Abstract
Objectives
Juvenile idiopathic arthritis (JIA) is a common chronic childhood disease and chronic pain is a debilitating feature. A strong link has been shown between poor sleep and pain in JIA. However, the causal direction is unknown. This study’s aim was to determine if, in adolescents with JIA, a recommended healthful sleep duration leads to reductions in pain when compared with the restricted sleep (RS) duration that is commonly seen.
Methods
Patients with JIA (12–18 years old; pain score of ≥1 on a visual analogue scale) participated in a randomised, crossover sleep manipulation protocol. The 3-week protocol comprised a baseline week (BL), a week with healthy sleep duration (HSD; 9.5 hours in bed/night) and a RS week (RS; 6.5 hours in bed/night). After BL, participants were randomly assigned to either HSD or RS, and then crossed over to the other condition. Pain was self-assessed using the iCanCope with Pain app. We used Bayesian hierarchical models to estimate the effect of sleep duration on pain.
Results
Participants (n=31; mean age=15.0±1.8 years) averaged 1.4 (95% credible interval (CrI) 1.2–1.6) more hours of sleep per night during HSD relative to RS. Compared with RS, HSD resulted in a favourable effect on pain scores (OR 0.61, 95% CrI 0.39–0.95).
Conclusion
It is possible to have adolescents with childhood arthritis get a healthier sleep duration, and this longer sleep results in reduced pain. These findings complement prior correlational studies and confirm a causal relationship between reduced sleep duration and increased pain.
Trial registration number
Keywords: arthritis, juvenile; patient reported outcome measures; arthritis
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Children with arthritis face debilitating chronic pain, despite modern biologic treatments. Many adolescents have poor sleep practices, and their inadequate sleep is associated with negative outcomes, including pain. Poor quantity of sleep, combined with childhood arthritis, may lead to even worse health outcomes. Studies have shown a strong link between sleep duration and pain in children with arthritis, but the direction of the causal relationship is not clear.
WHAT THIS STUDY ADDS
Adolescents with arthritis can increase their sleep duration and, with this increased sleep, their pain is reduced. This provides causal evidence that short sleep, in children with arthritis, contributes to worse pain.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Findings support interventions that improve the sleep of adolescents with chronic arthritis.
Childhood arthritis (classified worldwide as juvenile idiopathic arthritis (JIA)1 is the most common paediatric rheumatic disease. It affects 1 to 4 in 1000 children,2 making it one of the most common chronic diseases of childhood.
JIA is associated with high morbidity. For example, Swedish children with arthritis have significantly more physical limitations and reduced independence compared with other European children.3 Sixty per cent of Dutch children with JIA suffer from fatigue and low energy levels and over 25% have a very high degree of limitation.4 These limitations continue into adulthood with the greatest impacts in the areas of pain, anxiety/depression, activity limitation and mobility limitation.5 JIA is a cause of functional limitation, sometimes severe, and pain; it rarely remits and most affected children will suffer into adulthood or old age.6
Although modern biologic (anticytokine) treatments reduce the complications of JIA, pain continues to be a highly prevalent morbidity.7 8 Among North American paediatric rheumatologists, 77% report that children with JIA have significant pain despite aggressive therapy.9 Pain is the most frequent and distressing symptom of JIA,10 fluctuating within and between days.7 8 11 As compared with healthy children, Icelandic children with JIA report more pain, painful body areas, pain intensity, pain interference and pain behaviours.12 In those with polyarticular JIA, pain is reported on an average of 73% of days, with most participants reporting pain on more than 60% of days.7 A significant proportion of patients (39%) report the experience of pain on all days, while only a small minority (5%) reported no pain. This pain is associated with impairment of physical, emotional, social and role functioning as well as sleep disturbance and fatigue.13 14 Overall, arthritis-related pain intensity is typically reported in the mild to moderate range.7 Critically, even a small reduction in pain is associated with improved health-related quality of life (HRQoL).15
Adolescents, in general, frequently have poor sleep practices16; their poor sleep has been associated with negative health outcomes, including pain.17–19 Furthermore, there is a strong relationship between sleep duration (and/or sleep quality) and pain in JIA.14 20–22 For example, in a survey of a random sample of 115 Canadian children with JIA, greater than 40% reported moderately severe fatigue and disturbed sleep (quality and/or quantity); HRQoL was reduced and 66% reported ongoing pain.14 Most importantly, both pain and reduced HRQoL were highly correlated with disturbed sleep. However, given the correlational nature of the study designs to date, the causal direction of the relationship is not clear. That is, it remains unclear if poor sleep leads to greater pain, or if pain in childhood arthritis leads to poor sleep, or both.
If poor sleep is a cause of worsened pain in JIA, an intervention that improves sleep would be expected to lead to a reduction in pain. Therefore, we studied the effects of a proven sleep intervention as a method to reduce pain in adolescents with JIA. We sought to determine whether, in adolescents with childhood arthritis, a healthful sleep duration—based on current recommendations—leads to clinically meaningful reductions in self-reported pain when compared with mild sleep restriction similar to what many adolescents experience on school nights. Our secondary objective was to determine whether, in adolescents with childhood arthritis, healthful sleep duration leads to clinically meaningful improvements in disease activity and HRQoL.
Methods
We conducted a randomised crossover trial, analysed using a Bayesian framework. We used the Consolidated Standards of Reporting Trials reporting guidelines in this manuscript.23 The Hospital for Sick Children Research Ethics Board reviewed the study, and all participants provided written informed consent. This project included a parent representative (author SP) at the funding application phase, during which feedback and guidance on the proposal were provided.
