Abstract
Objective
To investigate associations between baseline macular pigment optical density (MPOD) and retinal layer thicknesses in eyes with and without manifest primary open-angle glaucoma (POAG) in the Carotenoids in Age-Related Eye Disease Study 2 (CAREDS2).
Methods and analysis
MPOD was measured at CAREDS baseline (2001–2004) via heterochromatic flicker photometry (0.5° from foveal centre). Peripapillary retinal nerve fibre layer (RNFL), macular ganglion cell complex (GCC), ganglion cell layer (GCL), inner plexiform layer (IPL), and RNFL thicknesses were measured at CAREDS2 (2016–2019) via spectral-domain optical coherence tomography. Associations between MPOD and retinal thickness were assessed using multivariable linear regression.
Results
Among 742 eyes (379 participants), manifest POAG was identified in 50 eyes (32 participants). In eyes without manifest POAG, MPOD was positively associated with macular GCC, GCL and IPL thicknesses in the central subfield (P-trend ≤0.01), but not the inner or outer subfields. Among eyes with manifest POAG, MPOD was positively associated with macular GCC, GCL, IPL and RNFL in the central subfield (P-trend ≤0.03), but not the inner or outer subfields, and was positively associated with peripapillary RNFL thickness in the superior and temporal quadrants (P-trend≤0.006).
Conclusion
We observed a positive association between MPOD and central subfield GCC thickness 15 years later. MPOD was positively associated with peripapillary RNFL superior and temporal quadrant thicknesses among eyes with manifest POAG. Our results linking low MPOD to retinal layers that are structural indicators of early glaucoma provide further evidence that carotenoids may be protective against manifest POAG.
Keywords: glaucoma, epidemiology, optic nerve, retina, imaging
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Small, cross-sectional case–control studies have shown an association between macular pigment and ganglion cell complex thickness.
WHAT THIS STUDY ADDS
We observed a positive association between baseline macular pigment optical density and ganglion cell complex thickness within the central subfield measured 15 years later among healthy and glaucomatous eyes in a large cohort of older women.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study supports continued development of clinical trials to determine whether interventions to increase macular pigment may prevent glaucoma development or progression.
Introduction
Primary open-angle glaucoma (POAG) is a leading cause of irreversible blindness,1 estimated to affect 44 million adults globally.2 POAG is characterised by death of retinal ganglion cells (RGCs) and their axons in the retinal nerve fibre layer (RNFL).3 The highest density of RGCs exists in the macula, which is increasingly recognised as a site of early glaucoma pathogenesis.4 Thinning of the macular ganglion cell complex (GCC) and peripapillary RNFL can occur early in glaucoma, prior to detectable visual field defects.4 Consequently, interventions to prevent macular GCC thinning may prevent glaucomatous vision loss.
There have been conflicting reports regarding whether POAG is associated with lower levels of dietary carotenoids lutein and zeaxanthin (L/Z), which comprise macular pigment.5–7 L/Z are antioxidants that accumulate in neural tissues throughout the brain and retina,8 9 with highest density in the fovea.10 Macular pigment may protect RGCs and their axons by neutralising reactive-oxygen species and providing structural support in cell membranes.11 12 These postulated neuroprotective effects are consistent with growing evidence that macular pigment and greater dietary L/Z are positively associated with cognition13 and lower risk of age-related macular degeneration11 14 15 and Alzheimer’s disease.16 Thus, macular pigment may be a novel POAG risk factor for intervention, as it can be measured non-invasively and modified via diet or nutritional supplementation.11
Several studies have reported a positive relationship between macular pigment optical density (MPOD) and thickness of retinal layers affected in early-stage glaucoma.5 6 17 However, these studies have been predominantly small and cross-sectional. In this study, we investigated the association between baseline MPOD and thickness of the macular GCC and peripapillary RNFL approximately 15 years later among participants in the Carotenoids in Age-Related Eye Disease Study 2 (CAREDS2), an ancillary study of the prospective Women’s Health Initiative (WHI) Observational Study. Since glaucomatous neurodegeneration significantly reduces macular GCC and peripapillary RNFL thickness,1 4 we examined associations with MPOD separately in eyes with and without manifest POAG. We tested the hypothesis that lower MPOD would be associated with thinner macular GCC and peripapillary RNFL thicknesses 15 years later.
Materials and methods
CAREDS study design and sample
CAREDS is an ancillary study of the WHI, a multicentre prospective study of postmenopausal women in the USA.18 The CAREDS baseline study design and recruitment process has been previously described.19 CAREDS was originally designed to study the relationship between diet and lifestyle with macular degeneration and cataract development. Glaucoma measures were subsequently added in CAREDS2 and were not assessed at CAREDS baseline.
At CAREDS baseline (2001–2004), 2005 women were recruited from three WHI study sites (Iowa City, Iowa; Madison, Wisconsin; Portland, Oregon) and completed either: an in-person study visit with questionnaires on demographics, medical history, dietary intake and supplement use (n=1894) or questionnaires only (n=111). In the follow-up CAREDS2 study (2016–2019) (n=685 participants), 487 completed the CAREDS2 in-person study visit and questionnaires, while 198 completed questionnaires only. Those who did not participate in CAREDS2 (n=1320) were either deceased (48.4%), had been lost to follow-up or refused further contact (35.9%) or either declined participation or could not be contacted (15.7%). All participants provided written informed consent. Patients were involved in the design of our research in that we obtained input and a letter of support from the University of Wisconsin Glaucoma Patient Support Group. This study was approved by the University of Wisconsin-Madison Health Sciences Institutional Review Board (IRB) and was conducted in accordance with the tenets of the Declaration of Helsinki.
In this study, we analysed data from participants who attended the CAREDS2 in-person study visit. Exclusion criteria included missing MPOD in both eyes at CAREDS baseline or missing retinal thickness measures in both eyes, axial length >26 mm, presence of advanced age-related macular degeneration (ie, geographic atrophy or neovascular disease), insufficient data to adjudicate manifest POAG or either narrow angles or secondary glaucoma at CAREDS2 (online supplemental figure 1).
