Abstract
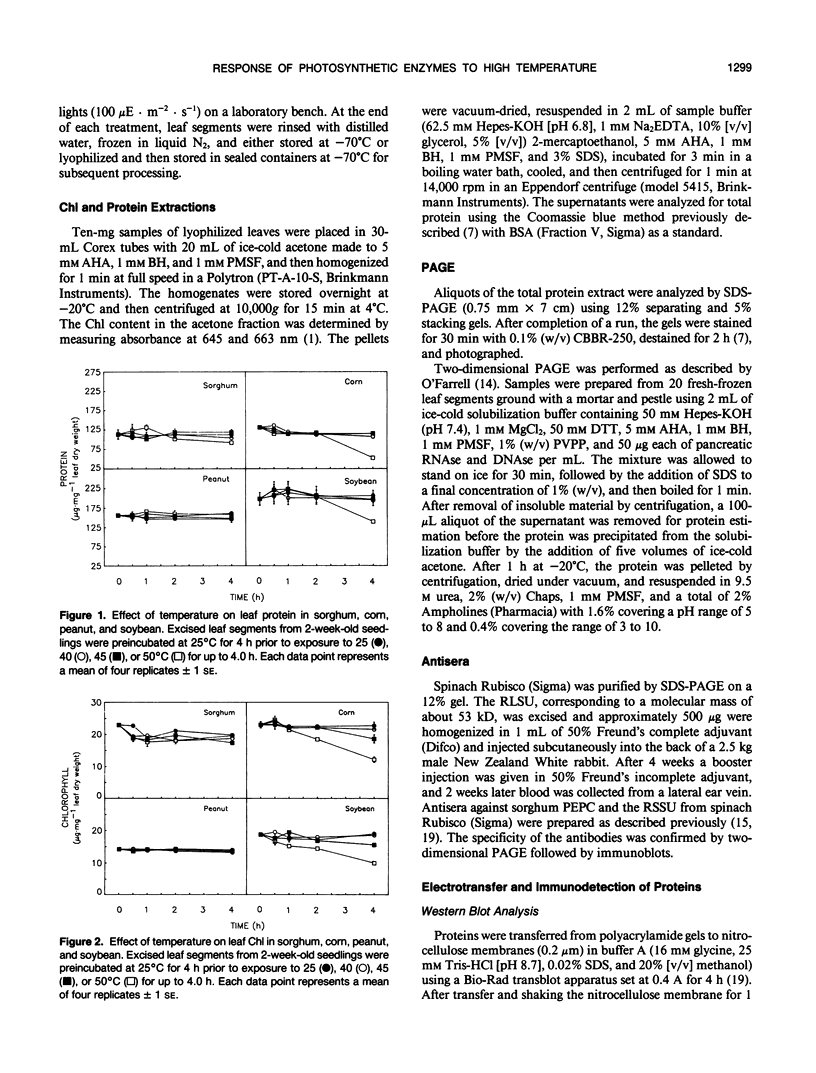
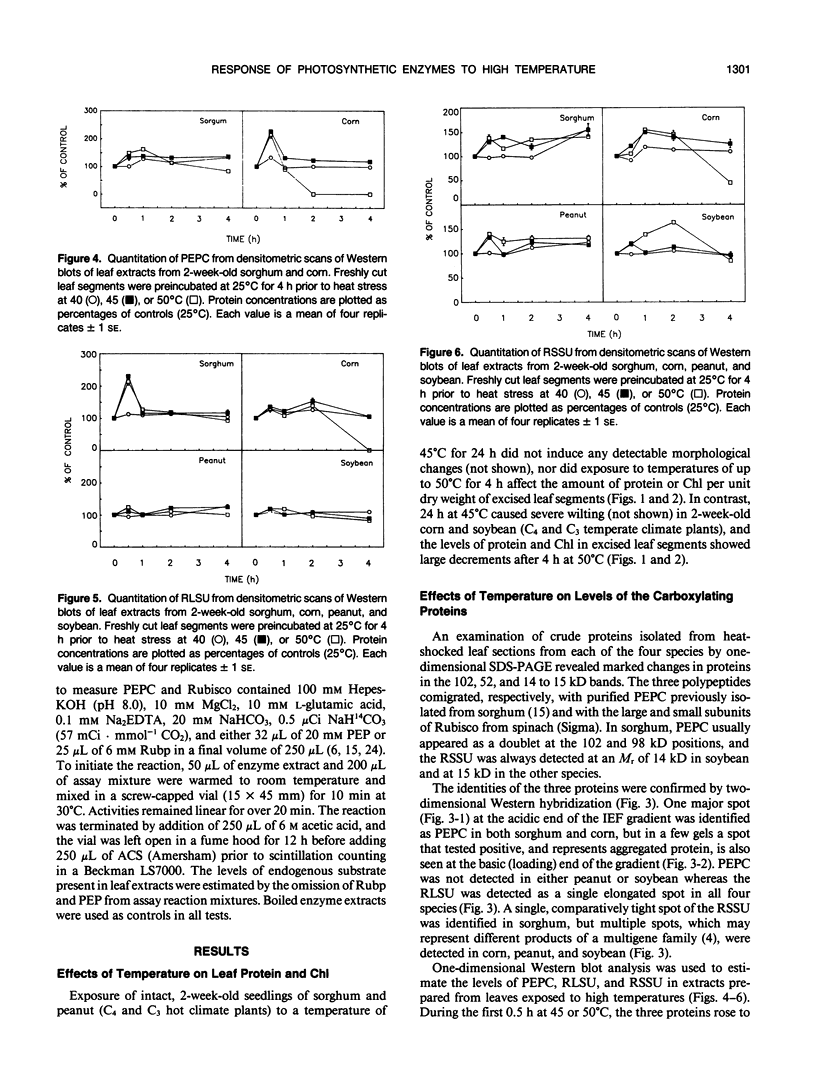
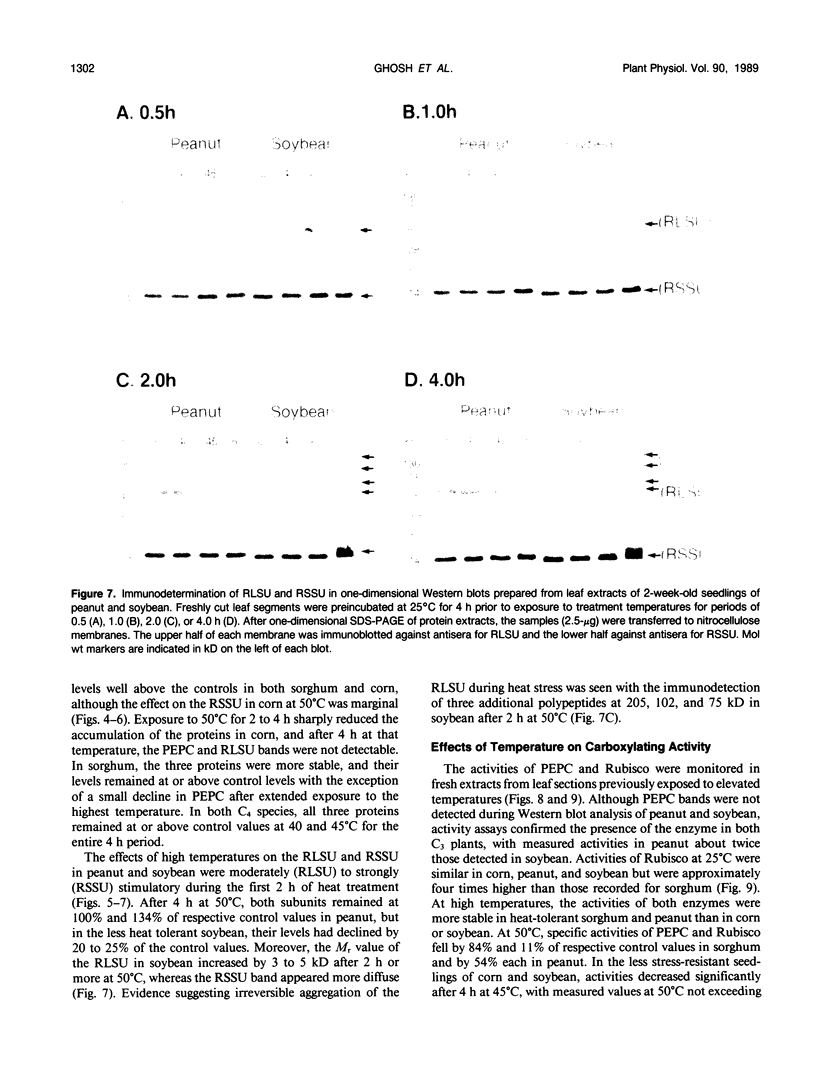
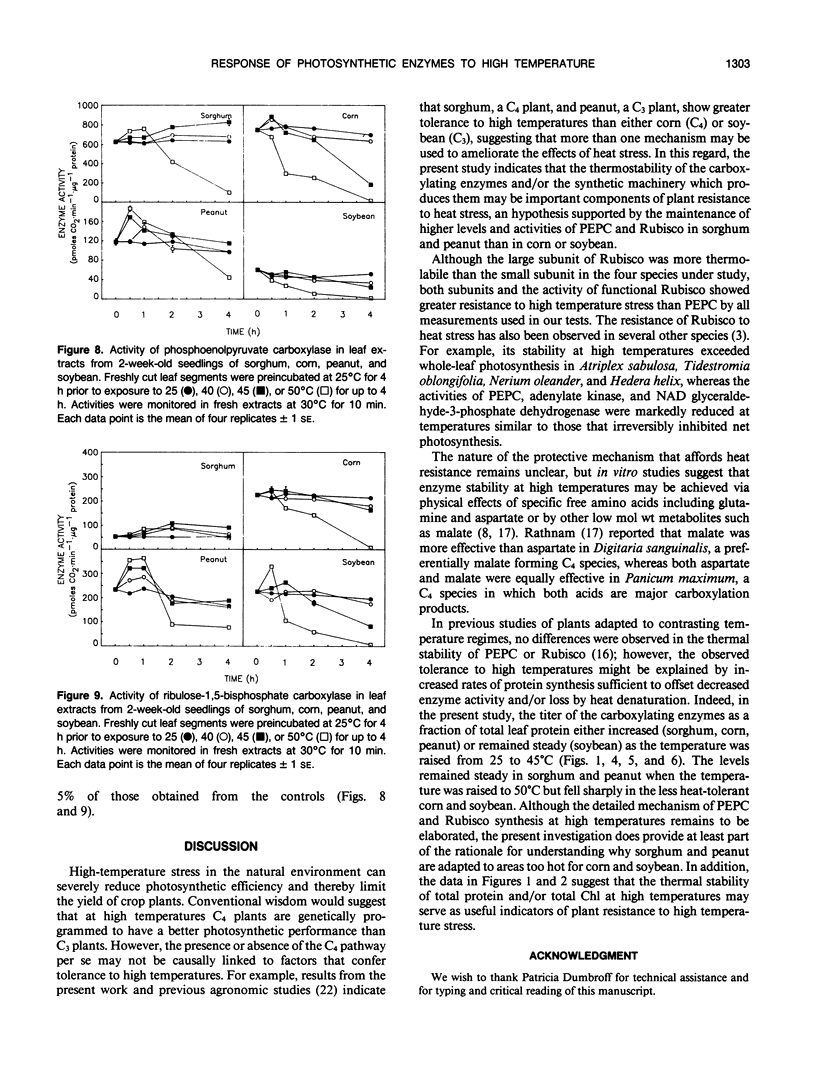
Exposure of leaf sections from 2-week-old seedlings of sorghum (Sorghum bicolor L.) (C4 plant), corn (Zea mays L.) (C4), peanut (Arachis hypogaea L.) (C3 plant), and soybean (Glycine max L.) (C3) to 40 or 45°C for up to 4 hours resulted in significant increases in the levels of 102 kilodalton (C4), 52 kilodalton (C3 and C4), and 15 kilodalton (C3 and C4) polypeptides. These proteins comigrated, respectively, with authentic phosphoenolpyruvate carboxylase (PEPC) and the large (RLSU) and small (RSSU) subunits of ribulose-1,5-bisphosphate carboxylase (Rubisco) during both one- and two-dimensional SDS-PAGE and reacted with antisera raised against these enzymes. After 4 hours at 50°C, levels of the polypeptides either remained relatively stable (PEPC, RLSU) or increased (RSSU) in sorghum and peanut (plants native to hot climates). In corn and soybean (plants native to temperate climates), levels of