Participants
Patients meeting inclusion criteria were consecutively approached in the rheumatology clinic at The Hospital for Sick Children in Toronto, Canada. The clinic—Canada’s largest of its kind—sees tertiary and quaternary referral patients. The inclusion criteria were: (1) a diagnosis of any subtype of JIA, as per the International League of Associations for Rheumatology criteria,1 (2) between 12 and 18 years of age, (3) capable of providing informed consent, as judged by the clinical team and (4) having a baseline pain score of ≥1 cm on a 10 cm visual analogue scale. This pain score was selected, as it represents at least some level of pain8 24 and previous work has shown that, on average, many patients with arthritis experience daily pain at this level.7 The age range was established for several reasons: all the questionnaires and tools used are valid for this age group, adolescents have particularly high base rates of inadequate sleep on school nights, and the sleep manipulation protocol has been shown to be feasible in this age group.18 25–27
Patients were excluded if they met at least one of the following criteria: (1) a known sleep disorder (eg, obstructive sleep apnoea), (2) a high probability of undiagnosed obstructive sleep apnoea as determined by the sleep-disordered breathing subscale of the parent-report Paediatric Sleep Questionnaire (PSQ),28 (3) taking medication with the intent to impact sleep (eg, zolpidem, benzodiazepines; taking melatonin was not an exclusion but was noted), (4) taking corticosteroids (which may adversely affect sleep), (5) have obligations that require a bed time later than 22:00 or a wake time earlier than 5:30 during the study period, (6) daily consumption of >1 coffee or ‘energy drink’ and/or >3 caffeinated carbonated beverages or (7) do not speak/understand English with enough proficiency to complete all study-related tasks, as judged by the clinical team. To minimise the effects of caffeine use while maintaining a representative sample, we allowed for mild use but excluded youth who consumed excessive caffeine.
Procedures
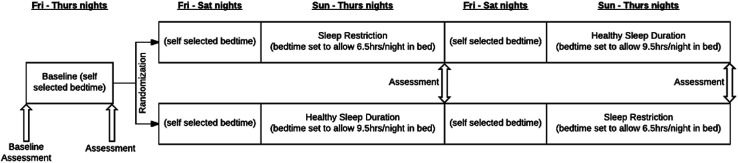
The sleep manipulation protocol used in this study has been successfully implemented for other chronic conditions.18 25 27 It consists of three sleep conditions, each lasting 1 week total (see figure 1). This study had both an in-person and virtual option; the virtual option became available after the onset of the COVID-19 pandemic. The virtual option had one additional week, a prebaseline week, before the baseline week, to allow a longer lead time into the study since the pandemic-related lock downs often resulted in unusual sleeping patterns.29 For the virtual option, participants were mailed a package of study materials prior to the scheduled prebaseline visit. Therefore, for each subject, there were four study visits over a 3-week period for the in-person participants (prior to COVID-19 pandemic) or five study visits over a 4-week period for the virtual option (COVID-19 study protocol). Study visits occurred on consecutive Fridays. All virtual visits were conducted using Zoom Healthcare (Zoom Video Communications, San Jose).
Figure 1.
Study protocol and duration of follow-up.
At the start of the study, participants identified a wake time that then remained constant throughout all parts of the study. The wake time was not allowed to be earlier than 5:30 or later than 9:30, and was generally guided by the time they awoke on school days. The Baseline week was a sleep stabilisation condition to establish baseline sleeping patterns. During this week, participants were allowed to set their own bedtime, while keeping the identified wake time. During the Pre-Baseline week of the virtual option, participants set their own bedtime, meaning those participants had a total of 2 weeks with self-selected bedtime and set wake-up time.
Following successful completion of the Baseline week (ie, the trial coordinator determined that participants adhered to the chosen constant wake up time within±30 min), participants were randomised in a 1:1 ratio to one of two sequences of experimental sleep conditions: a healthful sleep duration condition (healthy sleep duration; HSD) followed by a restricted sleep (RS) condition, or the reverse sequence (RS then HSD). Randomisation was based on an online random number generator (sealedenvelope.com) using permuted blocks of size 6 or 8.
During HSD, bedtimes were set to allow participants to be in bed for 9.5 hours each night, which allows for the 8–10 hours of nightly sleep that is recommended for adolescents.30 During RS, bedtimes were set to allow participants to be in bed for 6.5 hours each night, a mild but chronic level of sleep restriction as is commonly experienced on school nights. Bedtimes were calculated individually for each participant based on their preselected wake up time. Following completion of the first experimental week, the participants crossed over to the other experimental condition.
During each experimental week, the first two nights served as a washout period between conditions—during which participants were again allowed to self-select their bedtimes—and the experimental condition (HSD or RS) followed for five nights. Previous research has shown that two nights are sufficient to normalise neurobehavioural function, daytime sleepiness and other psychological outcomes in similar protocols.31 32
Due to the nature of the intervention, it was not feasible for participants to be blinded. To minimise measurement bias, all assessors and data analysts were blind to participant allocation. For trial management and implementation, the trial coordinators were not blinded to the participants’ allocation. The trial coordinators were required to explain the details of the sleep conditions to the participants and to determine appropriate bedtimes based on the allocated sleep condition and individual wake times.