bmjophth-2023-001331supp001.pdf (152.8KB, pdf)
Assessment of MPOD
MPOD at CAREDS baseline (2001–2004) was measured in both eyes via customised heterochromatic flicker photometry (Macular Metrics I, LLC, Rehoboth, Massachusetts, USA), a valid and reproducible psychophysical technique for MPOD measurement in older adults.20 MPOD was measured using a table-top densitometer similar to the device initially described by Wooten et al.21 In brief, MPOD was measured in the fovea at four targets, including 0.25°, 0.50°, 1.00° and 1.75° from the foveal centre, relative to a 7° reference measure. Measurements were completed first in the right eye, and then in the left eye (0.25° and 0.50° targets only). For each target, the MPOD measurement was calculated from five separate determinations, using a blue light-emitting diode with a peak wavelength of 460 nm, corresponding to the maximum absorption spectrum of macular pigment. For each participant, the flicker rate for MPOD testing was adjusted based on the individual’s critical flicker frequency at the foveal and parafoveal targets, which were measured prior to MPOD testing. A detailed protocol for MPOD measurement in CAREDS has been published previously.22 MPOD at 0.5° (central fovea) was utilised as the primary exposure for the analysis, as this target has the highest ratio of between-individual to within-individual variability.22
Assessment of retinal layer thicknesses
Macular volume and peripapillary RNFL thickness scans were obtained at the CAREDS2 in-person study visit via spectral-domain optical coherence tomography (SD-OCT) imaging using the Heidelberg Spectralis (Heidelberg Spectralis, Heidelberg Engineering, Heidelberg, Germany). All SD-OCT scans were obtained by a certified photographer following a Wisconsin Reading Centre (WRC)-approved protocol. SD-OCT scans, including volume scans centred on the macula and peripapillary RNFL thickness scans were obtained from 473 CAREDS2 participants (n=946 eyes). Segmentation of retinal layers in the macula was generated using Heidelberg Spectralis software (V.1.9.13.0). Manual adjustment for segmentation error was completed by masked WRC-certified graders. A quality score of 20 or higher was required to be considered acceptable for inclusion. Images from 40 eyes were excluded due to the presence of poor signal strength, imaging artefacts or other ocular pathology that made segmentation unreliable.23 Additional details pertaining to the macular SD-OCT imaging and segmentation in CAREDS2 have been published previously.23 For the peripapillary RNFL, one circular RNFL scan was obtained consisting of 1536 A-Scans at 12° at high resolution with a frame rate of 100.
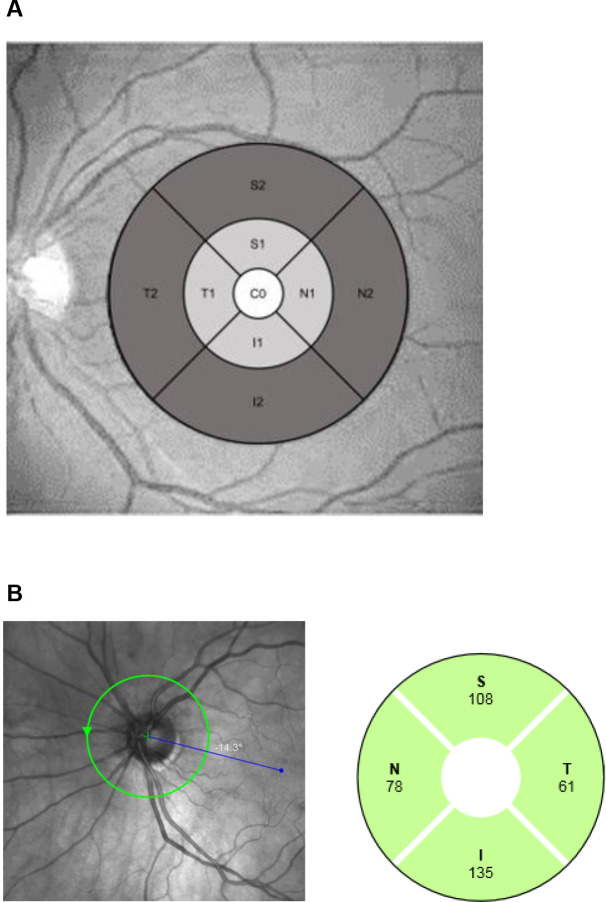
Macular thickness measurements were obtained for the RNFL, ganglion cell layer (GCL) and inner plexiform layer (IPL) for each subfield of the ETDRS (Early Treatment of Diabetic Retinopathy Study) grid, including the central (1 mm diameter), inner (3 mm diameter) and outer subfields (6 mm diameter) (figure 1A). The inner and outer macular subfield thicknesses were calculated as the average of the inferior, superior, nasal and temporal quadrants within each subfield, respectively. GCC was calculated as the sum of the measurements for macular RNFL, GCL and IPL thickness. Peripapillary RNFL thickness measurements were obtained for the inferior, superior, nasal and temporal quadrants, as well as the average of all quadrants (figure 1B).
Figure 1.
Maps of macular subfields* (A) and peripapillary retinal nerve fibre layer (B) thickness measurements using spectral-domain optical coherence tomography. *White: central subfield, light grey: inner subfield, dark grey: outer subfield. C0, central subfield; I, inferior quadrant; I1, inferior quadrant, inner subfield; I2, inferior quadrant, outer subfield; N, nasal quadrant; N1, nasal quadrant, inner subfield; N2, nasal quadrant, outer subfield; RNFL, retinal nerve fibre layer; S, superior quadrant; S1, superior quadrant, inner subfield; S2, superior quadrant, outer subfield; T, temporal quadrant; T1, temporal quadrant, inner subfield; T2, temporal quadrant, outer subfield.
Assessment of ocular characteristics
Ocular characteristics were evaluated by trained examiners at the CAREDS2 in-person study visit. These included intraocular pressure (IOP) (Tono-Pen, Reichert Inc, Depew, New York, USA), axial length (average of three measurements) (Gilras GRU-5000 A Biometer, US Ophthalmic, Coral, Florida, USA) and corneal pachymetry (PachPen, Accutome, Inc, Malvern, Pennsylvania, USA). A slit-lamp examination was conducted to ascertain whether an intraocular lens was implanted. Stereoscopic 30° digital colour fundus photographs of the optic nerve (Topcon TRC-DX50, Tokyo, Japan) were obtained by a WRC-certified photographer following pupil dilation with 2.5% phenylephrine and 1% tropicamide. The vertical cup-to-disc ratio was measured by a WRC-certified grader via IMAGEnet software (IMAGEnet 6, Topcon Healthcare, Oakland, NJ, USA) and the presence of disc haemorrhage or notching were identified following a standard protocol.24
Adjudication of manifest POAG
Detailed medical records were requested from eye care providers for participants who had at least one glaucoma risk factor. These risk factors including self-reported glaucoma or self-reported use of glaucoma medications, vertical cup-to-disc ratio ≥0.6 in either eye, vertical cup-to-disc asymmetry ≥0.2, disc haemorrhage or notching, IOP ≥22 mm Hg or peripapillary RNFL thickness <5th percentile for the average of all quadrants or for the inferior or superior quadrants. Medical records included visual field tests, peripapillary RNFL OCT imaging, fundus photographs and clinic notes. If a patient had either: no history of reproducible glaucomatous visual field defects or had unreliable visual fields (eg, >33% fixation losses, >25% false positives or >25% false negatives) or the most recent reliable visual fields were completed more than 1 year ago, the participant was invited to return for visual field testing. Humphrey visual field testing (Carl Zeiss Meditec, Inc, Jena, Germany) was completed in each eye using the SITA (Swedish Interactive Threshold Algorithm)-Standard 24-2 testing algorithm. Visual field testing was completed sequentially in the right eye and then left eye by a certified, trained examiner using each participants’ near refractive correction for each eye. Visual field testing was repeated for unreliable visual fields or if a rim artefact was suspected by the examiner.
