Abstract
Background
Tumor samples from the phase III IMpower010 study were used to compare two programmed death-ligand 1 (PD-L1) immunohistochemistry assays (VENTANA SP263 and Dako 22C3) for identification of PD-L1 patient subgroups (negative, positive, low, and high expression) and their predictive value for adjuvant atezolizumab compared with best supportive care (BSC) in resectable early-stage non-small cell lung cancer (NSCLC).
Methods
PD-L1 expression was assessed by the SP263 assay, which measured the percentage of tumor cells with any membranous PD-L1 staining, and the 22C3 assay, which scored the percentage of viable tumor cells showing partial or complete membranous PD-L1 staining.
Results
When examining the concordance at the PD-L1-positive threshold (SP263: tumor cell (TC)≥1%; 22C3: tumor proportion score (TPS)≥1%), the results were concordant between assays for 83% of the samples. Similarly, at the PD-L1–high cut-off (SP263: TC≥50%; 22C3: TPS≥50%), the results were concordant between assays for 92% of samples. The disease-free survival benefit of atezolizumab over BSC was comparable between assays for PD-L1-positive (TC≥1% by SP263: HR, 0.58 (95% CI: 0.40 to 0.85) vs TPS≥1% by 22C3: HR, 0.65 (95% CI: 0.45 to 0.95)) and PD-L1-high (TC≥50% by SP263: HR, 0.27 (95% CI: 0.14 to 0.53) vs TPS≥50% by 22C3: HR, 0.31 (95% CI: 0.16 to 0.60)) subgroups.
Conclusions
The SP263 and 22C3 assays showed high concordance and a comparable clinical predictive value of atezolizumab at validated PD-L1 thresholds, suggesting that both assays can identify patients with early-stage NSCLC most likely to experience benefit from adjuvant atezolizumab.
Trial registration number
Keywords: non-small cell lung cancer, immunohistochemistry, immune checkpoint inhibitors, tumor biomarkers, programmed cell death 1 receptor
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Patients with metastatic non-small cell lung cancer (NSCLC) who have tumors with high programmed death-ligand 1 (PD-L1) expression generally derive the greatest clinical benefit from anti-programmed death-1/PD-L1 drugs. Previous studies in advanced NSCLC have demonstrated concordance between the SP263, 22C3, and 28–8 immunohistochemistry assays for identifying PD-L1 expression on tumor cells. Even though the assessment of PD-L1 expression in patients’ tumors could help inform treatment decisions, direct data for PD-L1 assay comparisons does not exist for early-stage NSCLC.
WHAT THIS STUDY ADDS
Using tumor samples from IMpower010, this report shows that the SP263 and 22C3 immunohistochemistry assays have high concordance and a comparable clinical predictive value of atezolizumab over best supportive care at validated PD-L1 thresholds for patients with early-stage NSCLC.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
These data suggest that both the SP263 and 22C3 assays can be used clinically to identify patients with early-stage NSCLC who are most likely to experience benefit from adjuvant atezolizumab.
Introduction
Programmed death-ligand 1 (PD-L1) is an immune checkpoint protein expressed on tumor and tumor infiltrating immune cells. Binding of PD-L1 to its receptors programmed death-1 (PD-1) or B7.1 can lead to suppression of anti-cancer immune mechanisms.1 In advanced non-small cell lung cancer (NSCLC), inhibition of PD-L1 and its receptor, PD-1, has become an important therapeutic strategy for patients without driver mutations including EGFR and ALK. Across lines of therapy, PD-L1/PD-1 inhibitors atezolizumab, nivolumab, durvalumab, pembrolizumab, and cemiplimab either alone or in combination with chemotherapy, are recommended for use by current guidelines.2 3