Measures
Sleep monitoring (adherence)
Sleep was measured in several ways. First, an objective measure of sleep was obtained using the ActiGraph GT9X Link watch (ActiGraph Corp, Pensacola), which was worn on the non-dominant wrist 24 hours per day except while bathing for the duration of the trial. Actigraphic sleep outcomes, measured by the watch, included sleep onset time, sleep offset time, wake after sleep onset and total sleep time—time spent during awakenings was subtracted from time between sleep onset and sleep offset.17 25 We chose to use total time asleep, not including awakenings, for our analysis. Subjective sleep information was also collected using a self-report diary for participants to record their sleep and wake time, similar to previous studies.25 To obtain detailed habitual sleep pattern information, we used the Inattention and Sleepiness Behaviour Rating Form. This questionnaire has performed well in previous studies and has shown to be responsive to change in sleep conditions.26
Primary outcome—pain level
In a Bayesian trial, there is no statistical requirement for a primary outcome, as there is no concern about type I (alpha) error as there is in a frequentist analysis.33 However, our primary interest was in the effect of HSD versus RS on pain level in JIA. As such, the primary outcome measure was pain level as measured on a 10-point (0–9) scale on the iCanCope with Pain mobile application, a comprehensive pain self-management platform. Only the daily diary feature of the app was used. Participants were instructed to log their pain symptoms at least once, and up to three times daily throughout the trial. The app includes a diary component that tracks pain (location, character, triggers, intensity), sleep, mood, physical activity, pain interference and energy. The app is a user-friendly way for adolescents to log their daily pain symptoms and has check-in reminders embedded within.34
Before beginning data analysis, we made the decision to use pain level from the iCanCope app as the primary outcome on the grounds that it is completed frequently and is more reflective of pain at the time, rather than pain summarised in retrospect over the past week.
Secondary outcomes—pain interference and pain behaviour
Participants completed questionnaires at each study visit. Pain interference and pain behaviour were measured with the Patient-Reported Outcomes Measurement Information System (PROMIS) Pain Interference (PI)35 and Pain Behaviour (PB)36 scales. The PROMIS-PI is a well-validated tool that measures the level of interference that pain may cause in a patient’s everyday life. We used a modified version of the PROMIS-PI questionnaire, removing the question about the impacts of pain on sleep to eliminate the chance of scores changing due to the sleep condition in any particular week. Like the PROMIS-PI, the PROMIS-PB is well validated. The PROMIS-PB looks at the construct of pain behaviour, or what actions a patient may engage in that communicate to others that they are experiencing pain.36
Additional secondary outcomes—disease activity and health-related quality of life
Disease activity was measured at each study visit using a modified JIA Core Set and the clinical Juvenile Arthritis Disease Activity Score (cJADAS). The JIA Core Set includes physician global assessment of disease activity, parent/patient assessment of overall well-being, functional ability, number of joints with active arthritis, number of joints with limited range of motion and erythrocyte sedimentation rate (ESR).37 The cJADAS has been shown to be highly correlated with the validated JADAS score.38 The cJADAS comprises the physician assessment of disease activity, the patient/parent assessment of general well-being and the number of joints with active arthritis. At each of the four in-person study visits, a physical joint examination was performed by a physician or advanced practice physiotherapist who specialises in JIA. For virtual study visits, a virtual paediatric gait, arms, legs and spine (pGALS)39 assessment was done by an advanced practice physiotherapist. We also collected functional status information (via the Childhood Health Assessment Questionnaire) and the number of joints with restricted range of motion, for completeness and in keeping with international recommendations.37 Hence, the modified JIA Core Set included five of six measures, without ESR, to increase clinical feasibility.
HRQoL was measured with the Quality of My Life (QoML) questionnaire. The QoML is a validated instrument to measure overall quality of life and HRQoL as separate constructs, and provides complementary information beyond traditional measures of HRQoL.40
Statistical analysis
The primary analyses used Bayesian hierarchical models to estimate the effect of the study intervention on the iCanCope pain score. This score was treated as a 10-point ordinal variable in a proportional odds model with fixed effects for period (1 or 2), intervention (HSD or RS) and (in some models), carryover and accounting for repeated assessment of scores through random effects for participant and day within participant. In this model, the treatment effect captures how the odds of a higher pain score are affected by the intervention; the assumption is that whatever dichotomising threshold is used for ‘higher pain score’ (eg, >1 vs ≤1, >5 vs ≤5), the OR for treatment remains the same. As there can be uncertainty about the best model on which to base estimation in a crossover study, we fit models with and without a carryover effect, then chose the model with the best measure of fit (assessed by the deviance information criterion) to make our primary inference for the treatment effect. The main analyses used diffuse normal priors for period and intervention effects and the carryover parameter and diffuse half-t distributions on all random effects. Sensitivity analyses examined the robustness of the findings to archetypal priors,41 representing enthusiasm and scepticism about the intervention effect. Continuous outcomes (eg, sleep duration) were analysed using similar Bayesian models, but with linear regression replacing the proportional odds model. The estimated posterior mean treatment effects are presented with 95% credible intervals (CrI) and posterior probabilities of any benefit and benefit exceeding a minimum important difference, where this value is available. Analyses of the trial data used R V.4.2.142 and Stan.43 44
Sample size determination
Although, strictly speaking, Bayesian analyses do not require sample size specification,41 45 we wanted to make sure we had adequate precision. We based our sample size on the PROMIS pain interference score as the necessary published values were available (but not available at that time for iCanCope). Using the published values of 6 for the minimally important difference on the PROMIS pain interference score35 (although some studies report an MCID as low as 1) and 10 for its cross-sectional SD, we examined the performance of several sample sizes under varying assumptions about the amount of attrition (10%, 15%, 20%) and the within-person correlation of repeated measurement of PROMIS pain interference (0.8, 0.6)46; we simulated 1000 bivariate normal datasets, for each total sample size between 20 to 30 participants, with a period effect and a specified correlation between period 1 and period 2. For each dataset, we fitted our Bayesian no-carryover, random-participants model in the Just Another Gibbs Sampler (JAGS)47 statistical language, and computed the lower end of the 97.5% CrI. Finally, we calculated the “Bayesian power” as the proportion of the 1000 intervals that lay above and excluded the value zero; this is analogous to calculating power for a one-sided frequentist hypothesis test of no effect. For a sample size of 30 participants, a correlation of 0.6 between measurements in the two periods, and 20% dropout, Bayesian power was 85%. With a correlation of 0.8 and 20% dropout, this sample size gave power>98%.