Two fellowship-trained glaucoma specialists (YL and CT) who were masked to baseline MPOD measurements completed adjudication of manifest POAG status based on medical records review, visual field testing, fundus photography, SD-OCT imaging and ocular examination characteristics from CAREDS2. Manifest POAG was defined based on similar criteria as used in the Nurses’ Health Study, including the presence of glaucomatous visual field defects (ie, temporal, nasal, arcuate or paracentral).25 Defects were reproducible from at least one prior visual field test, were not related to other ocular conditions and were consistent with the pattern of optic nerve thinning from stereoscopic optic disc photographs and/or peripapillary RNFL OCT measurements. Disagreements on manifest POAG diagnosis were resolved by achieving consensus on joint review of participant data.
Statistical analysis
Age-adjusted multivariable regression models were used to assess associations between covariates and MPOD at CAREDS baseline, as well as manifest POAG status at CAREDS2. To assess potential survival bias, we also compared baseline characteristics among CAREDS participants (n=2005) who were included and those who were excluded from the analysis.
We used multiple linear regression to investigate associations between MPOD at CAREDS baseline and retinal layer thickness measurements approximately 15 years later at CAREDS2 among eyes with and without manifest POAG. Least-square means were calculated by quartile of MPOD, and β-coefficients and P-trends were calculated per one SD increase in MPOD as a continuous variable. All models were first adjusted for age and then other potential confounding covariates based on previously reported associations with MPOD in CAREDS and/or plausible causal associations with glaucoma or retinal thickness.19 Covariates were added individually to the age-adjusted model and retained if there was evidence of confounding (ie, change in the β-coefficients ≥10%). Only axial length met this criterion, and thus the final model included adjustment for age and axial length. Generalised estimating equations were used to account for correlations between eyes. A sensitivity analysis was conducted to exclude participants (approximately 16%) who reported using L/Z supplements (≥1 mg/day) prior to CAREDS2.
All participants had MPOD measurement in the right eye. For participants with missing baseline MPOD values in the left eye (n=17, 4.5%), the missing value was replaced with corresponding right eye value, as MPOD has high intereye correlation.22 Missing values for covariates were rare (approximately 1%–2% of observations) and were replaced using the median value. All analyses were conducted using SAS V.9.4 (Cary, North Carolina, USA). Statistical significance was set at P-trend <0.05.
Results
We included 742 eyes from 379 women in the analysis (online supplemental figure 1). The median age at CAREDS baseline was 65 years (range: 55–81 years) (table 1). Participants were predominantly white (97.9%) and non-Hispanic (99.5%) and had completed some college or vocational education (87.1%). MPOD at CAREDS baseline (0.5°, right eye) was associated with intraocular lens implantation (P-trend=0.01), larger waist circumference (P-trend <0.001) and higher body mass index (BMI) (P-trend <0.001). Those excluded from the analysis were slightly older, had lower levels of educational attainment and income, were slightly more likely to have an intraocular lens, to smoke, have diabetes, have a larger waist circumference and have a higher BMI (online supplemental table 1).
Table 1.
Participant characteristics by quartile of MPOD at CAREDS baseline (n=379)
| Variable (mean±SD or percentage) |
Full sample (n=379) |
MPOD (optical density units), 0.5° | P-trend | |||
| Quartile 1 (0.00–0.24) |
Quartile 2 (0.24–0.38) |
Quartile 3 (0.39–0.52) |
Quartile 4 (0.52–1.00) |
|||
| Age (years) | 65.4±0.3 | 64.4±0.6 | 65.9±0.6 | 65±0.6 | 66.3±0.6 | 0.10 |
| Race | ||||||
| Asian | 0.8% | 1.2% | 0.0% | 1.5% | 1.0% | 0.21 |
| Black | 0.8% | 2.3% | 1.2% | 0.0% | 0.0% | |
| White | 97.9% | 95.7% | 98.8% | 97.1% | 99.0% | |
| More than one race | 0.3% | 0.0% | 0.0% | 1.5% | 0.0% | |
| Unknown/not reported | 0.3% | 0.8% | 0.0% | 0.0% | 0.0% | |
| Ethnicity | ||||||
| Non-Hispanic | 99.5% | 100.0% | 100.0% | 99.1% | 99.2% | 0.55 |
| Hispanic | 0.5% | 0.0% | 0.0% | 0.9% | 0.8% | |
| Education | ||||||
| High school graduate or less | 12.9% | 16.8% | 10.4% | 13.0% | 12.9% | 0.14 |
| College or vocational training | 48.0% | 47.1% | 56.3% | 46.5% | 37.5% | |
| Post college | 39.1% | 36.0% | 33.3% | 40.6% | 49.6% | |
| Household Income | ||||||
| <US$75 000 | 72.0% | 73.6% | 68.0% | 75.2% | 70.8% | 0.51 |
| >US$75 000 | 28.0% | 26.4% | 32.0% | 24.8% | 29.2% | |
| Pack years smoked | ||||||
| Non-smoker | 58.0% | 52.6% | 60.9% | 58.7% | 61.6% | 0.18 |
| <7 pack years | 24.5% | 28.1% | 23.0% | 21.3% | 26.7% | |
| ≥7 pack years | 17.4% | 19.3% | 16.1% | 20.0% | 11.7% | |
| Intraocular lens implantation* | 6.6% | 11.4% | 6.1% | 7.8% | 2.7% | 0.01 |
| Intraocular pressure (mm Hg)* | 14.4±0.2 | 14.1±0.3 | 14.4±0.3 | 14.4±0.3 | 14.8±0.3 | 0.11 |
| Axial length (mm)* | 23.6±0.1 | 23.6±0.1 | 23.6±0.1 | 23.8±0.1 | 23.5±0.1 | 0.83 |
| Corneal thickness (µm)* | 558.2±1.9 | 556.2±3.8 | 560.7±3.7 | 556.1±3.7 | 559.7±3.7 | 0.61 |
| Waist circumference (in.) | 34.1±0.3 | 35.5±0.5 | 34.3±0.5 | 33.8±0.5 | 32.7±0.5 | <0.001 |
| Body mass index (kg/m2) | 27.5±0.3 | 29.2±0.5 | 27.6±0.5 | 26.8±0.5 | 26.2±0.5 | <0.001 |
| Self-reported hypertension | 21.4% | 28.2% | 18.1% | 22.3% | 17.2% | 0.09 |
| Self-reported diabetes | 3.7% | 3.4% | 5.0% | 2.6% | 2.9% | 0.46 |
*Values for ocular characteristics are shown only for the right eye.