For early-stage NSCLC, the standard of care is adjuvant chemotherapy,2 3 despite only modest 5-year survival benefits of ≈5%.4 5 IMpower010 was the first phase III study to demonstrate the clinical benefit of cancer immunotherapy (atezolizumab) as measured by improved disease-free survival (DFS) in adjuvant early-stage NSCLC.6 Adjuvant atezolizumab improved DFS compared with best supportive care (BSC) for patients with stage II–III NSCLC whose tumors expressed PD-L1 on ≥1% of tumor cells (median DFS, non-estimable (NE) (95% CI: 36.1 to NE) vs 35.3 months (95% CI: 29.0 to NE); HR 0.66 (95% CI: 0.50 to 0.88)); for patients with stage II–III NSCLC whose tumors expressed PD-L1 on ≥50% of tumor cells (median DFS, NE (95% CI: 42.3 to NE) vs 35.7 months (29.7–NE); HR, 0.43 (95% CI: 0.27 to 0.68)); and among all patients in the stage II–IIIA population (median DFS, 42.3 months (95% CI: 36.0 to NE) vs 35.3 months (95% CI: 30.4 to 46.4); stratified HR, 0.79; (95% CI: 0.64 to 0.96)). At this analysis, statistical significance for DFS was not met in the intention-to-treat (ITT) population (stage IB–IIIA).6 Based on these results, atezolizumab was approved in the USA, China, Japan, Brazil, Russia, New Zealand, and several other countries for adjuvant treatment following surgery and platinum-based chemotherapy for patients with stage II–IIIA NSCLC whose tumors express PD-L1 on ≥1% of tumor cells7; in the UK, Canada, Australia, and Switzerland for patients whose tumors express PD-L1 on ≥50% of tumor cells8; and in the European Union for patients whose tumors express PD-L1 on ≥50% of tumor cells and do not harbor EGFR mutations or ALK alterations.9 Further, the companion diagnostic test VENTANA PD-L1 SP263 immunohistochemistry (IHC) assay received US Food and Drug Administration approval for use in early-stage NSCLC to identify patients with PD-L1 expression on tumor cells.10
Since patients with metastatic NSCLC who have high PD-L1 expression generally derive the greatest clinical benefit from anti-PD-L1 drugs,11–15 assays to determine tumor PD-L1 expression are an important tool to identify patients who may achieve benefit from these treatments. In addition to SP263 (atezolizumab),10 other PD-L1 IHC assays that have been clinically validated as companion or complementary diagnostics with their associated PD-L1/PD-1 inhibitors are Dako 22C3 (pembrolizumab),12 16 17 Dako 28–8 (nivolumab),15 VENTANA SP263 (durvalumab),11 and VENTANA SP142 (atezolizumab).13 14 18 This one assay-one drug structure has limited clinicians to prescribe a treatment based on which assay is available to them. Since each assay uses a unique clone, detection instrument, and scoring algorithm, questions have arisen about the interchangeability of one test result with another. A better understanding of concordance between assays provides physicians with a greater flexibility to choose which assay to use when prescribing PD-L1/PD-1 inhibitors.
Previous studies in advanced NSCLC have demonstrated an alignment between assays, showing that the SP263, 22C3, and 28–8 assays were highly concordant for identifying PD-L1 expression on tumor cells.19–21 Additionally, in a retrospective analysis of patients with metastatic NSCLC (OAK trial; NCT02008227), atezolizumab administered as a second-line or third-line treatment improved survival compared with docetaxel regardless of whether the SP142 or 22C3 assay was used to determine tumor PD-L1 expression.18 Despite the fact that the assessment of PD-L1 expression in patients' tumors could help inform treatment decisions, direct data for PD-L1 assay comparisons does not exist in early-stage NSCLC.
The goal of the current analysis was to evaluate concordance and compare the predictive value of two PD-L1 assays, SP263 and 22C3, for benefit from atezolizumab in patients with stage II–IIIA NSCLC. Here, we show comparable clinical efficacy of atezolizumab over BSC between assays for each PD-L1 expression subgroup. Further, the comparable concordance between the SP263 and 22C3 assays suggests that both assays can identify patients with early-stage NSCLC who are most likely to experience benefit from adjuvant atezolizumab.
Methods
Patients and treatment
IMpower010 was a randomized, multicenter, open-label phase III study, which examined clinical outcomes of atezolizumab compared with BSC following adjuvant cisplatin-based chemotherapy.6 Detailed methods were previously described.6 Patients were aged ≥18 years with completely resected stage IB–IIIA NSCLC per the AJCC Cancer Staging Manual, seventh edition, and had an Eastern Cooperative Oncology Group performance status (ECOG PS) ≤1. Patients were randomly assigned (1:1) to receive adjuvant atezolizumab (1200 mg every 3 weeks for 16 cycles or 1 year) or BSC following one to four cycles of adjuvant platinum-based chemotherapy.