Results
Eighty-six potential participants were approached in clinic and 35 (41%) consented to participate between July 2019 and January 2021. Of the 35 consented participants, 4 (11%) screened positive (score>0.33) on the sleep-disordered breathing subscale of the PSQ and did not start the trial. Of the 31 participants who started the study, 30 completed the study in its entirety. Fourteen participants completed the study with in-person study visits and 16 completed the study with virtual study visits and a Pre-Baseline week; one participant who had virtual study visits did not complete the second experimental week. Table 1 describes the demographic and clinical characteristics of the study sample.
Table 1.
Demographic and clinical characteristics of the study sample
| Mean (SD) or count (%) | |
| Age (years) | 15.0 (1.8) |
| Age at diagnosis (years) | 7.3 (5.2) |
| Female sex | 24 (77.4) |
| BMI (kg/m2) | 23.0 (6.3) |
| JIA type | |
| Oligoarticular | 5 (16.1) |
| Polyarticular RF negative | 13 (41.9) |
| Polyarticular RF positive | 3 (9.7) |
| Enthesitis-related arthritis | 4 (12.9) |
| Psoriatic arthritis | 0 (0.0) |
| Systemic arthritis | 1 (3.2) |
| Other | 5 (16.1) |
| Medications for JIA | |
| Biologic | 16 (51.6) |
| Methotrexate | 8 (25.8) |
| NSAID | 8 (25.8) |
| Leflunomide | 6 (19.4) |
| Sulfasalazine | 1 (3.2) |
| Prednisone | 0 (0.0) |
| None | 7 (22.6) |
| Taking melatonin | 2 (6.5) |
BMI, body mass index; JIA, juvenile idiopathic arthritis; NSAID, non-steroidal antiinflammatory drugs; RF, rheumatoid factor.
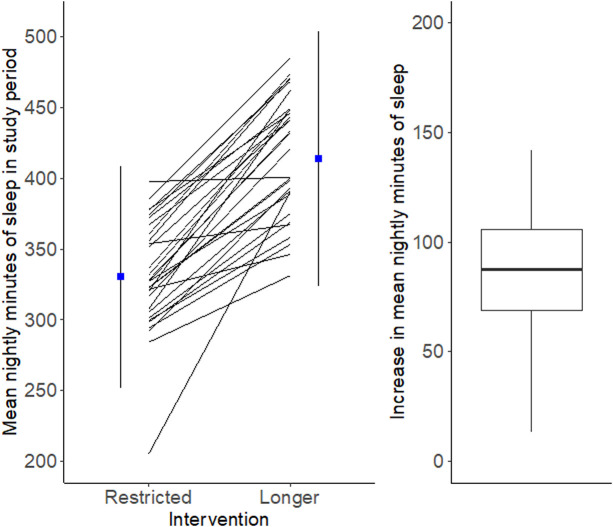
Participants averaged 1.4 (95% CrI 1.2–1.6) more hours of total sleep time per night (ie, time spent during awakenings was subtracted from time between sleep onset to sleep offset) during the healthful sleep condition relative to the RS condition (see figure 2). The mean difference between total time in bed and total time asleep was 69.4 min with an SD of 23.9 min, and there was a strong linear relationship between the two (Pearson r=0.90, p<0.0001).
Figure 2.
Mean nightly minutes of sleep per patient in each sleep condition (left). Mean increase in nightly minutes of sleep per patient (right).
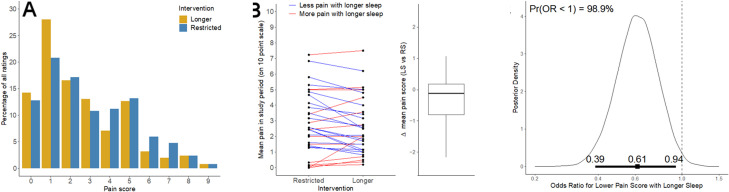
The distribution of all pain scores shifted towards higher values during the RS week and towards lower values during the HSD week (figure 3A). Daily pain, as reported using the iCanCope app, decreased in the HSD week compared with the RS week with a probability of 98.9% (figure 3C). Participants had a 40% reduction (OR 0.61, 95% CrI 0.39–0.95) in the odds of reporting higher pain scores in the HSD condition. However, the difference was small for several participants (see figure 3B).
Figure 3.
(A) Observed distribution of all pain ratings in both sleep conditions. (B) Mean pain level for each study participant during each sleep condition and change in mean pain score with healthful sleep duration. (C) Posterior (probability) density of the OR for the effect of HSD versus RS on the pain level on the iCanCope with Pain app. The vertical axis is proportional to probability; the horizontal axis is the OR for a lower pain score during the HSD when compared with RS. The plot shows that most evidence lies on values of the OR less than 1 (98.9% probability, visible as the area under the curve to the left of 1), indicating lower values of pain during the HSD week than in the RS week. The horizontal heavy bar displays the estimated mean OR and 95% CrI. CrI, credible interval; HSD, healthy sleep duration; RS, restricted sleep.