CAREDS, Carotenoids in Age-Related Eye Disease Study; MPOD, macular pigment optical density.
bmjophth-2023-001331supp003.pdf (199.8KB, pdf)
Manifest POAG was identified in 50 eyes (6.7%) from 32 participants. Participants with manifest POAG were slightly older, more likely to be a non-smoker, to have an intraocular lens and to have lower MPOD at CAREDS baseline (online supplemental table 2). Among participants with manifest glaucoma (n=32), the average visual field mean deviation in the worse eye was −5.85 dB (66% mild, MD ≥−6 dB, 28% moderate, MD <−6 dB and ≥−12 dB, and 6% severe, <−12 dB, following the Hodapp-Parrish-Anderson glaucoma severity classification).26 Compared with eyes without manifest POAG, those with manifest POAG had thinner peripapillary RNFL thicknesses in all quadrants (p<0.001) (table 2). Eyes with manifest POAG had significantly thinner macular RNFL, GCL, IPL and total GCC thickness (p≤0.006) in the inner and outer subfields. Modestly lower macular RNFL (p=0.07) and total GCC thickness (p=0.09) in the central subfield were also observed for glaucomatous eyes.
Table 2.
Retinal layer thickness* by manifest POAG status at CAREDS2
| Retinal layer thickness* (μm, mean±SE) |
No manifest POAG (n=692 eyes) |
Manifest POAG (n=50 eyes) |
β (SE) | P value |
| Peripapillary RNFL | ||||
| Average | 93.4±0.5 | 72.5±1.7 | 20.8±1.8 | <0.001 |
| Inferior | 120.8±0.9 | 83.7±3.1 | 37.1±3.3 | <0.001 |
| Superior | 110.7±0.8 | 85.3±2.5 | 25.5±2.6 | <0.001 |
| Nasal | 71.6±0.6 | 58.6±2.3 | 13.0±2.3 | <0.001 |
| Temporal | 70.5±0.7 | 62.5±1.9 | 8.0±2.0 | <0.001 |
| Macular RNFL | ||||
| Central | 13.2±0.1 | 12.1±0.6 | 1.1±0.6 | 0.07 |
| Inner | 23.6±0.1 | 22.4±0.4 | 1.2±0.4 | 0.006 |
| Outer | 39.6±0.3 | 32.3±1.0 | 7.4±1.1 | <0.001 |
| Macular GCL | ||||
| Central | 16.1±0.2 | 15.1±1.0 | 1.1±1.0 | 0.28 |
| Inner | 46.2±0.3 | 38.7±1.3 | 7.5±1.4 | <0.001 |
| Outer | 30.3±0.2 | 26.0±0.6 | 4.3±0.7 | <0.001 |
| Macular IPL | ||||
| Central | 21.4±0.2 | 20.7±1.1 | 0.7±1.1 | 0.51 |
| Inner | 38.1±0.2 | 34.0±0.9 | 4.1±0.9 | <0.001 |
| Outer | 25.5±0.1 | 23.1±0.3 | 2.5±0.4 | <0.001 |
| Macular GCC | ||||
| Central | 50.5±0.5 | 46.6±2.2 | 3.8±2.3 | 0.09 |
| Inner | 107.8±0.5 | 95.0±2.5 | 12.7±2.5 | <0.001 |
| Outer | 95.3±0.5 | 81.5±1.8 | 13.8±1.9 | <0.001 |
*Adjusted for age and axial length.
CAREDS, Carotenoids in Age-Related Eye Disease Study; GCC, ganglion cell complex; GCL, ganglion cell layer; IPL, inner plexiform layer; POAG, primary open angle glaucoma; RNFL, retinal nerve fiber layer.
In eyes without manifest POAG, MPOD at CAREDS baseline was not associated with peripapillary RNFL thickness (P-trend ≥0.26) nor with macular RNFL thickness (P-trend ≥0.13) (table 3). MPOD was positively associated with macular IPL (P-trend <0.001), GCL (P-trend=0.01) and total GCC (P-trend=0.003) thickness in the central subfield, but not the inner or outer subfields (P-trend ≥0.15) (online supplemental figure 2). In the sensitivity analysis, excluding eyes from L/Z supplement users, there were no significant changes in these results (online supplemental table 3).
Table 3.
Retinal layer thickness* by quartile of MPOD at CAREDS baseline among eyes without manifest POAG (n=347 participants)
| Retinal layer thickness* (μm, mean±SE) |
Number of eyes | MPOD-CAREDS baseline, 0.5° (optical density units) | β±SE (1−SD increase) |
P-trend | |||
| Quartile 1 (0.00–0.23) |
Quartile 2 (0.23–0.38) |
Quartile 3 (0.38–0.51) |
Quartile 4 (0.51–1.00) |
||||
| Peripapillary RNFL | |||||||
| Average | 631 | 93.9±1.0 | 93.6±1.0 | 92.8±1.0 | 93.2±0.9 | 0.3±0.5 | 0.53 |
| Inferior | 631 | 120.9±1.4 | 121.2±1.5 | 120.1±1.6 | 121.1±1.7 | 0.2±0.7 | 0.77 |
| Superior | 631 | 111.6±1.5 | 110.9±1.4 | 109.8±1.4 | 110.9±1.4 | 0.2±0.8 | 0.80 |
| Nasal | 631 | 72.3±1.2 | 72.3±1.2 | 70.7±1.2 | 71.2±1.1 | 0.7±0.6 | 0.26 |
| Temporal | 631 | 71.4±1.3 | 70.3±1.3 | 70.8±1.2 | 69.7±1.2 | 0.4±0.6 | 0.50 |
| Macular RNFL | |||||||
| Central | 668 | 13.2±0.3 | 12.9±0.2 | 13.1±0.2 | 13.7±0.2 | 0.2±0.1 | 0.13 |
| Inner | 668 | 23.9±0.3 | 23.5±0.3 | 23.5±0.2 | 23.6±0.2 | 0.1±0.1 | 0.53 |
| Outer | 666 | 40.1±0.6 | 39.1±0.5 | 39.7±0.5 | 39.6±0.5 | 0.0±0.3 | 0.88 |
| Macular GCL | |||||||
| Central | 667 | 15.7±0.5 | 15.6±0.5 | 16.2±0.3 | 17.2±0.4 | 0.5±0.2 | 0.01 |
| Inner | 668 | 46.0±0.5 | 46.0±0.5 | 46.3±0.5 | 46.6±0.4 | 0.3±0.2 | 0.26 |
| Outer | 665 | 30.2±0.3 | 30.1±0.3 | 30.5±0.3 | 30.3±0.3 | 0.1±0.1 | 0.38 |
| Macular IPL | |||||||
| Central | 669 | 20.9±0.4 | 20.8±0.3 | 21.4±0.3 | 22.4±0.3 | 0.6±0.2 | <0.001 |
| Inner | 669 | 38.0±0.3 | 38.0±0.3 | 38.1±0.3 | 38.4±0.2 | 0.2±0.1 | 0.23 |
| Outer | 668 | 25.4±0.2 | 25.5±0.2 | 25.6±0.2 | 25.7±0.2 | 0.1±0.1 | 0.15 |
| Macular GCC | |||||||
| Central | 656 | 49.2±1.0 | 48.9±1.0 | 50.6±0.8 | 53.1±0.8 | 1.3±0.4 | 0.003 |
| Inner | 657 | 107.6±0.9 | 107.4±1.0 | 107.8±1.0 | 108.5±0.7 | 0.4±0.4 | 0.35 |
| Outer | 655 | 95.5±0.8 | 94.7±0.9 | 95.6±1.0 | 95.6±0.8 | 0.3±0.4 | 0.50 |
*Adjusted for age and axial length.
