Abstract
Introduction
Rheumatic heart disease (RHD) affects over 39 million people worldwide, the majority in low-income and middle-income countries. Secondary antibiotic prophylaxis (SAP), given every 3–4 weeks can improve outcomes, provided more than 80% of doses are received. Poor adherence is strongly correlated with the distance travelled to receive prophylaxis. Decentralising RHD care has the potential to bridge these gaps and at least maintain or potentially increase RHD prophylaxis uptake. A package of implementation strategies was developed with the aim of reducing barriers to optimum SAP uptake.
Methods and analysis
A hybrid implementation-effectiveness study type III was designed to evaluate the effectiveness of a package of implementation strategies including a digital, cloud-based application to support decentralised RHD care, integrated into the public healthcare system in Uganda. Our overarching hypothesis is that secondary prophylaxis adherence can be maintained or improved via a decentralisation strategy, compared with the centralised delivery strategy, by increasing retention in care. To evaluate this, eligible patients with RHD irrespective of their age enrolled at Lira and Gulu hospital registry sites will be consented for decentralised care at their nearest participating health centre. We estimated a sample size of 150–200 registrants. The primary outcome will be adherence to secondary prophylaxis while detailed implementation measures will be collected to understand barriers and facilitators to decentralisation, digital application tool adoption and ultimately its use and scale-up in the public healthcare system.
Ethics and dissemination
This study was approved by the Institutional Review Board (IRB) at Cincinnati Children’s Hospital Medical Center (IRB 2021-0160) and Makerere University School of Medicine Research Ethics Committee (Mak-SOMREC-2021-61). Participation will be voluntary and informed consent or assent (>8 but <18) will be obtained prior to participation. At completion, study findings will be communicated to the public, key stakeholders and submitted for publication.
Keywords: valvular heart disease, adult cardiology, health informatics, registries, preventive medicine
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The study methodology outlines an evaluation approach for decentralised care programmes for rheumatic heart disease, which has not been described before, integrating an electronic rheumatic heart disease registry for primary healthcare.
A range of implementation strategies are incorporated within a robust and iterative methodology that address known barriers to care.
Two different geographical settings are used for the implementation in Uganda, increasing the external validity.
The study is limited by the pre/post design and lacks an external control group.
The small number of participating facilities and patients will limit understanding the effectiveness of the intervention.
Introduction
Rheumatic heart disease (RHD) remains the most commonly acquired heart disease in people under 25 years of age.1 The median age at death, 28 years in sub- Saharan Africa,2 translates into a large toll on the economically productive age groups, resulting in rippled economic effects for already impoverished families.3 Furthermore, RHD is a disease associated with marked disparities, disproportionally affecting socioeconomically disadvantaged populations including children, women, poverty-stricken and marginalised minority ethnic groups.4–8 It is estimated that there are 39 million people with RHD globally, surpassing the number of people currently living with HIV/AIDS.4 9 Unlike HIV, which has seen sustained efforts towards control, RHD was not a priority on the international health development agenda for many years. Most low-income countries have no RHD programmes in place, resulting in a gross underestimation of the prevalent cases and poor RHD knowledge among the healthcare workforce.
The first global resolution on rheumatic fever and RHD was adopted at the 71st World Health Assembly in 2018. Outlined among the broad clauses of this resolution is for countries to invest in community and primary healthcare (PHC) workers as well as access to medicines for the prevention and control of RHD.10 11 Secondary antibiotic prophylaxis (SAP), in the form of monthly intramuscular benzathine penicillin G (BPG), has been shown to be effective in preventing recurrent streptococcal infections—‘strep throat’, acute rheumatic fever (ARF) and progression of RHD.12–14 These benefits are contingent on achieving an optimum adherence, at least ≥80% coverage of prescribed injections over many years of treatment.14 15 However, adherence is often suboptimal, leaving patients vulnerable to recurrent ARF and disease progression, a significant risk factor for death within 8 months of diagnosis.16 Several factors have been shown to impact optimal BPG adherence—including drug supply shortages, distances travelled to the health facilities and associated costs of attending hospitals for monthly injections.17–19
Previous research in Uganda identified the distance people currently have to travel to receive routine monthly SAP is a major barrier, and a strong predictor of retention.20 21 This is due in part to the absent district-level RHD programmes in Uganda, where PHC nurses do not have practical skills and tools to efficiently manage BPG delivery for patients with RHD, despite the fact that this is well within their scope of practice. Moreover, registries have been identified as an important part of RHD control measures22–24 and set forth as a priority by RHD experts.25 In practice, centralised registries have often taken the form of static data collection,21 and not geared to scale to the community at large. While RHD programmes are not yet operational at the district level in Uganda, we have an opportunity to improve access and uptake of BPG prophylaxis for the small fraction (1%–2% of estimated total cases nationally) of people who have been identified and are active in the national RHD disease registry.20 Decentralisation of care to primary health facilities has been employed for other diseases, the most widespread in the region being decentralisation of HIV treatment to PHC nurses, allowing for major scale-up and availability of HIV services to those in-need.26
Demonstrating that a new approach to RHD care is effective and implementable is important for scaling RHD services more broadly. The capacity within the current centralised approach is insufficient to serve the approximately 200–400 thousand persons estimated to be living with RHD in Uganda. Thus, there is a need to bring RHD care into the digital age, where technology-enhanced dynamic tools can be employed to improve RHD care delivery. The ADD-RHD (Active Case Detection and Decentralised Dynamic Registry to Improve the Uptake of Rheumatic Heart Disease Secondary Prevention) study was designed to address the above-mentioned challenges, including long distances to regional hospitals and the lack of a dynamic record system. The study is called ‘ADD-RHD’ in part because the study ‘adds’ RHD care to the list of competencies of PHC nurses in the study sites. As a major component of this study, the Active community case management tool (ACT) that was recently developed and piloted will be introduced in this setting, intended to support clinicians with technology-enhanced support tools.27
Aims and hypothesis
This study aims to evaluate the effectiveness of a package of implementation strategies that includes: assessment of site readiness, decentralisation of service sites, a new mode of electronic record- keeping, healthcare worker (HCW) training, iterative feedback during implementation, identification of champions and physical supply of medicines for improving SAP delivery for RHD care. We hypothesise that this package of strategies will be equivalent to or improve the current SAP adherence and related outcomes for enrolees in the decentralised study locations.
