechanisms controlling light-stimulated gravitropism: role of calcium. CRC Crit Rev Plant Sci. 1987;5(3):205–236. doi: 10.1080/07352688709382240. [DOI] [PubMed] [Google Scholar]
- Smith D. L., Chen C. C., Bruegger B. B., Holtz S. L., Halpern R. M., Smith R. A. Characterization of protein kinases forming acid-labile histone phosphates in Walker-256 carcinosarcoma cell nuclei. Biochemistry. 1974 Aug 27;13(18):3780–3785. doi: 10.1021/bi00715a025. [DOI] [PubMed] [Google Scholar]
- Smith L. S., Kern C. W., Halpern R. M., Smith R. A. Phosphorylation on basic amino acids in myelin basic protein. Biochem Biophys Res Commun. 1976 Jul 26;71(2):459–465. doi: 10.1016/0006-291x(76)90809-3. [DOI] [PubMed] [Google Scholar]
- Yamauchi T., Fujisawa H. Self-regulation of calmodulin-dependent protein kinase II and glycogen synthase kinase by autophosphorylation. Biochem Biophys Res Commun. 1985 May 31;129(1):213–219. doi: 10.1016/0006-291x(85)91424-x. [DOI] [PubMed] [Google Scholar]