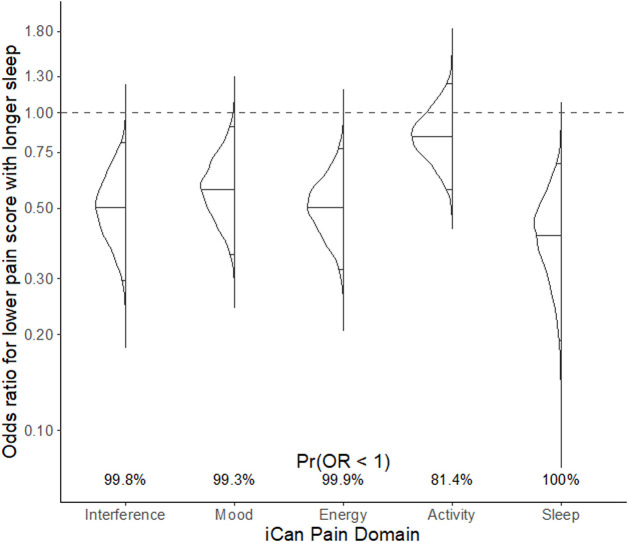
Responses to all other questions on the iCanCope app (ie, related to pain interference, mood, energy, physical activity and sleep) showed significant improvement during the HSD week relative to the RS week. Figure 4 displays the posterior density plots for each outcome measure, including the ORs and probability of the OR being less than 1.
Figure 4.
Posterior density plots (rotated 90 degrees) for outcome measures from the iCanCope with Pain app, including pain interference, mood, energy, activity and sleep. Please see figure 3C for a description of posterior density plots. Here, the solid horizontal lines inside each density show the mean OR and 95% CrI. CrI, credible interval.
The two PROMIS measures—PI and PB—had mixed results. Pain interference significantly improved during the HSD week (average improvement ~1, probability of improvement=87%), whereas pain behaviour appeared somewhat worse during the HSD week compared with the RS week. Online supplemental appendix figure 1 displays the posterior density plots for effects on each outcome measure, along with mean differences in PROMIS scores between RS and HSD periods and probabilities of the treatment being beneficial (ie, having a difference less than 0).
rmdopen-2023-003352supp001.pdf (63.7KB, pdf)
The components of the cJADAS, and the QoL and HRQoL measures all showed improved scores during the HSD week, meaning that these measures of disease activity and life quality improved with more sleep (but, to a small degree). Online supplemental appendix figure 2 displays the posterior density plots for the clinical measures, patient-report measures and HRQoL measures.
Discussion
To our knowledge, this is the first study in adolescents with JIA to use a sleep manipulation protocol to determine the effect on pain. This study found that pain improves with longer sleep for adolescents with JIA who report at least some daily pain. Improvements were found after an average increase of 84 min of sleep per night in our primary outcome, pain level, pain interference and also disease activity and HRQoL. Therefore, it can be concluded that a healthful duration of sleep is causally associated with (ie, leads to) reduced pain in adolescents with JIA when compared with the mild chronic sleep restriction experienced by many adolescents on school nights; improving sleep duration is an important part of ancillary care for children with arthritis.
It is interesting to speculate why adolescents with JIA reported worse pain behaviours when they slept more. This is especially puzzling since the iCanCope measures of pain interference, mood, energy and activity (putative mediators of pain behaviours) all improved significantly and with high probability in the longer sleep condition. It is possible that the duration of the intervention periods was too short to observe behaviour changes. In a recently published study of over 300 adults (median age 39 years) undergoing sports-related knee or shoulder surgery, changes in sleep disturbance (presumably from the surgery) predicted PROMIS pain behaviour changes 6 weeks post-surgery.48 Perhaps our washout period did not correctly address the lag time between sleep disturbance and pain behaviour. Also, the PROMIS questionnaire asks about pain behaviours ‘when I was in pain’. As such, it may be that while pain level improves with a HSD, there is no change in behaviour when pain actually occurs.
Many papers report the total time in bed, but we reported total time asleep. There was a tight correlation to the two times in our subjects, and one could just add 69.4 min to our results to see the total time in bed.
Adequate quality and quantity of sleep is now recognised as one of the most important contributors to health.49 Conversely, both poor quality sleep and reduced sleep time are associated with many impairments in health and well-being. For example, there is increasing awareness of the link between chronic sleep deprivation and its association with impairments in affective states, including increased anxiety, depression and mood lability, poorer cognitive function and worse functional impairment scores.16 50 51 Sleep deprivation is also associated with impaired HRQoL.50 Here, we show the importance of healthful sleep duration in JIA as well.
The mechanism by which longer sleep duration reduces pain, in general, has not been fully worked out. While the association seems sure and, when studied, poor sleep is temporally more predictive of pain than the other way around, the mechanisms have not been fully established.52 Several studies, mostly in healthy adults, have examined psychological states (eg, negative affect, depressive and/or anxiety symptoms, increase pain attention, pain helplessness) as potential mediators of the relationship of sleep duration and pain. Whibley et al, did a systematic review of these mediation studies and concluded that they, while suggestive, could not be considered conclusive because of limitations in the methods (eg, most studies have been cross-sectional.53 More recently, Krause et al, examined 25 healthy adults with a balanced, repeated measures experimental design using functional MRI (fMRI). In this study, the participants were subject to 1 night of sleep deprivation and had pain thresholds (using a standard thermal test) determined while in the fMRI scanner. The same subjects were tested in the same way after a full night’s sleep. Following the sleep deprivation condition, pain thresholds were relatively reduced (ie, pain levels were increased to thermal stimulation). On imaging, this was associated with increased pain reactivity in the contralateral primary somatosensory cortex and reduced activity in the thalamus and nucleus accumbens (consistent with a change in the decision threshold as to what constitutes a painful stimuli). Higher order cortical evaluation of pain was reduced in the insula and cingulate during the sleep deprivation condition, suggesting disinhibition of pain.54 While we did not investigate brain mechanisms in our clinically diagnosed youth, we speculate that healthy sleep restores normal inhibitory pain responses in JIA, while reduced sleep leads to disinhibited pain sensations.
Pain is difficult to measure,55 and we used a state-of-the-art, real-time method.34 Many investigators treat numerical and visual analogue scale measures as though they are digital and have ratio, or at least interval, properties, which is often not true56; for example, small changes near the ends of these scales carry more importance than similar changes in the middle of the scales. Treating ordinal measures as though they have interval properties may have some empirical support when tolerant statistical testing methods are used57; however, we chose to be true to the nature of the measurements and analysed our results as ordinal,58 and using a Bayesian framework. In this way our results may be more conservative but are more robust.