Immunohistochemistry assays
For patient enrollment and stratification in the IMpower010 study,6 PD-L1 expression on tumor tissue samples was determined using the SP142 IHC assay (Ventana Medical Systems, Tucson, Arizona, USA). The SP142 assay expresses PD-L1 as a percentage of total tumor cells (TC) and tumor-infiltrating immune cells (IC). Tumor PD-L1 expression by SP142 was defined by TC/IC cut-off values of PD-L1 negative (TC0 and IC0), PD-L1 positive (TC1/2/3 or IC1/2/3), or PD-L1 high (TC3 or IC3).
Staining with the SP263 assay (Ventana Medical Systems) was performed retrospectively on freshly cut tissue sections or formalin-fixed paraffin-embedded tumor tissue sections <1 year old, based on the manufacturer’s cut slide stability instructions.22 Of the 1005 patients in the IMpower010 study,6 979 patients (97%) had tissue available for evaluation by the SP263 assay. SP263 assesses the percentage of tumor cells with membranous PD-L1 staining of any intensity. Categories were defined by TC cut-off values of PD-L1 negative (TC<1%), PD-L1 positive (TC≥1%), PD-L1 low (TC 1%–49%), or PD-L1 high (TC≥50%).
Staining with the 22C3 assay (Dako pharmDx 22C3 IHC; Dako North America, Carpinteria, California, USA) was performed retrospectively on freshly cut tissue sections or formalin-fixed paraffin-embedded tumor tissue sections <6 months old, based on the manufacturer’s cut slide stability instructions.23 Tumors were scored in terms of the tumor proportion score (TPS), which represents the percentage of viable tumor cells showing partial or complete membranous PD-L1 staining. Tumor PD-L1 expression was defined by TPS cut-off values of PD-L1 negative (TPS<1%), PD-L1 positive (TPS≥1%), PD-L1 low (TPS 1%–49%), or PD-L1 high (TPS≥50%).
Staining and scoring occurred at a central laboratory (Q2 Solutions in Beijing, China, for patients in China and CellCarta in Montreal, Quebec, Canada, for all other patients). Although most patients (97%) had samples available for SP263 analysis, the 22C3-BEP was smaller because the Human Genetic Resources Administration of China exploratory application was not in place at the time of testing, and therefore, the China cohort was not included in the 22C3 analysis. Others were not analyzed by 22C3 due to insufficient tissue.
Statistical analysis
Analyses were performed in the 22C3-biomarker-evaluable population (22C3-BEP; patients with available tissue that was analyzed by 22C3), the combined-BEP (patients with available tissue that was analyzed by both 22C3 and SP263 assays), and the non-22C3-BEP (patients whose tumors were analyzed by SP263 only). Kaplan-Meier estimates and corresponding medians for DFS were calculated within the 22C3-BEP, combined-BEP, and non-22C3-BEP according to the predefined PD-L1 cut-off values with each assay. All HRs and 95% CIs were derived from unstratified and unadjusted Cox models.
Results
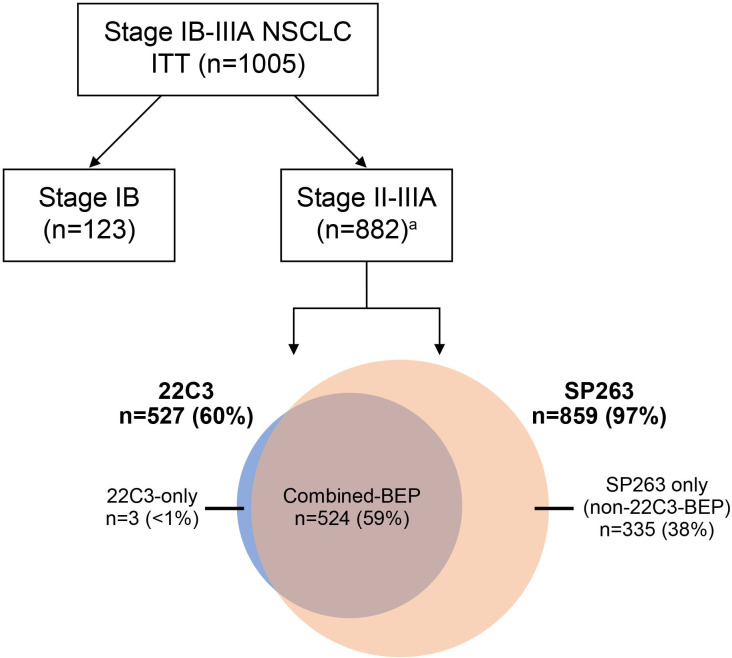
Patient enrollment occurred between October 7, 2015, and September 19, 2018. The ITT population comprised 1005 patients. Within the ITT population, 882 patients had stage II–IIIA NSCLC. Among the 882 stage II–IIIA patients, 527 patients had samples analyzed by 22C3 (22C3-BEP), and of those, 524 patients had samples also analyzed by SP263 (combined-BEP). Finally, 335 of the 882 patients had tumors without 22C3 assessment (non-22C3-BEP). Twenty patients had samples that were non-evaluable by both assays (figure 1).
