Abstract
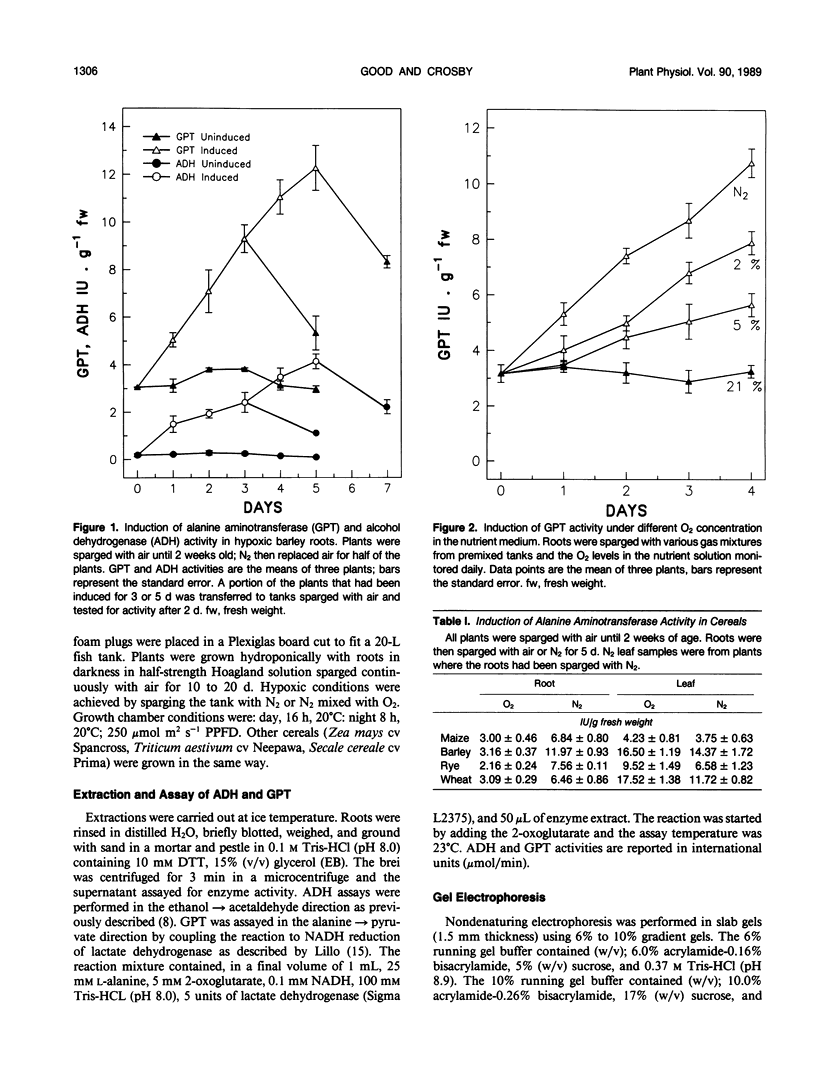
Alanine aminotransferase, otherwise called glutamate-pyruvate aminotransferase (GPT), activity increases up to fourfold during several days of anaerobic induction in barley (Hordeum vulgare L.) roots, reaching a maximum activity of 13 international units per gram fresh weight. This increase in activity paralleled the increase in alcohol dehydrogenase activity in the same root tissue. Upon return to aerobic conditions, the induced GPT activity declined with an apparent half-life of 2 days. The isozyme profile of GPT in barley root tissue comprised one band of activity; in maize there were three bands of activity, the bands with greater mobility had much lower activity. Native polyacrylamide gel electrophoresis indicated that the induction of GPT activity results from an increase in the level of activity of these bands; no other activities were detected. When root tissue was induced under different levels of hypoxia (0%, 2%, 5%, and 21% O2), changes in GPT activity were found to increase with lower levels of oxygen. Comparisons of GPT induction in barley, maize (Zea mays), rye, (Secale cereale) and wheat (Triticum aestivum) indicate that this enzyme is induced in the root tissue of all of these cereals; however, anaerobic root conditions do not result in the induction of GPT activity in leaf tissue. The dependence of GPT induction on high levels of nitrate in the media was tested by comparing activity levels in Hoagland solution and a nitrate-free nutrient solution. GPT activity was induced to similar levels under both conditions. These results indicate that alanine aminotransferase shows a very similar pattern of induction to alcohol dehydrogenase in barley root tissue and may be important in anaerobic glycolysis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Effer W. R., Ranson S. L. Some effects of oxygen concentration on levels of respiratory intermediates in buckwheat seedlings. Plant Physiol. 1967 Aug;42(8):1053–1058. doi: 10.1104/pp.42.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach W. L., Pryor A. J., Dennis E. S., Ferl R. J., Sachs M. M., Peacock W. J. cDNA cloning and induction of the alcohol dehydrogenase gene (Adh1) of maize. Proc Natl Acad Sci U S A. 1982 May;79(9):2981–2985. doi: 10.1073/pnas.79.9.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm E. A., Vose B. M., Chu E. W., Wilson D. J., Lotze M. T., Rayner A. A., Rosenberg S. A. The human mixed lymphocyte-tumor cell interaction test. I. Positive autologous lymphocyte proliferative responses can be stimulated by tumor cells as well as by cells from normal tissues. Cancer Immunol Immunother. 1984;17(2):83–89. doi: 10.1007/BF00200041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson A. D., Jacobsen J. V., Zwar J. A. Regulated expression of three alcohol dehydrogenase genes in barley aleurone layers. Plant Physiol. 1984 Jul;75(3):573–581. doi: 10.1104/pp.75.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D., Mau S. L. Activity, location, and role of asparate aminotransferase and alanine aminotransferase isoenzymes in leaves with C4 pathway photosynthesis. Arch Biochem Biophys. 1973 May;156(1):195–206. doi: 10.1016/0003-9861(73)90357-3. [DOI] [PubMed] [Google Scholar]
- Hatch M. D. Separation and properties of leaf aspartate aminotransferase and alanine aminotransferase isoenzymes operative in the C4 pathway of photosynthesis. Arch Biochem Biophys. 1973 May;156(1):207–214. doi: 10.1016/0003-9861(73)90358-5. [DOI] [PubMed] [Google Scholar]
- Hoffman N. E., Bent A. F., Hanson A. D. Induction of lactate dehydrogenase isozymes by oxygen deficit in barley root tissue. Plant Physiol. 1986 Nov;82(3):658–663. doi: 10.1104/pp.82.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley P. M., Freeling M. Anaerobic expression of maize fructose-1,6-diphosphate aldolase. J Biol Chem. 1984 Nov 25;259(22):14180–14183. [PubMed] [Google Scholar]
- Kelley P. M., Freeling M. Anaerobic expression of maize glucose phosphate isomerase I. J Biol Chem. 1984 Jan 10;259(1):673–677. [PubMed] [Google Scholar]
- Roberts J. K., Callis J., Wemmer D., Walbot V., Jardetzky O. Mechanisms of cytoplasmic pH regulation in hypoxic maize root tips and its role in survival under hypoxia. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3379–3383. doi: 10.1073/pnas.81.11.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs M. M., Freeling M., Okimoto R. The anaerobic proteins of maize. Cell. 1980 Jul;20(3):761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Sakano K., Tazawa M. Metabolic Conversion of Amino Acids Loaded in the Vacuole of Chara australis Internodal Cells. Plant Physiol. 1985 Aug;78(4):673–677. doi: 10.1104/pp.78.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]