CAREDS, Carotenoids in Age-Related Eye Disease Study; GCC, ganglion cell complex; GCL, ganglion cell layer; IPL, inner plexiform layer; MPOD, macular pigment optical density; POAG, primary open-angle glaucoma; RNFL, retinal nerve fibre layer.
bmjophth-2023-001331supp002.pdf (99.2KB, pdf)
Among eyes with manifest POAG, MPOD was positively associated with macular RNFL, IPL, GCL and GCC thickness in the central subfield (P-trend ≤0.03), but not the inner or outer subfields (P-trend ≥0.15) (table 4) (online supplemental figure 2). MPOD was also positively associated with peripapillary RNFL thickness in the superior (P-trend=0.005) and temporal quadrants (P-trend=0.006), but not in the average, inferior or nasal quadrants (P-trend ≥0.11). After excluding eyes from L/Z supplement users, the association with GCL thickness in the central subfield remained statistically significant, while associations with the macular IPL thickness (P-trend=0.09) and total GCC thickness (P-trend=0.07) were somewhat attenuated (online supplemental table 4).
Table 4.
Retinal layer thickness* by quartile of MPOD at CAREDS baseline among eyes with manifest POAG (n=32 participants)
| Retinal layer thickness* (μm, mean±SE) |
Number of eyes | MPOD-CAREDS baseline, 0.5° (optical density units) | β±SE (1−SD increase) |
P-trend | |||
| Quartile 1 (0.00–0.07) |
Quartile 2 (0.08–0.30) |
Quartile 3 (0.32–0.48) |
Quartile 4 (0.48–0.81) |
||||
| Peripapillary RNFL | |||||||
| Average | 40 | 67.0±3.9 | 73.2±2.1 | 75.3±2.4 | 72.2±3.2 | 2.3±1.4 | 0.11 |
| Inferior | 40 | 75.5±6.0 | 89.8±4.9 | 89.6±5.7 | 72.6±5.9 | 0.0±2.8 | 0.99 |
| Superior | 40 | 75.7±5.2 | 80.5±3.2 | 94.1±3.6 | 90.0±3.6 | 6.0±2.1 | 0.005 |
| Nasal | 40 | 61.0±4.0 | 57.6±3.2 | 56.6±5.0 | 58.0±3.5 | 0.8±2.0 | 0.69 |
| Temporal | 40 | 56.1±3.4 | 64.9±2.4 | 60.7±2.4 | 68.7±3.5 | 4.0±1.5 | 0.006 |
| Macular RNFL | |||||||
| Central | 45 | 11.0±0.7 | 12.6±1.0 | 10.6±1.1 | 14.0±1.1 | 1.1±0.5 | 0.03 |
| Inner | 45 | 21.2±0.7 | 24.1±0.8 | 22.5±0.5 | 22.4±0.6 | 0.3±0.3 | 0.30 |
| Outer | 45 | 29.4±2.0 | 37.2±1.8 | 32.4±1.7 | 31.0±1.4 | 0.0±1.0 | 0.96 |
| Macular GCL | |||||||
| Central | 45 | 12.0±1.0 | 15.5±1.9 | 13.8±1.9 | 18.5±1.7 | 2.3±0.7 | 0.002 |
| Inner | 45 | 34.1±1.8 | 42.9±1.7 | 40.1±1.3 | 36.7±2.5 | 0.7±1.0 | 0.52 |
| Outer | 45 | 25.4±0.7 | 27.0±0.8 | 26.7±0.5 | 23.9±0.9 | 0.6±0.4 | 0.15 |
| Macular IPL | |||||||
| Central | 45 | 17.6±0.8 | 22.5±2.5 | 20.3±2.1 | 22.1±1.8 | 1.8±0.7 | 0.01 |
| Inner | 45 | 30.7±1.0 | 37.4±1.4 | 34.4±0.6 | 32.0±1.9 | 0.3±0.7 | 0.63 |
| Outer | 45 | 22.5±0.5 | 23.6±0.5 | 23.0±0.4 | 22.2±0.4 | 0.3±0.3 | 0.33 |
| Macular GCC | |||||||
| Central | 43 | 40.7±2.2 | 48.4±4.3 | 43.6±4.4 | 54.1±4.1 | 4.8±1.7 | 0.005 |
| Inner | 43 | 86.1±3.1 | 103.5±3.3 | 97.1±2.3 | 91.8±5.1 | 1.7±2.0 | 0.38 |
| Outer | 43 | 77.4±2.8 | 87.8±2.7 | 82.1±2.4 | 77.2±2.7 | 0.7±1.4 | 0.63 |
*Adjusted for age and axial length.
CAREDS, Carotenoids in Age-Related Eye Disease Study; GCC, ganglion cell complex; GCL, ganglion cell layer; IPL, inner-plexiform layer; MPOD, macular pigment optical density; POAG, primary open-angle glaucoma; RNFL, retinal nerve fiber layer.
Discussion
We assessed the relationship between MPOD and retinal thickness measured approximately 15 years later among a sample of older women with and without manifest POAG. We observed a significant positive association between baseline MPOD and macular GCC thickness in CAREDS2 within the central subfield, but not the inner or outer macular subfields. This finding was consistent among eyes with and without manifest POAG, despite considerable differences in GCC thickness between these groups. Additionally, eyes with manifest POAG showed a positive association between MPOD and superior and temporal peripapillary RNFL thickness.
Our results contribute to growing evidence of the association between MPOD and macular GCC thickness.5 6 17 Notably, we observed that MPOD at 0.5° was positively associated with GCC thickness in the central subfield, but not the inner and outer macular subfields. This may reflect higher MPOD in the fovea compared with the peripheral macula.27 Consequently, it is possible that MPOD may be a risk factor for foveal-involved POAG (causing paracentral vision loss), but not other types of glaucoma. This is consistent with the results from Siah et al5 that showed glaucomatous eyes with foveal GCC thinning (vs those without foveal GCC thinning) had nearly 50% lower MPOD in an Irish case–control study. In addition, Ji et al6 observed that MPOD was positively correlated with macular GCC thickness among eyes with POAG and those from age-matched controls in a small Chinese cross-sectional study. Most recently, Nagai et al17 reported that MPOD was positively correlated with macular GCC volume among healthy eyes from young Japanese participants (aged 22–48 years).