Methods and analysis
Study design
This is a mixed-methods, hybrid type III effectiveness-implementation study that will be integrated into rural and semiurban primary health centres (HCs). This design primarily focuses on the effectiveness of a package of implementation strategies while collecting secondary data on clinical outcomes.28 Decentralisation of SAP delivery is postulated to at least preserve the level of adherence, while building capacity to scale up service delivery. The primary implementation endpoints will look at the postimplementation healthcare utilisation outcomes among enrolled patients, with a particular focus on SAP adherence (defined as proportion of days covered), which is strongly associated with the clinical outcomes of recurrence of ARF and progression of disease.14 The study will determine whether adherence to SAP postimplementation is non-inferior to the current, centralised care. We estimated a total of 150–200 persons with RHD Lira and Gulu will be eligible for decentralisation (see the Statistical considerations section). Further, we will evaluate the acceptability, penetration, adoption and cost of the implementation.
For secondary clinical outcomes, we will explore the relationship between programme and adverse cardiovascular events (recurrent ARF, new or worsening heart failure, atrial fibrillation) and mortality compared with the baseline period. Because of the relatively small number of identified people with RHD in Uganda, and the centralised nature of secondary prophylaxis delivery currently at a small number of referral hospitals, we developed a non-randomised experiment using pre/post methods to demonstrate the impact of a package of strategies on implementation outcomes as well as intermediate clinical outcomes.
Study setting
Current RHD care provision: national registry at central and referral regional hospitals
Presently, a national RHD registry, a collection of clinical data for patients with RHD enrolled and known to the healthcare system in Uganda is run centrally by research staff at the Uganda Heart Institute (Kamplala). Initially established in 2010, the registry subsequently expanded to include a satellite centre in Kampala (Lebowa)—and regional registry sites within three districts across the country (Mbarara, Gulu and Lira). The current RHD registry-based care in Uganda was initiated to capture people presenting with RHD to tertiary care and has served to establish numbers of those affected together with informing patient status. Dedicated research staff provide, coordinate and monitor routine BPG prophylaxis and RHD-related patient care in the country. The RHD registry is hosted electronically on REDCap, and involves both direct data entry and transfer of paper records into REDCap.29 30 However, the majority of records are paper-based and limited to the centres, which has proven to be outdated and ineffective (table 1).
Table 1.
Comparison of current and proposed SAP delivery approaches
| Current approach—National RHD Registry | Proposed approach—Decentralised RHD Registry at district-level health facilities | |
| Location | Limited to central and regional referral centres | Expansion to Health centres III/IV (Lira and Gulu districts) |
| Staff | Dedicated research staff regionally | This approach will incorporate existing MOH staff at HCIII/IV at district and regional hospitals, as well as administrators and different stakeholders from Ministerial representatives |
| Patient records and data | REDCap/paper-based clinical records
|
ACT application
|
ACT, active community case management tool; BPG, benzathine penicillin G; HC III/IV, health centre III/IV; HCW, healthcare worker; MOH, Ministry of Health; RHD, rheumatic heart disease; SAP, secondary antibiotic prophylaxis.
The new approach: ADD-RHD
Existing RHD registrants based in Lira and Gulu districts will have their monthly SAP visits decentralised from the current research-nurse led regional hospitals to outpatient settings of selected PHCs staffed by ministry of health nurses (table 1). In this study, decentralisation is defined as the change of service sites for delivery of SAP for RHD registry patients, from current regional hospitals to district level PHC facilities. As part of the ADD-RHD package, the ACT application (see below) will be introduced.
The intervention
The evidenced-based practice, SAP, has been proven to be effective in reducing the recurrence of strep throat and ARF, the cascade of events that can progress to RHD.14 15 31 In addition to reducing the progression of RHD, there is evidence that SAP can also induce the regression of clinical RHD.32 Unless contraindicated, BPG is the gold standard and most widely used for RHD secondary prevention.14
Preparation for decentralisation
In preparation for the study, facility visits, engagement of local and district health officers and consideration of the existing registry were done in order to inform important aspects of the implementation strategy.