Figure 1.
Study design for analysis of patient samples from the IMpower010 trial using the SP263 and 22C3 IHC assays to measure tumor cell PD-L1 levels. a Twenty samples were non-evaluable by both the 22C3 and SP263 assays. BEP, biomarker-evaluable population; IHC, immunohistochemistry; ITT, intention to treat; NSCLC, non-small cell lung cancer; PD-L1, programmed death-ligand 1.
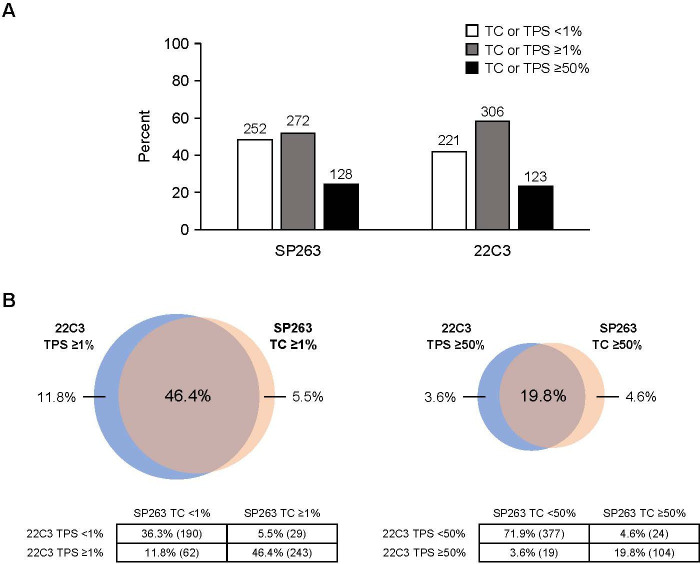
We examined the prevalence of PD-L1 expression levels by the SP263 and 22C3 assays in the stage II–IIIA combined-BEP and 22C3-BEP, respectively. The SP263 assay identified 51.9% (272/524) of patients in the combined-BEP who were PD-L1 positive (TC≥1%) compared with 58.1% (306/527) of patients in the 22C3-BEP who were 22C3 PD-L1 positive (TPS≥1%). Likewise, 24.4% (128/524) of patients in the combined-BEP were SP263 PD-L1 high (TC≥50%) compared with 23.3% (123/527) of patients in the 22C3-BEP who were 22C3 PD-L1 high (TPS≥50%) (figure 2A).
Figure 2.
(A) Prevalence of patients identified in the programmed death-ligand 1 (PD-L1)-negative, PD-L1-positive, and PD-L1-high subgroups among the patients with stage II–IIIA NSCLC in the combined-biomarker-evaluable population (BEP) according to the SP263 assay or among the 22C3-BEP according to the 22C3 assay. (B) Venn diagrams show the percentage of patients in the stage II–IIIA combined-BEP that were defined as having tumors that were PD-L1 positive or PD-L1 high according to both, just one, or neither of the SP263 and 22C3 assays. TC, tumor cell; TPS, tumor proportion score.
Next, we assessed the stage II–IIIA combined-BEP for the overlap of patients based on each PD-L1 assay. When using a cut-off of TC≥1% by SP263 and TPS≥1% by 22C3, 46.4% (243/524) of patients were identified as having PD-L1-positive tumors by both assays. Each assay identified a unique population of patients who were non-overlapping between the assays; 5.5% (29/524) of the patients were defined as SP263 PD-L1 positive (TC≥1%) but 22C3 PD-L1 negative (TPS<1%), and 11.8% (62/524) of the patients were defined as 22C3 PD-L1 positive (TPS≥1%) but SP263 PD-L1 negative (TC<1%). Finally, 36.3% (190/524) of patients were characterized as PD-L1 negative by both assays (TC<1% by SP263 and TPS<1% by 22C3). In total, at the PD-L1 TC or TPS≥1% cut-off, 82.6% (433/524) of patients were characterized into the same PD-L1 tumor expression level category by both assays (figure 2B).