Whereas our results demonstrate a very highly likely improvement in pain with a HSD, the improvement noted on the PROMIS pain interference measure is rather small, and less than the minimal important difference reported in most studies. One might expect that a greater increase in the actual sleep experienced by participants (during the longer sleep duration week) and a longer duration of healthy sleep, would lead to larger improvements in pain interference, but this remains unproven. Interestingly, the improvement in pain interference as measured by the iCanCope showed a larger improvement; this may reflect the improved measurement properties of repeatedly measuring in real time (ecologic momentary assessment).59
A single systematic review has investigated sleep quality in JIA, however, of the 10 studies included, there were no intervention trials that specifically targeted improved sleep with the aim of reducing pain.21 This review reported that several sleep abnormalities are seen when children with JIA are compared with healthy children—shorter phases of restorative sleep, more periodic leg movements and more arousals. Children with JIA also have increased periods of alpha wave intrusion during non-REM sleep. The review concluded that compared with healthy controls, children with arthritis experience increased sleep disturbances, and that these sleep disturbances are often associated with pain.21 However, the quality of all the studies reviewed was suboptimal; importantly, no experimental study had been done to establish the direction of causality between pain and disturbed sleep. Our study adds to this literature and suggests a causal link between reduced sleep duration and increase pain; sleep interventions that target healthful sleep duration should, based on our findings, lead to reduced pain in our patients.
Our study findings should be interpreted in light of some possible limitations. First, this study only focused on duration of sleep and did not examine other aspects of sleep which may also impact pain, such as wake periods (arousals) after sleep onset. Second, we had continuous recruitment over the course of 1.5 years and did not differentiate between periods when school was in session or not. It is not known whether this population experiences a difference in pain levels between times when school is in session (winter months) versus not in session (summer months). Finally, we were able to continue enrolling participants into the study after the onset of the COVID-19 pandemic by completing all study procedures virtually; however, it is known that lockdowns due to the pandemic had a significant effect on levels of physical activity experienced by children and adolescents,60 which may have in-turn contributed to pain levels. Future research may consider examining how sleep manipulation protocols may impact adolescents with varying levels of physical activity.
Conclusion
This study has shown a positive causal effect of healthful sleep duration on reduced pain scores. Although less striking, we have also shown improvements of healthful sleep on measures of disease activity and HRQoL in adolescents with childhood arthritis. This study provides strong evidence supporting the practice of sleep hygiene counselling for adolescents with chronic arthritis with a goal of achieving a healthful sleep duration.
Footnotes
Contributors: BMF was the study’s principal investigator and guarantor. HC and SD collected the data for the study and drafted the initial manuscript. GT and BMF performed the data analysis for the study. DB, BC, RML, DL, IN, SP, RS, LS, SS, JS, ST, SW and KW were involved in study design, reviewed the manuscript and approved the final submitted manuscript.
Funding: This study was funded by a CIHR Project Grant (PJT-156121).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data are available upon reasonable request to the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by The Hospital for Sick Children Research Ethics Board (REB#1000061386). Participants gave informed consent to participate in the study before taking part.
References
- 1.Petty RE. International League of associations for rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton. J Rheumatol 2001;31:390–2. [PubMed] [Google Scholar]
- 2.Manners PJ, Bower C. Worldwide prevalence of juvenile arthritis why does it vary so much. J Rheumatol 2002;29:1520–30. [PubMed] [Google Scholar]
- 3.Lundberg V, Eriksson C. Health-Related quality of life among Swedish children with juvenile idiopathic arthritis: parent–child discrepancies, gender differences and comparison with a European cohort. Pediatr Rheumatol 2017;15:26. 10.1186/s12969-017-0153-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armbrust W, Lelieveld OHTM, Tuinstra J, et al. Fatigue in patients with juvenile idiopathic arthritis: relationship to perceived health, physical health, self-efficacy, and participation. Pediatr Rheumatol 2016;14:65. 10.1186/s12969-016-0125-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barth S, Haas J-P, Schlichtiger J, et al. Long-term health-related quality of life in German patients with juvenile idiopathic arthritis in comparison to German general population. PLoS One 2016;11:e0153267. 10.1371/journal.pone.0153267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oen K, Malleson PN, Cabral DA. Disease course and outcome of juvenile rheumatoid arthritis in a multicenter cohort. J Rheumatol 2002;29:1989–99. [PubMed] [Google Scholar]
- 7.Schanberg LE, Anthony KK, Gil KM, et al. Daily pain and symptoms in children with polyarticular arthritis. Arthritis Rheum 2003;48:1390–7. Available http://doi.wiley.com/10.1002/art.v48:5 10.1002/art.10986 [DOI] [PubMed] [Google Scholar]