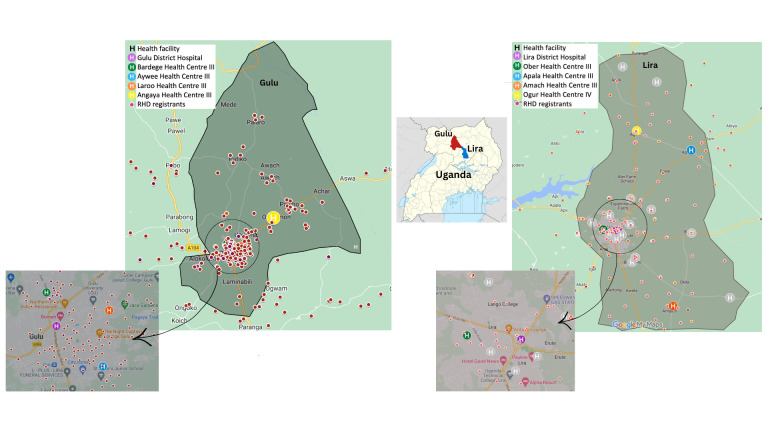
Existing RHD registrants were mapped by residence, and consequently, four levels 3 and 4 HCs (HCIII/IV) were chosen in each district based on diversity of location (city and rural parts of the districts) and RHD registrant geographical density (figure 1). This was done in coordination with the local government, involving the District Health Administrator. In Uganda, rural and semiurban areas are served by primary care health facilities with designated levels 1–4, where level 1 is the lowest basic dispensary and 4 with more services such as maternity care.
Figure 1.
Geographical location of implementation sites in two districts within Uganda. The four selected health centres (H) in Lira and Gulu (colour coded above) were chosen according to RHD registrants’ geographical density. RHD, rheumatic heart disease.
Initial collection of 12–18 months of intensive baseline data of existing RHD registry patients will be done at the Lira and Gulu regional hospitals, where RHD patient care is currently based. Where necessary, patients will be contacted by phone for confirmation and completeness of information in order to determine baseline BPG adherence and retention data prior to decentralisation. This data will be collected on a quarterly basis for important primary and secondary metrics defined.
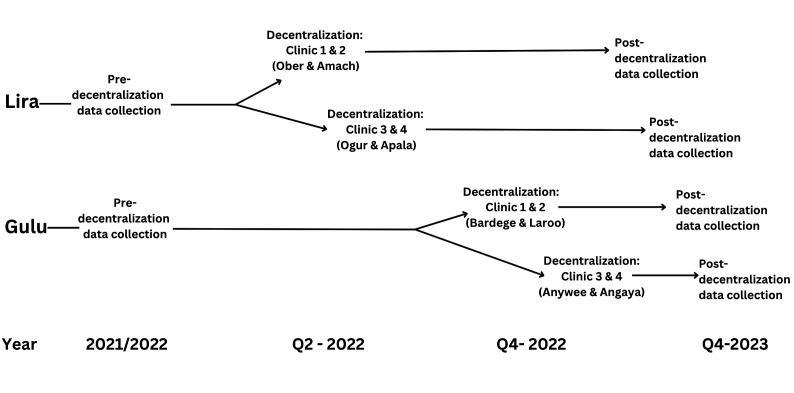
Decentralisation will be a phased process starting with two of the four clinics in Lira district, followed by the remaining two, with an approximate 4-week gap in-between. Thereafter, this will be replicated in Gulu after a period of 4–6 months to allow incorporation of planned formative feedback from decentralisation in the Lira district (figure 2).
Figure 2.
Sequential outline of ADD-RHD implementation plans for health centres in Lira and Gulu districts, Uganda. ADD-RHD, Active Case Detection and Decentralised Dynamic Registry to Improve the Uptake of Rheumatic Heart Disease Secondary Prevention.
In this study, a package of implementation strategies (table 2) will be employed to support a decentralised SAP delivery strategy to HC III/IV. This will be centred around the introduction of the ACT application further elaborated below.
Table 2.
ADD-RHD implementation strategies mapped according to expert recommendations for implementing change (ERIC) discreet implementation strategies40
| Strategy | Details |
| Assess for readiness and identify barriers and facilitators | Assessment for readiness will be done through facility visits, surveys and engagement of local and district health officers. Patient and provider interviews will identify barriers and facilitators to decentralisation. Key stakeholder and community engagement on decentralisation logistics will serve to establish key components of the process. |
| Change of service sites (decentralisation) | Four health facilities were identified based on patient clusters and distances from their residences primarily geared at increasing access and reducing distances travelled. |
| Training healthcare workers (HCWs)/develop educational materials | HCW training was planned to include the development of education materials on RHD clinical knowledge, BPG preparation and injection skills, penicillin adverse events recognition and first aid management. A detailed description is provided below. |
| Change record systems | ACT application was specifically developed as a clinical tool for HCWs through stakeholder engagement and piloting.27 It encompasses in-built tools to enhance patient engagement, including clinicians’ monitoring of adherence and quality metrics for monitoring supply stocks. This will replace the current regional registry. A detailed description is provided below. |
| Purposefully re-examine the implementation | We built in milestones to re-examine implementation activities, identify challenges, and provide feedback and support to health facilities in order to continuously improve the quality of care. This includes looking at the use of ACT for patient management, identifying challenges and giving feedback to healthcare workers in health centres. |
| Identify and prepare champions | Initial assessment for readiness informed the need for local champions at each health centre, selected to drive the implementation by providing support and driving quality improvement activities such as updating stock and supplies data for quality metrics on the ACT application. |
| Physical supply of medicines* | Although historically used to treat other conditions, such as syphilis, its consistent availability is variable in public facilities in Uganda. Hence, through stakeholder and local engagement, temporary BPG supply was found to be an essential initial component to the success of the intervention at a few facilities. This marked an iterative adaptation in light of short-term regulatory constraints. For some facilities, increasing BPG supply through the government system was motivated by history of use, and hence a gap in supply was inevitable during the initial post-decentralisation period. Covering this gap was an important aspect to implementation. |
*Not a specific ERIC implementation strategy.