When tumor samples were characterized using a cut-off of PD-L1 TC≥50% by SP263 and TPS≥50% by 22C3, 19.8% (104/524) of patients were identified as having PD-L1-high tumors by both assays. Each assay also identified a unique population of patients who were non-overlapping between the assays at this PD-L1 cut-off: the SP263 assay identified 4.6% (24/524) of samples as PD-L1 high (TC≥50%) that were TPS<50% by 22C3, and the 22C3 assay identified 3.6% (19/524) of samples as PD-L1 high (TPS≥50%) that were TC<50% by SP263. Lastly, 71.9% (377/524) of patients did not have PD-L1-high tumors according to both assays (TC<50% by SP263 and TPS<50% by 22C3). In total, 91.8% (481/524) of patients were characterized into the same PD-L1 tumor expression level category by both the 22C3 and SP263 assays at the PD-L1 TC or TPS≥50% expression level cut-off (figure 2B).
Since patients in IMpower010 were stratified by SP142,6 we also examined the overlap of patients identified as PD-L1 positive or PD-L1 high using SP142 with that of SP263 or 22C3 in the combined-BEP. When comparing the SP142 and SP263 assays, 50.4% (264/524) of patients were identified as having PD-L1-positive tumors by both assays (TC1/2/3 or IC1/2/3 by SP142 and TC≥1% by assay) and 17.4% (91/524) of patients were identified as having PD-L1-high tumors by both assays (TC3 or IC3 by SP142 and TC≥50% by SP263). In total, the SP142 and SP263 assays characterized 54.8% (287/524) of patients into the same PD-L1 tumor expression level category at the PD-L1-positive cut-off and 80.7% (423/524) of patients into the same PD-L1 tumor expression level category at the PD-L1-high cut-off (online supplemental figure 1A,B). When comparing the SP142 and 22C3 assays, 57.1% (299/524) of patients were identified as having PD-L1-positive tumors by both assays (TC1/2/3 or IC1/2/3 by SP142 and TPS≥1% by 22C3) and 16.2% of patients (85/524) were identified as having PD-L1-high tumors by both assays (TC3 or IC3 by SP142 and TPS≥50% by 22C3). In total, the SP142 and 22C3 assays characterized 61.8% (324/524) of patients into the same PD-L1 tumor expression level category at the PD-L1-positive cut-off and 79.4% (416/524) of patients into the same PD-L1 tumor expression level category at the PD-L1-high cut-off (online supplemental figure 1C,D).
jitc-2023-007047supp001.pdf (201.7KB, pdf)
jitc-2023-007047supp002.pdf (1.3MB, pdf)
The baseline characteristics among the SP263-defined tumor PD-L1 subgroups (TC≥1%, TC 1%–49%, TC≥50%) in the stage II–IIIA patients were generally well balanced (<10% difference in prevalence) between treatment arms. However, there were a few exceptions. In the PD-L1 TC 1%–49% subgroup, the atezolizumab arm had a higher proportion of ECOG PS 1 and stage III patients compared with the BSC arm, while the BSC arm had a higher proportion of stage II, ECOG PS 0, and patients aged ≥65 years compared with the atezolizumab arm (table 1). Within the PD-L1 TC≥50% subgroup, the only imbalance observed was for ECOG PS, where there was a higher proportion of patients with an ECOG PS 0 in the atezolizumab arm and a higher proportion of patients with an ECOG PS 1 in the BSC arm (table 1).
Table 1.
Demographics and baseline characteristics among patients with stage II–IIIA non-small cell lung cancer in the combined-biomarker-evaluable population (BEP) when tumor cell programmed death-ligand 1 (PD-L1) expression was defined by the SP263 assay and in the 22C3-BEP when tumor PD-L1 expression was defined by the 22C3 assay.