We also found an association between MPOD and thickness of the superior and temporal peripapillary RNFL quadrants in glaucomatous eyes, but not among healthy eyes. Prior small, cross-sectional studies have provided limited evidence regarding this relationship.5 6 Ji et al found no statistically significant associations between MPOD and peripapillary RNFL thickness6 except for a marginal association between MPOD and temporal RNFL thickness among glaucomatous eyes. Notably, this study did not include adjustment for age or axial length, known predictors of peripapillary RNFL thickness, which may explain the difference in our results. Siah et al reported a positive correlation between MPOD (0.5°) and peripapillary RNFL thickness in the inferior quadrant among patients with glaucoma, although this finding did not reach statistical significance.5 Glaucoma preferentially affects the superior and inferior quadrants of the peripapillary RNFL, which makes the association of these regions with MPOD notable.4 Peripapillary RNFL and GCC thinning are often detectable in early glaucoma before detectable visual field defects.4 Thus, our findings relating MPOD to peripapillary RNFL and GCC thickness support the hypothesis that low MPOD may contribute to or serve as a biomarker of glaucoma.
There are several plausible biological mechanisms that may underlie the association between MPOD and glaucomatous structural changes. Under the ‘protective’ hypothesis, macular pigment may mitigate the age-related decline in neural retinal thickness by reducing oxidative stress that contributes to neural cell death.28 L/Z promote antioxidant defenses through multiple pathways including direct scavenging of free radicals,29 30 suppressing proinflammatory signalling pathways,31 and filtering short-wavelength blue light.32 L/Z are also incorporated into cell membranes and increase their rigidity,12 and consequently may provide structural support for RGCs under elevated IOP. Alternatively, under the ‘structural’ hypothesis, greater retinal thickness in the fovea may facilitate accumulation of macular pigment by providing additional binding sites for L/Z. In a study of 11 individuals with Stargardt disease, Aleman et al33 reported that greater retinal thickness was associated with greater likelihood for increasing MPOD with L/Z supplementation.33 Likewise, thinning of the inner retina in glaucoma may contribute to lower MPOD (ie, reverse causation). Our results provide important clues concerning the underlying biological relationship between MPOD and manifest POAG, and support ongoing clinical trials to determine whether increasing MPOD through L/Z intake or low-cost supplementation may be effective in preventing POAG development or progression.34–36
Limitations of our study include that participants were older women and predominantly white and non-Hispanic. Thus, our results may not generalise to those who are younger, male or from other racial/ethnic groups. In addition, our statistical power may have been limited due to the relatively small sample size in the subgroup with manifest POAG. Yet, we did find significant associations, which supports the robustness of these relationships. Further, our results may have been affected by survival bias due to loss to follow-up and mortality, which was associated with slightly lower MPOD at CAREDS baseline that was not statistically significant (p=0.08).37 Since assessment of glaucoma measures (eg, RNFL OCT and visual field testing) were not performed at CAREDS baseline, we were unable to assess for progression of glaucomatous changes. We also cannot rule out the possibility that glaucomatous changes to the retina influenced MPOD measurements at CAREDS2 baseline. However, only 20.9% of participants with manifest POAG at CAREDS2 had at least one eye with a cup to disc ratio ≥0.6 at CAREDS baseline, an indicator of possible glaucomatous optic neuropathy.38 Finally, as with any observational study, our results may be affected by residual confounding. However, the similarity of our findings with those from prior smaller, case–control studies support the associations identified.
In conclusion, we observed a positive association between baseline MPOD and central subfield GCC thickness measured approximately 15 years later in eyes with and without manifest POAG. In addition, MPOD was positively associated with the thickness of the superior and temporal quadrants within the peripapillary RNFL among eyes with manifest POAG. Our results linking low MPOD to retinal layers that are structural indicators of early glaucoma provide further evidence that L/Z may provide protection against manifest POAG. Additional studies, including clinical trials, are needed to further elucidate the relationship between MPOD and the integrity of retinal layers associated with glaucoma, which may facilitate the development of novel interventions for manifest POAG.
Acknowledgments
The authors are grateful for the time and energy that the CAREDS2 participants devoted to collecting the data which informed this work, as well as the support of the University of Wisconsin Glaucoma Patient Support Group. We also acknowledge Minbo Bai, MD; Nitasha Gupta, MD; Saira Khanna, MD; Madison Kurth, BS; Alexandra Michalik, DO; Tran (Lisa) Nguyen, DO; and Jacob Schumacher, BS who contributed to data collection and medical records abstraction from CAREDS2 participants.
Footnotes
Collaborators: *The Second Carotenoids in Age-Related Eye Disease Study Research Group:University of Wisconsin-Madison: Julie Mares PhD, Barbara Blodi MD, Yao Liu MD MS, Amitha Domalpally MD PhD, Corinne Engelman PhD, Ronald Gangnon PhD, Gloria Sarto MD PhD; Oregon Health Sciences University: Steven Bailey MD, Erin LeBlanc (Kaiser-Permanente); University of Iowa: Karen Gehrs MD, Jennifer Robinson MD; Medical College of Wisconsin: Catherine Thuruthumaly MD; Women’s Health Initiative: Lesley Tinker PhD, RD; University of Texas: D. Max Snodderly PhD; University of Georgia: Randy Hammond PhD; University at Buffalo: Amy Millen PhD; Brown University: Bill Wooten, PhD; Tufts University: Elizabeth Johnson PhD; CAREDS 2 Examiners and Clinical Coordinators: Portland, OR: Jennifer Maykoski, BS; Ann Lundquist, BS; Madison, WI: Chris Smith, BS; Kim Wood, BS; Jennie Perry-Raymond, BS; Iowa City, IA: Heather Stockman, BS; Jean Walshire, BS; Christine Sinkey, BSNCAREDS2 Coordinating Center Staff at the University of Wisconsin-Madison. Courtney Blomme, MS; Kim Wood, BS; Kristen Hall, BS; Diane Pauk, BS; Esther Mezhibovsky, MS; Scientists: Krista Christensen, PhD; Marine Nalbandyan, MD, PhD. *Short list of Women’s Health Initiative Investigators: Program Office National Heart, Lung, and Blood Institute, Bethesda, Maryland: Jacques Rossouw; Shari Ludlam; Joan McGowan; Leslie Ford; and Nancy Geller Clinical Coordinating Center Fred Hutchinson Cancer Research Center, Seattle, WA: Garnet Anderson; Ross Prentice; Andrea LaCroix; and Charles Kooperberg Investigators and Academic Centers Brigham and Women’s Hospital, Harvard Medical School, Boston, MA: JoAnn E. Manson; MedStar Health Research Institute/Howard University, Washington, DC: Barbara V. Howard; Stanford Prevention Research Center, Stanford, CA: Marcia L. Stefanick; The Ohio State University, Columbus, OH: Rebecca Jackson; University of Arizona, Tucson/Phoenix, AZ: Cynthia A. Thomson; University at Buffalo, Buffalo, NY: Jean Wactawski-Wende; University of Florida, Gainesville/Jacksonville, FL: Marian Limacher; University of Iowa, Iowa City/Davenport, IA: Jennifer Robinson; University of Pittsburgh, Pittsburgh, PA: Lewis Kuller; Wake Forest University School of Medicine, Winston-Salem, NC: Sally Shumaker; University of Nevada, Reno, NV: Robert Brunner Women’s Health Initiative Memory Study Wake Forest University School of Medicine, Winston-Salem, NC: Mark Espeland.