ACT, active community case management tool; ADD, Active Case Detection and Decentralised Dynamic Registry; BPG, benzathine penicillin G; RHD, rheumatic heart disease.
ACT application
The ACT application is a digital tool-kit designed to build on REDCap, the current research database. The application incorporates several important features for RHD control including; (1) availability of a simplified, interactive record of patients’ administered BPG injections with automatic adherence calculations and relevant patient details, investigations and management; (2) a ‘manage my patient’ feature that allows clinicians to track patient status by due or missed visits that integrates with a clinician-facing reminder function and (3) monitoring of medicinal and supply stocks at facility and central levels. ACT is a small-scale medical record application built with the overarching goal of its integration for RHD care nationally and internationally, with potential to be replicated for use in other chronic disease management. Table 1 summarises the current and new approach with regards to ACT as a novel electronic tool. The use of technology enhanced tools in this setting will require some additional efforts. This was informed by the pilot training where many HCWs were not conversant with digital tools and apps. The feedback was then used to develop a simplified application version for HCWs.27 Second, an offline feature was added to ACT to ensure interruptions are minimised given the instability with internet connectivity in this setting. Further, initial ongoing site support visits have been planned at predetermined intervals (frequently at first and then more spaced out) and will serve to provide refresher training on RHD and ACT. Data will be collected around these and incorporated into the implementation evaluation.
Provider education
Training of health workers from HCs will be central to this project. While advocacy and awareness of RHD has been increasing due to established research efforts, a large gap remains in provider RHD competency. Recent research found that less than 25% of facilities across several Ugandan districts had received any RHD training in the past 2 years and only 11% and 8% HC III and IVs had any RHD guidelines.33 Further, limited RHD knowledge was a prominent theme in published healthcare provider interviews, who expressed a strong desire for training.33
Currently, service provision of BPG for RHD in HC III/IV is not systematically undertaken. This informed an initial pilot training of representatives from each of the selected HCIII/IV facilities for decentralisation in Lira. The pilot identified deficiencies in specific areas that were instrumental in tailoring educational materials developed for pre-decentralisation training. We found variable but low RHD knowledge and experience among the HCWs. BPG is known to be a difficult injection to administer due to its nature to crystallise and presents challenges for unexperienced workers. A substantial portion of the planned training (and refresher sessions planned periodically thereafter), will focus on the practical aspects of BPG administration, as well as recognition and triage of potential BPG allergic reactions and anaphylaxis. The second set of training materials will consist of introduction to the ACT application. Additionally, a standard operating procedure was developed, which will serve as the guideline for all decentralisation procedures in health facilities in both districts.
‘Champions’ will be identified by the RHD research nurses based at the regional hospitals. The relationship between them and the staff at the HCs has been fostered overtime, giving them an advantage to identify overall motivated staff in different aspects of decentralisation, with a particular emphasis on electronic record patient management and follow-up. Champions will be identified for each HC in the first few months after decentralisation and geared to drive the implementation and continuous support Inclusion of medical records personnel was not initially planned for. However, the pilot training revealed that these personnel are more technologically competent and including them would facilitate support at the HCs throughout the ‘implementation’.
Study population and recruitment
All eligible RHD registrants will be approached by trained study staff. After an explanation of the study is provided, the registrants will be invited to participate in decentralisation. Registrants will be presented with the option to receive their care at one of the four community HCs selected in each district that is closest to them. Participation for all participants is voluntary and informed consent or assent from a parent or guardian (for those >8 but <18 years) will be sought and signed before enrolment.
Participant eligibility
Inclusion criteria
Eligible participants for decentralisation will be all RHD registrants who live within 20 km of a participating HCs.
Exclusion criteria
Registrants will be ineligible for participation if they have severe RHD—shown to be associated with an increased risk of a vasovagal mediated sudden deterioration during or immediately after a BPG injection; caution has been issued on BPG use in this population.34 According to these recommendations, we will exclude patients with severe mitral stenosis, severe aortic regurgitation or stenosis, ventricular dysfunction (Measured ejection fraction (EF) <50%) or with advanced symptoms (New York Heat Association (NYHA) class III/IV)34 as ascertained by echocardiography performed within 6 months prior to decentralisation.
Registrants consenting to decentralisation will have the necessary information regarding their care at participating HCs given to them during their last visit at the regional hospital. Following this, registration of HC nurses to the ACT application will commence. Given the novelty of systematic RHD care delivery in clinics, the research team will be on-site (at the HCs) for the first week and frequently thereafter, according to a documented schedule in order to provide the necessary support during this period. This will be phased out slowly over 3–6 months. As one of the key implementation strategies, we purposefully planned to re-examine implementation activities—including patient flow at clinics and the use of ACT for patient management. Any challenges identified will be attended to through a feedback process between HCWs in HCs, the research team and study administrators.
Implementation outcomes
For the primary implementation outcome, BPG adherence will be measured as a proxy for post-implementation healthcare utilisation among registrants. The annualised proportion of persons who have ≥80% of days covered pre-decentralisation and post-decentralisation will be compared. At the individual level, adherence is calculated as the proportion of days covered over days prescribed BPG (table 3). Data will be obtained from the ACT application and RHD REDCap registry for baseline pre-decentralisation adherence data. Based on our hypothesis, we will be testing for non-inferiority of SAP adherence, postimplementation.