| Characteristic | Assay | PD-L1 TC or TPS ≥1% |
PD-L1 TC or TPS 1%–49% |
PD-L1 TC or TPS ≥50% |
|||
| Atezo | BSC | Atezo | BSC | Atezo | BSC | ||
| n | SP263 | 142 | 130 | 76 | 68 | 66 | 62 |
| 22C3 | 157 | 149 | 95 | 88 | 62 | 61 | |
| Age, median (range), years | SP263 | 61 (34–80) | 61 (26–84) | 60 (40–80) | 63 (26–84) | 62 (34–76) | 60 (36–79) |
| 22C3 | 62 (34–80) | 62 (26–84) | 62 (40–80) | 62 (26–84) | 62 (34–76) | 62 (39–79) | |
| Age, ≥65 years | SP263 | 51 (35.9) | 48 (36.9) | 27 (35.5) | 31 (45.6) | 24 (36.4) | 17 (27.4) |
| 22C3 | 57 (36.3) | 58 (38.9) | 36 (37.9) | 36 (40.9) | 21 (33.9) | 22 (36.1) | |
| Sex, male | SP263 | 98 (69.0) | 88 (67.7) | 47 (61.8) | 44 (64.7) | 51 (77.3) | 44 (71.0) |
| 22C3 | 107 (68.2) | 103 (69.1) | 63 (66.3) | 55 (62.5) | 44 (71.0) | 48 (78.7) | |
| Race, white | SP263 | 133 (93.7) | 126 (96.9) | 72 (94.7) | 66 (97.1) | 61 (92.4) | 60 (96.8) |
| 22C3 | 148 (94.3) | 145 (97.3) | 89 (93.7) | 86 (97.7) | 59 (95.2) | 59 (96.7) | |
| ECOG PS 0 | SP263 | 76 (53.5) | 73 (56.2) | 35 (46.1) | 42 (61.8) | 41 (62.1) | 31 (50.0) |
| 22C3 | 87 (55.4) | 87 (58.4) | 52 (54.7) | 54 (61.4) | 35 (56.5) | 33 (54.1) | |
| Histology, squamous | SP263 | 62 (43.7) | 56 (43.1) | 35 (46.1) | 26 (38.2) | 27 (40.9) | 30 (48.4) |
| 22C3 | 71 (45.2) | 69 (46.3) | 45 (47.4) | 37 (42.0) | 26 (41.9) | 32 (52.5) | |
| Histology, non-squamous | SP263 | 80 (56.3) | 74 (56.9) | 41 (54.0) | 42 (61.8) | 39 (59.1) | 32 (51.6) |
| 22C3 | 86 (54.8) | 80 (53.7) | 50 (52.6) | 51 (58.0) | 36 (58.1) | 29 (47.5) | |
| Stage, II | SP263 | 67 (47.2) | 69 (53.1) | 33 (43.4) | 37 (54.4) | 34 (51.5) | 32 (51.6) |
| 22C3 | 81 (51.6) | 80 (53.7) | 51 (53.7) | 48 (54.5) | 30 (48.4) | 32 (52.5) | |
| Stage, IIIA | SP263 | 75 (52.8) | 61 (46.9) | 43 (56.6) | 31 (45.6) | 32 (48.5) | 30 (48.4) |
| 22C3 | 76 (48.4) | 69 (46.3) | 44 (46.3) | 40 (45.5) | 32 (51.6) | 29 (47.5) | |
| Smoker status, current or previous | SP263 | 118 (83.1) | 109 (83.8) | 61 (80.3) | 54 (79.4) | 57 (86.4) | 55 (88.7) |
| 22C3 | 132 (84.1) | 124 (83.2) | 78 (82.1) | 67 (76.1) | 54 (87.1) | 57 (93.4) | |
| EGFR mutation status, positive | SP263 | 10 (7.0) | 9 (6.9) | 8 (10.5) | 4 (5.9) | 2 (3.0) | 5 (8.1) |
| 22C3 | 8 (5.1) | 12 (8.1) | 6 (6.3) | 8 (9.1) | 2 (3.2) | 4 (6.6) | |
| EGFR mutation status, negative | SP263 | 67 (47.2) | 64 (49.2) | 33 (43.4) | 36 (52.9) | 34 (51.5) | 28 (45.2) |
| 22C3 | 75 (47.8) | 69 (46.3) | 41 (43.2) | 43 (48.9) | 34 (54.8) | 26 (42.6) | |
| ALK rearrangement status, positive | SP263 | 3 (2.1) | 6 (4.6) | 2 (2.6) | 5 (7.4) | 1 (1.5) | 1 (1.6) |
| 22C3 | 2 (1.3) | 6 (4.0) | 1 (1.1) | 5 (5.7) | 1 (1.6) | 1 (1.6) | |
| ALK rearrangement status, negative | SP263 | 69 (48.6) | 59 (45.4) | 36 (47.4) | 30 (44.1) | 33 (50.0) | 29 (46.8) |
| 22C3 | 74 (47.1) | 67 (45.0) | 39 (41.1) | 39 (44.3) | 35 (56.5) | 28 (45.9) | |
Values reported are n (%) unless otherwise specified.