- 8.Tupper SM, Rosenberg AM, Pahwa P, et al. Pain intensity variability and its relationship with quality of life in youths with juvenile idiopathic arthritis. Arthritis Care Res 2013;65:563–70. Available https://acrjournals.onlinelibrary.wiley.com/toc/21514658/65/4 10.1002/acr.21850 [DOI] [PubMed] [Google Scholar]
- 9.Kimura Y, Walco GA, Sugarman E, et al. Treatment of pain in juvenile idiopathic arthritis: a survey of pediatric rheumatologists. Arthritis Rheum 2006;55:81–5. Available https://onlinelibrary.wiley.com/toc/15290131a/55/1 10.1002/art.21689 [DOI] [PubMed] [Google Scholar]
- 10.Stinson JN, Luca NJC, Jibb LA. Assessment and management of pain in juvenile idiopathic arthritis. Pain Res Manag 2012;17:391–6. 10.1155/2012/237258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stinson JN, Stevens BJ, Feldman BM, et al. Construct validity of a multidimensional electronic pain diary for adolescents with arthritis. Pain 2008;136:281–92. 10.1016/j.pain.2007.07.002 [DOI] [PubMed] [Google Scholar]
- 12.Oskarsdottir SA, Kristjansdottir A, Gudmundsdottir JA, et al. Musculoskeletal pain and its effect on daily activity and behaviour in Icelandic children and youths with juvenile idiopathic arthritis: a cross-sectional case-control study. Pediatr Rheumatol 2022;20:48. 10.1186/s12969-022-00706-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sawyer MG, Whitham JN, Roberton DM, et al. The relationship between health-related quality of life, pain and coping strategies in juvenile idiopathic arthritis. Rheumatology (Oxford) 2004;43:325–30. 10.1093/rheumatology/keh030 [DOI] [PubMed] [Google Scholar]
- 14.Butbul Aviel Y, Stremler R, Benseler SM, et al. Sleep and fatigue and the relationship to pain, disease activity and quality of life in juvenile idiopathic arthritis and juvenile dermatomyositis. Rheumatology (Oxford) 2011;50:2051–60. 10.1093/rheumatology/ker256 [DOI] [PubMed] [Google Scholar]
- 15.Dhanani S, Quenneville J, Perron M, et al. Minimal difference in pain associated with change in quality of life in children with rheumatic disease. Arthritis Care Res 2002;47:501–5. Available https://onlinelibrary.wiley.com/toc/15290131a/47/5 10.1002/art.10661 [DOI] [PubMed] [Google Scholar]
- 16.Owens J, Adolescent Sleep Working Group, Committee on Adolescence . Insufficient sleep in adolescents and young adults: an update on causes and consequences. Pediatrics 2014;134:e921–32. 10.1542/peds.2014-1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beebe DW. Sleep problems as consequence, contributor, and Comorbidity: introduction to the special issue on sleep, published in coordination with special issues in clinical practice in pediatric psychology and. J Pediatr Psychol 2016;41:583–7. 10.1093/jpepsy/jsw037 10.1093/jpepsy/jsw037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meltzer LJ, Faino A, Szefler SJ, et al. Experimentally manipulated sleep duration in adolescents with asthma: feasibility and preliminary findings. Pediatr Pulmonol 2015;50:1360–7. 10.1002/ppul.23179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perfect MM, Levine-Donnerstein D, Archbold K, et al. The contribution of sleep problems to academic and psychosocial functioning. Psychol Sch 2014;51:273–95. 10.1002/pits.21746 [DOI] [Google Scholar]
- 20.Bromberg MH, Gil KM, Schanberg LE. Daily sleep quality and mood as predictors of pain in children with juvenile Polyarticular arthritis. Health Psychol 2012;31:202–9. 10.1037/a0025075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stinson JN, Hayden JA, Ahola Kohut S, et al. Sleep problems and associated factors in children with juvenile idiopathic arthritis: a systematic review. Pediatr Rheumatol 2014;12. 10.1186/1546-0096-12-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward TM, Beebe DW, Chen ML, et al. Sleep disturbances and neurobehavioral performance in juvenile idiopathic arthritis. J Rheumatol 2017;44:361–7. 10.3899/jrheum.160556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulz KF, Altman DG, Moher D. Withdrawn: consort 2010 statement: updated guidelines for reporting parallel group randomised trials. International Journal of Surgery 2010. 10.1016/j.ijsu.2010.09.006 [DOI] [Google Scholar]
- 24.Boonstra AM, Schiphorst Preuper HR, Balk GA, et al. Cut-off points for mild, moderate, and severe pain on the visual analogue scale for pain in patients with chronic musculoskeletal pain. PAIN 2014;155:2545–50. 10.1016/j.pain.2014.09.014 [DOI] [PubMed] [Google Scholar]
- 25.Beebe DW, Fallone G, Godiwala N, et al. Feasibility and behavioral effects of an At‐Home Multi‐Night sleep restriction protocol for adolescents. J Child Psychol Psychiatry 2008;49:915–23. 10.1111/j.1469-7610.2008.01885.x [DOI] [PubMed] [Google Scholar]
- 26.Beebe DW, Field J, Milller MM, et al. Impact of multi-night experimentally induced short sleep on adolescent performance in a simulated classroom. Sleep 2017;40:zsw035. 10.1093/sleep/zsw035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beebe DW, Simon S, Summer S, et al. Dietary intake following experimentally restricted sleep in adolescents. Sleep 2013;36:827–34. 10.5665/sleep.2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chervin RD, Weatherly RA, Garetz SL, et al. Pediatric sleep questionnaire: prediction of sleep apnea and outcomes. Arch Otolaryngol Head Neck Surg 2007;133:216–22. 10.1001/archotol.133.3.216 [DOI] [PubMed] [Google Scholar]
- 29.Ramos Socarras L, Potvin J, Forest G. COVID-19 and sleep patterns in adolescents and young adults. Sleep Med 2021;83:26–33. 10.1016/j.sleep.2021.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirshkowitz M, Whiton K, Albert SM, et al. National sleep foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health 2015;1:40–3. 10.1016/j.sleh.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 31.Jay SM, Lamond N, Ferguson SA, et al. The characteristics of recovery sleep when recovery opportunity is restricted. Sleep 2007;30:353–60. 10.1093/sleep/30.3.353 [DOI] [PubMed] [Google Scholar]