Table 3.
Key metrics collected during baseline data collection
| Primary metric | Operational definition | Collection method |
| BPG adherence | The proportion of persons who have 80% of days covered. Each registrants’ days of coverage will be calculated as: Days of coverage (%)=days with adequate BPG coverage/*/days prescribed BPG. |
National Registry and ACT application, based on dates of injections as compared with prescription |
| Secondary metrics | Operational definition | Collection method |
| Retention | Defined as being seen at least twice in a 12-month period for clinical review (outside or in conjunction with BPG delivery) | National Registry and ACT application |
| Composite adverse CV events | Combination of new or worsening heart failure, atrial fibrillation, infective endocarditis and/or recurrent ARF | National Registry and ACT application, supplemented as needed by patient interview |
| RHD mortality | Death of an RHD registrant that is determined to be the direct or indirect result of RHD | Multimodality, direct report from family or hospital/clinic if death was witnessed by medical staff |
| BPG stockouts | Number of days with no BPG or BPG-related supplies (needles, syringes, dilutant, lidocaine, etc) to be tracked individually, and number of days at <20% supply (based on anticipated number of RHD registrants assigned to that clinical location) | ONLY tracked during decentralised care, through both stock inventory by our research staff (monthly surveillance) and reports on the ACT application |
*Adequate BPG coverage defined as the prescribed interval between BPG injection (ie, 28, 21 or 14 days).
ACT, active community case management tool; BPG, benzathine penicillin G; CV, cardiovascular; RHD, rheumatic heart disease.
The taxonomy of implementation constructs proposed by Proctor et al 35 will be used to guide the data collection, levels of analysis and measurement of implementation outcomes, with particular emphasis on acceptability, adoption, penetration and implementation cost.
Preimplementation, formative research was planned and undertaken among stakeholders in the two districts to inform the design and logistical aspects of the project. At the start of the study, facility surveys will collect monthly data on clinic staff numbers and roles, availability of drugs (which will inform drug-stock outs) and consumables—with particular emphasis on RHD care-relevant supplies. We will evaluate the implementation outcomes of acceptability, adoption and penetration by conducting a concurrent mixed-methods evaluation of the ADD-RHD programme (table 4). Pre/post decentralisation patient and provider qualitative interviews using semistructured questionnaires have been planned within a month prior to decentralisation to get in-depths perspectives from potential participants, including foreseen barriers to programme roll-out. This was intended to be formative, and no formal framework will be used. However, six key areas of interest pertaining to decentralisation were used to develop a priori data extraction template. A matrix-based rapid qualitative analysis will be done and themes and subthemes generated will enable the incorporation of findings in real-time to optimise the decentralisation of RHD care. This was planned as part of implementation iteration (together with a staggered roll-out in Lira and then Gulu district), for quality improvement. Further, database queries, anecdotes, user inquiries and field diaries from support staff will be kept to inform determinants of implementation. In particular, we will collect data on patient use of HCs and return rates (if any) to the regional hospital that will inform acceptability (table 4). A substudy will evaluate costs, as an important implementation outcome. High out of pocket costs have been previously documented to be associated with the current centralised care.3 It is postulated that a decentralised model will result in reduction of out-of-pocket expenditures. To enable this evaluation, preplanned patient surveys, time and motion studies36 and facility cost data will be used in an embedded economic evaluation and reported separately from the main study. Furthermore, time and motion studies will be incorporated to evaluate any disruptions to care and potential distribution of valuable manpower resources which will be valuable for planning and scaling the intervention if it were successful.
Table 4.
The evaluation of implementation outcomes
| Definition (Proctor et al 2011)35 | Provider level | Patient level | Facility level | ACT application audits | Record audit at RRH visits | |
| Acceptability | Relates to perceptions on suitability and agreeability of the innovation and the satisfaction among stakeholders. Analysis will be at the patient and provider level. | Provider pre/post decentralisation interviews | Patient pre/post decentralisation interviews Coded under acceptability and health setting preferences |
Administrator interview | ACT usage audit/data queries. Documented ACT application changes. |
RRH injection record audits Self-decentralisation rates and rates of return to RRH post-decentralisation |
| Adoption | Defined as the intention to take up an innovation; also quoted as ‘uptake’ (Proctor et al, 2011, p.69).35 In this study adoption will be analysed at the provider level at the health facilities. | Provider pre/post decentralisation interviews Observation Time and Motion study |
N/A | Monthly Health facility survey—organisation and appropriateness of RHD medicinal and supplies order based on need. Direct observation. |
ACT usage audit
|
Examination of entered data on BPG card versus ACT application |
| Penetration | Refers to the absorption or incorporation of the practice into the service setting and looks at the integration of the innovation in question. The level of analysis is at the health facility level. | Provider pre/post decentralisation interviews. Attendance to RHD cases. Numbers of providers trained vs numbers delivering service. |
N/A | Monthly Health facility survey—Improvements in medicinal (BPG) and supply shortages Direct observation Regular attendance of patients with RHD |
Query-generation rates on ACT application Use of paper records for RHD care ACT usage by HC nurses—completion of information and other usage parameters overtime Rates of re-education for HC nurses on deficient areas identified |
Reduction in BPG visits to RRH services for decentralised registrants |
ACT, active community case management tool; BPG, benzathine penicillin G; HC, health centre; RHD, rheumatic heart disease; RRH, regional referral hospital.