Atezo, atezolizumab; BSC, best supportive care; ECOG PS, Eastern Cooperative Oncology Group performance status; TC, tumor cell; TPS, tumor proportion score.
In the 22C3-BEP, baseline characteristics among the patients defined by tumor PD-L1 subgroups within the stage II–IIIA NSCLC population were well balanced between treatment arms (<10% difference in prevalence; table 1). The only exception observed was within the PD-L1 TPS≥50% subgroup, where a higher proportion of patients in the atezolizumab arm had non-squamous histology, a negative ALK mutation status, and a negative EGFR mutation status, and a greater proportion of patients in the BSC arm had squamous histology and an unknown ALK mutation status (table 1).
Finally, we evaluated the baseline characteristics in the patients defined by the SP263 assay within the non-22C3-BEP (online supplemental table 1) and observed a few of the categories with ≥10% imbalances between arms in the three PD-L1 subgroups. Specifically, in the PD-L1 TC 1%–49% subgroup, the atezolizumab arm had a higher proportion of ECOG PS 0 and stage II patients compared with the BSC arm, while the BSC arm had a higher proportion of ECOG PS 1 and stage III patients compared with the atezolizumab arm. In addition, in the PD-L1 TC≥1% and ≥50% subgroups, the imbalances between arms were mainly observed in categories such as stage, race, age, histology, and EGFR status (online supplemental table 1).
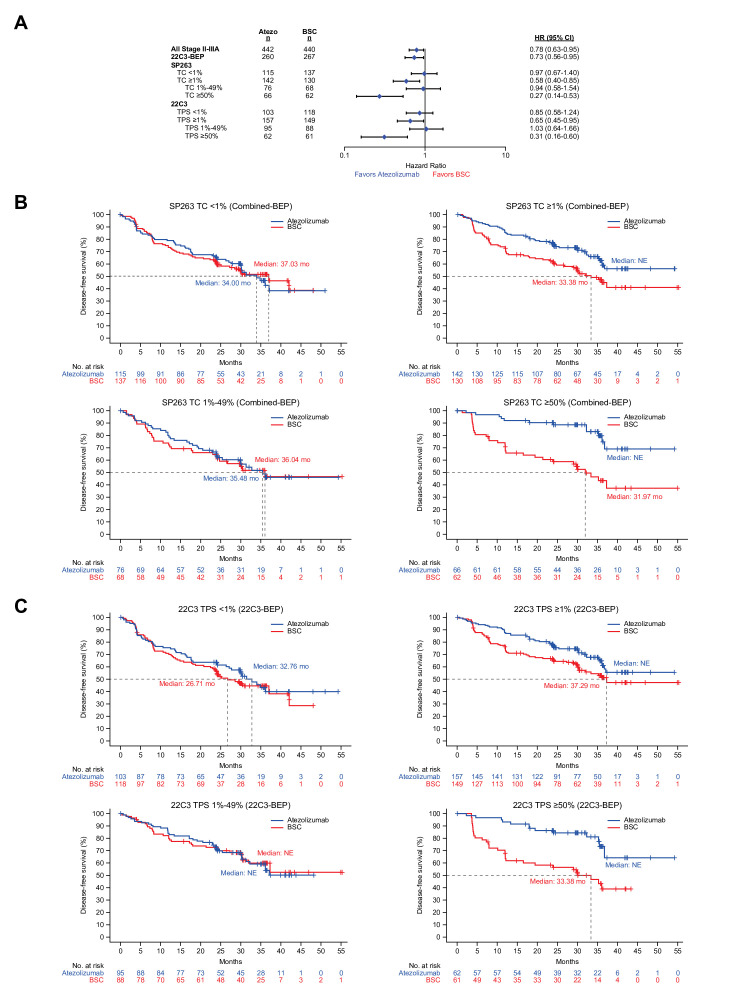
At data cut-off (January 21, 2021), clinical outcomes for atezolizumab compared with BSC among the stage II–IIIA 22C3-BEP (n=527) were similar to those of all stage II–IIIA patients (n=882), with similar DFS HRs (0.73; 95% CI: 0.56 to 0.95 vs 0.78; 95% CI: 0.63 to 0.95; figure 3A). When patient tumors were defined according to PD-L1 expression levels, DFS HRs for atezolizumab compared with BSC were similar regardless of whether the SP263 assay was used to analyze the combined-BEP or the 22C3 assay was used to analyze the 22C3-BEP (figure 3A). Specifically, for the PD-L1-negative subgroup, the DFS HR was 0.97 (95% CI: 0.67 to 1.40) when subgroups were defined by SP263 (TC<1%) versus 0.85 (95% CI: 0.58 to 1.24) when defined by 22C3 (TPS<1%). In the SP263-defined PD-L1-positive subgroup (TC≥1%), the DFS HR was 0.58 (95% CI: 0.40 to 0.85) versus 0.65 (95% CI: 0.45, 0.95) in the 22C3-defined PD-L1-positive subgroup (TPS≥1%). In the PD-L1-low subgroup, the DFS HR was 0.94 (95% CI: 0.58 to 1.54) per SP263 (TC 1%–49%) versus 1.03 (95% CI: 0.64 to 1.66) per 22C3 (TPS 1%–49%). Finally, in the SP263-defined PD-L1-high subgroup (TC≥50%), the DFS HR was 0.27 (95% CI: 0.14 to 0.53) versus 0.31 (95% CI: 0.16 to 0.60) in the 22C3-defined PD-L1-high subgroup (TPS≥50%). Likewise, Kaplan-Meier estimates for the atezolizumab and BSC arms among patients with stage II–IIIA NSCLC in the combined-BEP and 22C3-BEP (figure 3B,C) were similar regardless of whether tumor PD-L1 expression (PD-L1 negative, positive, low, or high), was identified by the SP263 or 22C3 assay, respectively.