the proteins either fell sharply (corn) or showed strong evidence of incomplete processing and/or aggregation (soybean). In addition to changes in levels of the proteins, the activities of PEPC and Rubisco in extracts of leaves exposed to 50°C fell by 84% and 11% of their respective control values in sorghum and by 54% each in peanut. In corn and soybean, the activities of both enzymes were depressed at 40°C, with measured values at 50°C not exceeding 5% of those from the nonstressed controls.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Lowe S. L., Mc Knight T. D., Shah D. M., Meagher R. B. The nucleotide sequence, expression, and evolution of one member of a multigene family encoding the small subunit of ribulose-1,5-bisphosphate carboxylase in soybean. J Mol Appl Genet. 1982;1(6):483–498. [PubMed] [Google Scholar]
- Cooper P., Ho T. H., Hauptmann R. M. Tissue specificity of the heat-shock response in maize. Plant Physiol. 1984 Jun;75(2):431–441. doi: 10.1104/pp.75.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. R., Seemann J. R. Differences between Wheat Genotypes in Specific Activity of Ribulose-1,5-bisphosphate Carboxylase and the Relationship to Photosynthesis. Plant Physiol. 1984 Apr;74(4):759–765. doi: 10.1104/pp.74.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Gepstein S., Heikkila J. J., Dumbroff E. B. Use of a scanning densitometer or an ELISA plate reader for measurement of nanogram amounts of protein in crude extracts from biological tissues. Anal Biochem. 1988 Mar;169(2):227–233. doi: 10.1016/0003-2697(88)90278-3. [DOI] [PubMed] [Google Scholar]
- Goh C. J., Dumbroff E. B., Lepock J. R. Amino acid pools in CHL V79 cells during induction of thermotolerance: reduction in free intracellular glutamine. J Cell Physiol. 1988 Apr;135(1):139–144. doi: 10.1002/jcp.1041350120. [DOI] [PubMed] [Google Scholar]
- Mansfield M. A., Key J. L. Synthesis of the low molecular weight heat shock proteins in plants. Plant Physiol. 1987 Aug;84(4):1007–1017. doi: 10.1104/pp.84.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Shurtz-Swirski R., Gepstein S. Proteolysis of endogenous substrates in senescing oat leaves : I. Specific degradation of ribulose bisphosphate carboxylase. Plant Physiol. 1985 May;78(1):121–125. doi: 10.1104/pp.78.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T., Mizuno M., Hayashi M. Partitioning of Nitrogen among Ribulose-1,5-bisphosphate Carboxylase/Oxygenase, Phosphoenolpyruvate Carboxylase, and Pyruvate Orthophosphate Dikinase as Related to Biomass Productivity in Maize Seedlings. Plant Physiol. 1984 Jul;75(3):665–669. doi: 10.1104/pp.75.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell E. The 70-kd mammalian heat shock proteins are structurally and functionally related to the uncoating protein that releases clathrin triskelia from coated vesicles. EMBO J. 1985 Dec 16;4(13A):3385–3391. doi: 10.1002/j.1460-2075.1985.tb04094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A. Breakdown of Ribulose Bisphosphate Carboxylase and Change in Proteolytic Activity during Dark-induced Senescence of Wheat Seedlings. Plant Physiol. 1978 Oct;62(4):604–608. doi: 10.1104/pp.62.4.604. [DOI] [PMC free article] [PubMed] [Google Scholar]