Secondary clinical outcomes
Information on secondary clinical outcomes will be reported (table 3). We will assess the non-inferiority of the decentralised registry on rates of retention at 2 years postimplementation (table 3). Further, a composite of adverse cardiovascular events, including a combination of new or worsening heart failure, recurrent ARF, atrial fibrillation, infective endocarditis and mortality will be documented during decentralisation and records extracted for baseline period rates. In an exploratory analysis, using continuous measures of adherence, we will compare event rates among more adherent and less adherent participants to validate the purported dose–response relationship between SAP and ARF, clinical progression of RHD in this context.
Statistical considerations
The primary outcome of the study is to determine whether adherence after decentralisation is non-inferior to the baseline adherence recorded for national RHD registry. We propose a one-sided exact binomial test at alpha=0.025 of the null hypothesis that the proportion of adherent patients during the intervention period is less than baseline by more than 10%. Based on previous experience with this patient population, we expect approximately 150–200 persons with RHD will contribute data on adherence during the baseline and intervention periods in Lira and Gulu. We also approximate 75% of these patients will receive 80% of BPG injections. Based on these assumptions, a one-sided alpha of 0.025, taking a total of between 150–200 participants will provide between 72% and 86% power to reject the null hypothesis of inferiority when the baseline percent adherent is 75% and the NIM is −10%. Thus, we expect to be at least moderately powered to reject the null hypothesis of inferiority at the proposed non-inferiority margin.
Patient and public involvement
Within the current setup of RHD care in Uganda, patients attending the main hospital in both districts expressed the problems they faced with long distances travelled from residences (often in rural areas) to come for monthly RHD care at the main hospital. These patients’ experiences were incorporated into the design and informed the implementation strategies of this study. Furthermore, stakeholder and community engagement, local and district health administrators were consulted for input on the logistics of the study, including the use of ACT software to enhance decentralised RHD care to the rural and semiurban health facilities.
Results of the study will be made available to participants and the community through the health facilities and the main hospital in both districts where RHD follow-ups are done.
Ethics and dissemination
This study was approved by the Institutional Review Board at Cincinnati Children’s Hospital Medical Center (IRB 2021-0160) and Makerere University School of Medicine Research Ethics Committee (Mak-SOMREC-2021-61). Participation will be voluntary and informed consent or assent (>8 but <18) will be obtained and signed prior to participation.
At completion, study findings will be published in peer-reviewed journals and communicated to the public and key stakeholders.
The ADD-RHD study was initially approved on 4 March 2021. Decentralisation of study participants is currently being finalised in the second site and post-decentralisation data collection will follow for 12 months to December 2023. Data analysis is planned to start early 2024, with the full project due for completion in April 2024.
Discussion
Like many chronic diseases, the successful prevention of RHD entails optimum adherence to monthly BPG injections, the cornerstone of RHD control. Decentralisation of health services has long been advocated as a means to improve health service delivery and reach.37 Successful decentralisation of care to PHCs resulted in the widespread availability and accessibility of HIV treatments in similar settings, playing a key role in HIV programme successes.38 Disease registries have been previously advocated in the early 2000s by organisations such as World Health Federation and RHD Action,39 which outlined minimum standards and guidance for RHD registries. However, these efforts have seen variable country uptake, often characterised by centralised registries, fragile paper records and limitations in quality assurance and continuous monitoring. To date, no modern version of a decentralised national RHD registry currently exists in low-income and middle-income countries. The ACT application, one of the packages of implementation strategies, was designed to mitigate the static nature of registries and further aid health workers in managing RHD secondary prevention. If successful, this will modernise how we approach RHD secondary prevention in Uganda and other similar settings, where RHD is prevalent. Based on this, and through long-standing partnerships encompassing local, regional and district key stakeholder engagement, we established the feasibility and suitability of ADD-RHD.
ACT is a novel technology-enabled dynamic application that integrates features to empower health workers at all levels of care with supportive tools to track, monitor and better engage patients with RHD. In addition, the application will facilitate HC communication channels to responsible bodies, such as relevant persons in medical supplies and the ministry of health, an important aspect to ensure availability of medicines, supplies and quality improvement. Electronic medical records are yet to be incorporated widely in health facilities in Uganda, hence the tailored simplification for PHCs and initial support provided by the current research collaborative will be valuable and presents a potential for its absorption into future electronic medical records expansion plans.27 Ultimately, the study can be used as a model for chronic disease management by informing how we integrate these digital health systems to enhance patient care in similar settings where HCWs are not necessarily well-versed with computers or technology.
We acknowledge some limitations to this study. First, the study design did not use an external control group, and had a fixed sample size, reflecting the limited cases currently identified and established in the registry from previous screening efforts. The use of fairly robust and more comprehensive mixed-methods with the additional collection of more granular data was designed to mitigate some of these limitations. Another potential limitation is around the ACT application, requiring baseline user comfort with technology and smart phones, which was not the case with some community public health workers. The pilot training informed the development of a simplified application version tailored specifically to the roles of the HCWs.27 In addition, internet connectivity is often unstable is this setting, which informed the incorporation of an offline feature function that may enhance the functionality and uptake of the application. Lastly, several other system-level factors pose potential challenges, including long waiting times, staff shortages and drug availability that are generalisable country-wide, but which may impact implementation. Securing medication by use of external resources in the initial period limits the generalisability without modifications to the health system. However, it demonstrates the values of securing supplies to make improvements and signifies more work needs to be done in this area. Despite evidence regarding SAP, most governments have not developed nor scaled up RHD programmes, limiting access to healthcare available to patients. Ultimately, through the project, there is an opportunity to redesign and equip PHCs to overcome some of these barriers to healthcare and serve as a foundation for scaling up much needed RHD services to different parts of the country.