Contributors: Dr Liu had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis as the guarantor (YL). Concept and design: TL, JAM, ZL, TSV, AD, BRH, RBW, LFT, MN, YL. Acquisition, analysis or interpretation of data: All authors, the Second Carotenoids in Age-Related Eye Disease Study Research Group, and short list of Women’s Health Initiative Investigators. Drafting of the manuscript: TL, YL. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: TL, JAM, ZL, MN, YL. Obtained funding: JAM, ZL, RBW, LFT, YL, SB, KG. Administrative, technical or material support: TL, JAM, ZL, BRH, RBW, LFT, YL, the Second Carotenoids in Age-Related Eye Disease Study Research Group, and short list of Women’s Health Initiative Investigators. Supervision: JAM, YL, SB, KG.
Funding: This work was supported by National Eye Institute grants EY013018, EY016886 and EY025292, and a supplement to EY025292-01S1 from the Office of Dietary Supplements, as well as by the Molecular and Applied Nutrition Training Program (MANTP) NIH grant T32 DK007665, NIH/NCATS UL1 TR002373 (UW ICTR Basic and Clinical Translational Research Pilot Award), Lions Eye Bank of Wisconsin Gift of Sight Discovery Fund, American Glaucoma Society Mentoring for Advancement of Physician-Scientists (MAPS) Award. This work was also supported in part by an unrestricted grant from Research to Prevent Blindness, Inc. to the UW Madison Department of Ophthalmology and Visual Sciences, and in part by a National Eye Institute Vision Research Core grant (P30 EY016665) to the UW Madison Department of Ophthalmology and Visual Sciences. It is an ancillary study to Women’s Health Initiative (WHI). The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C. This work was also supported by a Shapiro Fellowship from the University of Wisconsin (UW) School of Medicine and Public Health and the Dan and Ellie Albert Medical Student Scholarship Fund from the McPherson Eye Research Institute.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
Collaborators: The Second Carotenoids in Age-Related Eye Disease Study Research Group, Julie Mares, Barbara Blodi, Yao Liu, Amitha Domalpally, Corinne Engelman, Ronald Gangnon, Julie Mares, Barbara Blodi, Yao Liu, Amitha Domalpally, Corinne Engelman, Ronald Gangnon, Gloria Sarto, Steven Bailey, Erin LeBlanc, Karen Gehrs, Jennifer Robinson, Catherine Thuruthumaly, Lesley Tinker, D Max Snodderly, Randy Hammond, Amy Millen, Bill Wooten, Elizabeth Johnson, Jennifer Maykoski Portland, Ann Lundquist, Chris Smith Madison, Kim Wood, Jennie Perry Raymond, Iowa City, Heather Stockman, Jean Walshire, Christine Sinkey, Courtney Blomme, Kim Wood, Kristen Hall, Diane Pauk, Esther Mezhibovsky, Krista Christensen, Marine Nalbandyan, Jacques Rossouw Bethesda, Shari Ludlam, Joan McGowan, Leslie Ford, Nancy Geller, Garnet Anderson Seattle, Ross Prentice, Andrea LaCroix, Charles Kooperberg, JoAnn E Manson, Barbara V Howard, Marcia L Stefanick, Rebecca Jackson Columbus, Tucson Phoenix, Cynthia A Thomson, Jean Wactawski Wende, Gainesville Jacksonville, Jennifer Robinson, Lewis Kuller Pittsburgh, Sally Shumaker Winston-Salem, Robert Brunner Reno, and Mark Espeland Winston-Salem
Data availability statement
Data are available upon reasonable request. The data are available upon reasonable request to those who have obtained all required approvals from the Women’s Health Initiative and the University of Wisconsin Health Sciences Institutional Review Board.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study involves human participants and was approved by University of Wisconsin Health Sciences IRB, ID: 2015-1293 Participants gave informed consent to participate in the study before taking part.
References
- 1.Quigley HA. Glaucoma. Lancet 2011;377:1367–77. 10.1016/S0140-6736(10)61423-7 [DOI] [PubMed] [Google Scholar]
- 2.Tham Y-C, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 2014;121:2081–90. 10.1016/j.ophtha.2014.05.013 [DOI] [PubMed] [Google Scholar]
- 3.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA 2014;311:1901–11. 10.1001/jama.2014.3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hood DC, Raza AS, de Moraes CGV, et al. Glaucomatous damage of the macula. Prog Retin Eye Res 2013;32:1–21. 10.1016/j.preteyeres.2012.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siah WF, Loughman J, O’Brien C. Lower macular pigment optical density in foveal-involved glaucoma. Ophthalmology 2015;122:2029–37. 10.1016/j.ophtha.2015.06.028 [DOI] [PubMed] [Google Scholar]
- 6.Ji Y, Zuo C, Lin M, et al. Macular pigment optical density in Chinese primary open angle glaucoma using the one-wavelength reflectometry method. J Ophthalmol 2016;2016:2792103. 10.1155/2016/2792103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Igras E, Loughman J, Ratzlaff M, et al. Evidence of lower macular pigment optical density in chronic open angle glaucoma. Br J Ophthalmol 2013;97:994–8. 10.1136/bjophthalmol-2013-303153 [DOI] [PubMed] [Google Scholar]
- 8.Snodderly DM, Auran JD, Delori FC. The macular pigment. II. spatial distribution in primate retinas. Investigative Ophthalmology & Visual Science 1984;25:674–85. [PubMed] [Google Scholar]
- 9.Jeon S, Ranard KM, Neuringer M, et al. Lutein is differentially deposited across brain regions following formula or breast feeding of infant rhesus macaques. J Nutr 2018;148:31–9. 10.1093/jn/nxx023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammond BR, Wooten BR, Snodderly DM. Individual variations in the spatial profile of human macular pigment. J Opt Soc Am A Opt Image Sci Vis 1997;14:1187–96. 10.1364/josaa.14.001187 [DOI] [PubMed] [Google Scholar]
- 11.Mares J. Lutein and zeaxanthin isomers in eye health and disease. Annu Rev Nutr 2016;36:571–602. 10.1146/annurev-nutr-071715-051110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabrielska J, Gruszecki WI. Zeaxanthin (dihydroxy-beta-carotene) but not beta-carotene rigidifies lipid membranes: a 1H-NMR study of carotenoid-egg phosphatidylcholine liposomes. Biochim Biophys Acta 1996;1285:167–74. 10.1016/s0005-2736(96)00152-6 [DOI] [PubMed] [Google Scholar]