- 32.Lamond N, Jay SM, Dorrian J, et al. The Dynamics of Neurobehavioural recovery following sleep loss. J Sleep Res 2007;16:33–41. 10.1111/j.1365-2869.2007.00574.x [DOI] [PubMed] [Google Scholar]
- 33.Ryan EG, Brock K, Gates S, et al. Do we need to adjust for interim analyses in a Bayesian adaptive trial design BMC Med Res Methodol 2020;20:150. 10.1186/s12874-020-01042-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lalloo C, Nishat F, Zempsky W, et al. Characterizing user engagement with a Digital intervention for pain self-management among youth with sickle cell disease and their Caregivers: Subanalysis of a randomized controlled trial. J Med Internet Res 2022;24:e40096. 10.2196/40096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain 2010;150:173–82. 10.1016/j.pain.2010.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Revicki DA, Chen W-H, Harnam N, et al. Development and Psychometric analysis of the PROMIS pain behavior item bank. Pain 2009;146:158–69. 10.1016/j.pain.2009.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giannini EH, Ruperto N, Ravelli A, et al. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum 1997;40:1202–9. [DOI] [PubMed] [Google Scholar]
- 38.McErlane F, Beresford MW, Baildam EM, et al. Validity of a three-variable juvenile arthritis disease activity score in children with new-onset juvenile idiopathic arthritis. Ann Rheum Dis 2013;72:1983–8. 10.1136/annrheumdis-2012-202031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foster HE, Jandial S. pGALS – paediatric gait arms legs and spine: a simple examination of the musculoskeletal system. Pediatr Rheumatol 2013;11:44. 10.1186/1546-0096-11-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gong GWK, Young NL, Dempster H, et al. The quality of my life questionnaire: the minimal clinically important difference for pediatric rheumatology patients. J Rheumatol 2007;34:581–7. [PubMed] [Google Scholar]
- 41.Spiegelhalter DJ, Abrams KR, Myles JP. Bayesian approaches to clinical trials and health-care evaluation. John Wiley & Sons, 2003. 10.1002/0470092602 [DOI] [Google Scholar]
- 42.R Core Team . R: a language and environment for statistical computing 2022.
- 43.Stan Development Team . Stan modeling language users guide and reference manual, 2.3.1 2022.
- 44.Stan Development Team . Rstan: the R interface to Stan. R package version 2.21.8 2023.
- 45.Lilford RJ, Thornton JG, Braunholtz D. Clinical trials and rare diseases: a way out of a conundrum. BMJ 1995;311:1621–5. 10.1136/bmj.311.7020.1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Broderick T, Pitman J, Jordan MI. Feature Allocations, probability functions, and Paintboxes. Bayesian Anal 2013;8:801–36. 10.1214/13-BA823 [DOI] [Google Scholar]
- 47.Plummer M. Rjags: Bayesian graphical models using MCMC. R Package Version 2022:4–13. [Google Scholar]
- 48.Highland KB, Parry J, Kent M, et al. Lagged effect of Patient‐Reported outcomes measurement information system (PROMIS) sleep disturbance on subacute Postsurgical PROMIS pain behavior. J Orthop Res 2023;41:711–7. 10.1002/jor.25412 [DOI] [PubMed] [Google Scholar]
- 49.Chaput J-P, Janssen I. Sleep duration estimates of Canadian children and adolescents. J Sleep Res 2016;25:541–8. 10.1111/jsr.12410 [DOI] [PubMed] [Google Scholar]
- 50.Stheneur C, Sznajder M, Spiry C. Sleep duration, quality of life and depression in adolescents: a school-based survey. Minerva Pediatr, 2017. [DOI] [PubMed] [Google Scholar]
- 51.Smaldone A, Honig JC, Byrne MW. Sleepless in America: inadequate sleep and relationships to health and well-being of our nation’s children. Pediatrics 2007;119 Suppl 1:S29–37. 10.1542/peds.2006-2089F [DOI] [PubMed] [Google Scholar]
- 52.Finan PH, Goodin BR, Smith MT. The Association of sleep and pain: an update and a path forward. J Pain 2013;14:1539–52. 10.1016/j.jpain.2013.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whibley D, AlKandari N, Kristensen K, et al. Sleep and pain: A systematic review of studies of mediation. Clin J Pain 2019;35:544–58. 10.1097/AJP.0000000000000697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krause AJ, Prather AA, Wager TD, et al. The pain of sleep loss: A brain characterization in humans. J Neurosci 2019;39:2291–300. 10.1523/JNEUROSCI.2408-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nair AS, Diwan S. Pain scores and statistical analysis—the conundrum. Ain-Shams J Anesthesiol 2020;12:35. 10.1186/s42077-020-00085-8 [DOI] [Google Scholar]
- 56.Kersten P, Küçükdeveci AA, Tennant A. The use of the visual analogue scale (VAS) in rehabilitation outcomes. J Rehabil Med 2012;44:609–10. 10.2340/16501977-0999 [DOI] [PubMed] [Google Scholar]
- 57.Norman G. Likert scales, levels of measurement and the “laws” of statistics. Adv in Health Sci Educ 2010;15:625–32. 10.1007/s10459-010-9222-y [DOI] [PubMed] [Google Scholar]
- 58.Manuguerra M, Heller GZ, Ma J. Continuous Ordinal regression for analysis of visual analogue scales: the R package ordinalCont. J Stat Softw 2020;96. 10.18637/jss.v096.i08 [DOI] [Google Scholar]
- 59.Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol 2008;4:1–32. 10.1146/annurev.clinpsy.3.022806.091415 [DOI] [PubMed] [Google Scholar]
- 60.Neville RD, Lakes KD, Hopkins WG, et al. Global changes in child and adolescent physical activity during the COVID-19 pandemic: A systematic review and meta-analysis. JAMA Pediatr 2022;176:886–94. 10.1001/jamapediatrics.2022.2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-003352supp001.pdf (63.7KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data are available upon reasonable request to the corresponding author.