Supplementary Material
Acknowledgments
The authors would like to thank all the local and international stakeholders who contributed to the refinement of this protocol, including the patients, staff at the central, district and local health centres who volunteered their time and inputs to the logistical aspects of decentralisation planning. Sincere thanks to Nicholas Felicelli, Kristen Tillman, Riley Morrison and other members from the design team who contributed to the ACT application build.
Footnotes
Twitter: @JafesPulle, @emmyrov
Contributors: AZB, DAW, EO, CTL, CS, JR and KD initially conceptualised the different aspects of the study. AZB, DAW, KD, NWM, JP, JR, EO, NO, JWD, SRdL, RS, NF and JAb contributed to the study design and proposal. NWM, JP, JR, NF, SRdL, RS, JAt, JK, DN, LO, FO, HN, YS, KD and DAW contributed to the implementation aspects of the study. NWM, DAW, AZB and KD wrote the initial draft of the protocol paper. All authors reviewed, updated and approved the final protocol.
Funding: The ADD-RHD study was funded through the AHA SFRN, grant 20SFRN35380042. Redcap registry was supported by Clinical and Translational Science Award (CTSC) UL1TR002548.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Ghamari S, Abbasi‐Kangevari M, Saeedi Moghaddam S, et al. Rheumatic heart disease is a neglected disease relative to its burden worldwide: findings from global burden of disease. JAHA 2022;11. 10.1161/JAHA.122.025284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zühlke L, Karthikeyan G, Engel ME, et al. Clinical outcomes in 3343 children and adults with rheumatic heart disease from 14 low-and middle-income countries: two-year follow-up of the global rheumatic heart disease Registry (the REMEDY study). Circulation 2016;134:1456–66. 10.1161/CIRCULATIONAHA.116.024769 [DOI] [PubMed] [Google Scholar]
- 3. Opara CC, Du Y, Kawakatsu Y, et al. Household economic consequences of rheumatic heart disease in Uganda. Front Cardiovasc Med 2021;8:636280. 10.3389/fcvm.2021.636280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Watkins DA, Johnson CO, Colquhoun SM, et al. Global, regional, & National burden of rheumatic heart disease, 1990-2015. N Engl J Med 2017;377:713–22. 10.1056/NEJMoa1603693 [DOI] [PubMed] [Google Scholar]
- 5. Okello E, Kakande B, Sebatta E, et al. Socioeconomic and environmental risk factors among rheumatic heart disease patients in Uganda. PLoS One 2012;7:e43917. 10.1371/journal.pone.0043917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Steer AC. Historical aspects of rheumatic fever. J Paediatr Child Health 2015;51:21–7. 10.1111/jpc.12808 [DOI] [PubMed] [Google Scholar]
- 7. Australian Institute of Health and Welfare (AIHW) . Rheumatic heart disease and acute rheumatic fever in Australia: 1996-2012. Cardiovasc Dis Ser 2013;36:60. [Google Scholar]
- 8. Carapetis JR, Beaton A, Cunningham MW, et al. Acute rheumatic fever and rheumatic heart disease. Nat Rev Dis Primers 2016;2. 10.1038/nrdp.2015.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization . HIV/AIDS key facts. 2021. Available: https://www.who.int/news-room/fact-sheets/detail/hiv-aids
- 10. Seventy-first World Health Assembly . Rheumatic Fever and Rheumatic Heart Disease, Available: https://apps.who.int/gb/ebwha/pdf/WHA71/A71_R14-en.pdf
- 11. White A. WHO resolution on rheumatic heart disease. Eur Heart J 2018;39:4233. 10.1093/eurheartj/ehy764 [DOI] [PubMed] [Google Scholar]
- 12. Gerber MA, Baltimore RS, Eaton CB, et al. Prevention of rheumatic fever and diagnosis and treatment of acute Streptococcal Pharyngitis: A scientific statement from the American heart Association rheumatic fever,Endocarditis,and Kawasaki disease committee of the Council on cardiovascular disease I. Circulation 2009;119:1541–51. [DOI] [PubMed] [Google Scholar]
- 13. Rothenbühler M, O’Sullivan CJ, Stortecky S, et al. Active surveillance for rheumatic heart disease in Endemic regions: a systematic review and meta-analysis of prevalence among children and adolescents. Lancet Glob Health 2014;2:e717–26. 10.1016/S2214-109X(14)70310-9 Available: 10.1016/S2214-109X(14)70310-9 [DOI] [PubMed] [Google Scholar]
- 14. Manyemba J, Mayosi BM. Penicillin for secondary prevention of rheumatic fever. Cochrane Database Syst Rev 2002;2002:CD002227. 10.1002/14651858.CD002227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Dassel JL, de Klerk N, Carapetis JR, et al. How many doses make a difference? an analysis of secondary prevention of rheumatic fever and rheumatic heart disease. JAHA 2018;7:24. 10.1161/JAHA.118.010223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zimmerman M, Kitooleko S, Okello E, et al. Clinical outcomes of children with rheumatic heart disease. Heart 2022;108:633–8. 10.1136/heartjnl-2021-320356 Available: http://heart.bmj.com/content/108/8/633.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Edwards JG, Barry M, Essam D, et al. Health system factors serving as Facilitators and barriers to rheumatic heart disease care in Sudan. Glob Health Res Policy 2021;6. 10.1186/s41256-021-00222-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kevat PM, Reeves BM, Ruben AR, et al. Adherence to secondary prophylaxis for acute rheumatic fever and rheumatic heart disease: A systematic review. Curr Cardiol Rev 2017;13:155–66. 10.2174/1573403X13666170116120828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huck DM, Nalubwama H, Longenecker CT, et al. n.d. A qualitative examination of secondary prophylaxis in rheumatic heart disease: factors influencing adherence to secondary prophylaxis in Uganda. Gh;10:63. 10.1016/j.gheart.2014.10.001 Available: 10.1016/j.gheart.2014.10.001 [DOI] [PubMed] [Google Scholar]