- 13.Johnson EJ. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr Rev 2014;72:605–12. 10.1111/nure.12133 [DOI] [PubMed] [Google Scholar]
- 14.Seddon JM, Ajani UA, Sperduto RD, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. JAMA 1994;272:1413–20. [PubMed] [Google Scholar]
- 15.Wu J, Cho E, Willett WC, et al. Intakes of lutein, zeaxanthin, and other carotenoids and age-related macular degeneration during 2 decades of prospective follow-up. JAMA Ophthalmol 2015;133:1415–24. 10.1001/jamaophthalmol.2015.3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qu M, Shi H, Wang K, et al. The associations of plasma/serum carotenoids with Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis 2021;82:1055–66. 10.3233/JAD-210384 [DOI] [PubMed] [Google Scholar]
- 17.Nagai N, Asato T, Minami S, et al. Correlation between macular pigment optical density and neural thickness and volume of the retina. Nutrients 2020;12:888. 10.3390/nu12040888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Design of the Women’s Health Initiative clinical trial and observational study. Controlled Clinical Trials 1998;19:61–109. 10.1016/S0197-2456(97)00078-0 [DOI] [PubMed] [Google Scholar]
- 19.Mares JA, LaRowe TL, Snodderly DM, et al. Predictors of optical density of lutein and zeaxanthin in retinas of older women in the Carotenoids in Age-Related Eye Disease study, an ancillary study of the Women’s Health Initiative. Am J Clin Nutr 2006;84:1107–22. 10.1093/ajcn/84.5.1107 [DOI] [PubMed] [Google Scholar]
- 20.Hammond BR, Wooten BR, Smollon B. Assessment of the validity of in vivo methods of measuring human macular pigment optical density. Optom Vis Sci 2005;82:387–404. 10.1097/01.opx.0000162652.85875.d2 [DOI] [PubMed] [Google Scholar]
- 21.Wooten BR, Hammond BR, Land RI, et al. A practical method for measuring macular pigment optical density. Investigative Ophthalmology & Visual Science 1999;40:2481–9. [PubMed] [Google Scholar]
- 22.Snodderly DM, Mares JA, Wooten BR, et al. Macular pigment measurement by heterochromatic flicker photometry in older subjects: the Carotenoids and Age-Related Eye Disease Study. Invest Ophthalmol Vis Sci 2004;45:531. 10.1167/iovs.03-0762 [DOI] [PubMed] [Google Scholar]
- 23.Etheridge T, Liu Z, Nalbandyan M, et al. Association of macular thickness with age and age-related macular degeneration in the Carotenoids in Age-Related Eye Disease Study 2 (CAREDS2), an ancillary study of the Women’s Health Initiative. Trans Vis Sci Tech 2021;10:39. 10.1167/tvst.10.2.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wisconsin Reading Center . Procedure for optic nerve head evaluations; 2008. Dept. of Ophthalmology and visual sciences, University of Wisconsin school of medicine and public health
- 25.Kang JH, Willett WC, Rosner BA, et al. Association of dietary nitrate intake with primary open-angle glaucoma: a prospective analysis from the Nurses' Health Study and Health Professionals Follow-up Study. JAMA Ophthalmol 2016;134:294–303. 10.1001/jamaophthalmol.2015.5601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodapp EA, Parrish RK. In: Anderson DR, ed. Clinical Decisions In Glaucoma. Mosby. 1993. [Google Scholar]
- 27.Hammond BR, Wooten BR, Snodderly DM. Individual variations in the spatial profile of human macular pigment. J Opt Soc Am A 1997;14:1187. 10.1364/JOSAA.14.001187 [DOI] [PubMed] [Google Scholar]
- 28.Li S-Y, Lo ACY. Lutein protects RGC-5 cells against hypoxia and oxidative stress. Int J Mol Sci 2010;11:2109–17. 10.3390/ijms11052109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khachik F, Bernstein PS, Garland DL. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Investigative Ophthalmology & Visual Science 1997;38:1802–11. [PubMed] [Google Scholar]
- 30.Chae SY, Park SY, Park G. Lutein protects human retinal pigment epithelial cells from oxidative stressinduced cellular senescence. Mol Med Rep 2018;18:5182–90. 10.3892/mmr.2018.9538 [DOI] [PubMed] [Google Scholar]
- 31.Kamoshita M, Toda E, Osada H, et al. Lutein acts via multiple antioxidant pathways in the photo-stressed retina. Sci Rep 2016;6:30226. 10.1038/srep30226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barker FM, Snodderly DM, Johnson EJ, et al. Nutritional manipulation of primate retinas, V: effects of lutein, zeaxanthin, and N-3 fatty acids on retinal sensitivity to blue-light-induced damage. Invest Ophthalmol Vis Sci 2011;52:3934–42. 10.1167/iovs.10-5898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aleman TS, Cideciyan AV, Windsor EAM, et al. Macular pigment and lutein supplementation in ABCA4-associated retinal degenerations. Invest Ophthalmol Vis Sci 2007;48:1319–29. 10.1167/iovs.06-0764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.NIH U.S. National Library of Medicine . Macular Pigment and Visual Performance in Glaucoma Patients, Available: https://ClinicalTrials.gov/show/NCT03959592 [Accessed 8 Jan 2022].
- 35.NIH U.S. National Library of Medicine . European Nutrition in Glaucoma Management Trial. Available: https://ClinicalTrials.gov/show/NCT04460365 [Accessed 8 Jan 2022].
- 36.NIH U.S. National Library of Medicine . Augmented Macular Pigment-containing Nutraceutical and Central Visual Function, Available: https://ClinicalTrials.gov/show/NCT04676126 [Accessed 8 Jan 2022].
- 37.Mares JA, Liu Z, Wallace RB, et al. The relationship of macular pigment optical density (MPOD) to mortality in the second Carotenoids in Age-Related Eye Disease Study (CAREDS 2), an ancillary study of the Women’s Health Initiative (WHI). Investigative Ophthalmology & Visual Science 2017;58:2982. [Google Scholar]
- 38.Vajaranant TS, Hallak J, Espeland MA, et al. An association between large optic nerve cupping and cognitive function. Am J Ophthalmol 2019;206:40–7. 10.1016/j.ajo.2019.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjophth-2023-001331supp001.pdf (152.8KB, pdf)
bmjophth-2023-001331supp003.pdf (199.8KB, pdf)
bmjophth-2023-001331supp002.pdf (99.2KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The data are available upon reasonable request to those who have obtained all required approvals from the Women’s Health Initiative and the University of Wisconsin Health Sciences Institutional Review Board.