- 20. Longenecker CT, Morris SR, Aliku TO, et al. Rheumatic heart disease treatment Cascade in Uganda. Circ: Cardiovascular Quality and Outcomes 2017;10:11. 10.1161/CIRCOUTCOMES.117.004037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Okello E, Longenecker CT, Scheel A, et al. Impact of Regionalisation of a national rheumatic heart disease Registry: the Ugandan experience. Heart Asia 2018;10:e010981. 10.1136/heartasia-2017-010981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Remenyi B, Carapetis J, Wyber R, et al. Position statement of the world heart Federation on the prevention and control of rheumatic heart disease. Nat Rev Cardiol 2013;10:284–92. 10.1038/nrcardio.2013.34 [DOI] [PubMed] [Google Scholar]
- 23. Karthikeyan G, Zühlke L, Engel M, et al. Rationale and design of a global rheumatic heart disease Registry: the REMEDY study. Am Heart J 2012;163:535–40. 10.1016/j.ahj.2012.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rémond MGW, Coyle ME, Mills JE, et al. Approaches to improving adherence to secondary prophylaxis for rheumatic fever and rheumatic heart disease: A literature review with a global perspective. Cardiol Rev 2016;24:94–8. 10.1097/CRD.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 25. Zühlke LJ, Watkins DA, Perkins S, et al. A comprehensive needs assessment tool for planning RHD control programs in limited resource settings. Glob Heart 2017;12:25–31. 10.1016/j.gheart.2016.10.028 [DOI] [PubMed] [Google Scholar]
- 26. Kredo T, Ford N, Adeniyi FB, et al. Decentralising HIV treatment in Lower- and middle-income countries. Cochrane Database Syst Rev 2013;2013:CD009987. 10.1002/14651858.CD009987.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Loizaga Sarah R, Jafes P, et al. Development and pilot of the active community case management tool (ACT platform): A dynamic tool for rheumatic heart disease case management in Uganda; submitted manuscript ID ACI-2022-12-RA-0339.R1. Appl Clin Inform 2023. [Google Scholar]
- 28. Curran GM, Bauer M, Mittman B, et al. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care 2012;50:217–26. 10.1097/MLR.0b013e3182408812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (Redcap)-A Metadata-driven methodology and Workflow process for providing Translational research Informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harris PA, Taylor R, Minor BL, et al. The Redcap consortium: building an international community of software platform partners. J Biomed Inform 2019;95:S1532-0464(19)30126-1. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Watkins DA, Beaton AZ, Carapetis JR, et al. Rheumatic heart disease worldwide. J AmCollege Cardiol 2018;72:1397–416. 10.1016/j.jacc.2018.06.063 [DOI] [PubMed] [Google Scholar]
- 32. Tompkins DG, Boxerbaum B, Liebman J. Long-term prognosis of rheumatic fever patients receiving regular Intramuscular Benzathine penicillin. Circulation 1972;45:543–51. 10.1161/01.cir.45.3.543 [DOI] [PubMed] [Google Scholar]
- 33. Ndagire E, Kawakatsu Y, Nalubwama H, et al. Examining the Ugandan health system’s readiness to deliver rheumatic heart disease-related services. PLoS Negl Trop Dis 2021;15:e0009164. 10.1371/journal.pntd.0009164 Available: 10.1371/journal.pntd.0009164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sanyahumbi A, Ali S, Benjamin IJ, et al. Penicillin reactions in patients with severe rheumatic heart disease: A Presidential advisory from the American heart Association. J Am Heart Assoc 2022;11:e024517. 10.1161/JAHA.121.024517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health 2011;38:65–76. 10.1007/s10488-010-0319-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gold HT, McDermott C, Hoomans T, et al. Cost data in implementation science: categories and approaches to costing. Implement Sci 2022;17:11. 10.1186/s13012-021-01172-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bossert T. Analyzing the decentralization of health systems in developing countries: decision space, innovation and performance. Soc Sci Med 1998;47:1513–27. 10.1016/s0277-9536(98)00234-2 [DOI] [PubMed] [Google Scholar]
- 38. Geng EH, Nash D, Kambugu A, et al. Retention in care among hiv-infected patients in resource-limited settings: emerging insights and new directions. Curr HIV/AIDS Rep 2010;7:234–44. 10.1007/s11904-010-0061-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. RHD Action . RHD Registers, Available: https://rhdaction.org/control/rhd-registers
- 40. Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the expert recommendations for implementing change (ERIC) project. Implement Sci 2015;10:21. 10.1186/s13012-015-0209-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.