Figure 3.
(A) Unstratified HRs for disease-free survival (DFS) observed for all stage II–IIIA patients, the 22C3-biomarker-evaluable population (BEP), and programmed death-ligand 1 (PD-L1) expression level subgroups in the combined-BEP by the SP263 assay and in the 22C3-BEP by the 22C3 assay. (B) DFS Kaplan-Meier curves for PD-L1 expression subgroups in the combined-BEP by the SP263 assay (C) and in the 22C3-BEP by the 22C3 assay. BSC, best supportive care; NE, non-estimable; TC, tumor cell; TPS, tumor proportion score.
Given the smaller BEP for the 22C3 assay compared with SP263, we also analyzed the non-22C3-BEP to provide additional context for interpreting the results described above. Among the non-22C3-BEP, the DFS treatment effect was an HR of 0.98 (95% CI: 0.58 to 1.67) in the PD-L1-negative (TC<1%) subgroup and an HR of 0.76 (95% CI: 0.49 to 1.16) in the PD-L1-positive (TC≥1%) subgroup. Among those in the PD-L1-positive subgroup, the treatment effect was 0.75 (95% CI: 0.42 to 1.32) for the PD-L1-low (TC 1%–49%) subgroup and 0.71 (95% CI: 0.37 to 1.37) for the PD-L1-high (TC≥50%) subgroup (online supplemental figure 2A,B).
Discussion
This retrospective analysis of the IMpower010 trial showed high assay concordance and a similar predictive value of the SP263 and 22C3 PD-L1 assays in demonstrating atezolizumab benefit in patients with early-stage NSCLC after surgery and adjuvant platinum-based chemotherapy. The assays had high concordance, identifying 83% of the same patients as having PD-L1-positive or PD-L1-negative (double positive or double negative) tumors and 92% of the same patients as having PD-L1-high or not PD-L1-high (double positive or double negative) tumors. These data expand on previous studies, primarily in advanced NSCLC, that showed concordance between the SP263 and 22C3 assays19–21 and demonstrate atezolizumab’s benefit over BSC in patients with PD-L1-positive or PD-L1-high tumors, regardless of which assay was used to define tumor PD-L1 expression levels in subgroups of the IMpower010 population. In this study, the SP142 assay was used to stratify patients by PD-L1 expression, but the assay was not used for primary efficacy analysis.6 Consistent with analyses in advanced NSCLC, the concordance of SP142 with SP263 or 22C3 was lower than that between SP263 and 22C3.19 20 24
While the 22C3 assay was used to analyze the 22C3-BEP and the SP263 assay was used to analyze the combined-BEP, these populations differed only by three additional patients in the 22C3-BEP; baseline characteristics were similar between groups (table 1). Accordingly, clinical differences between the 22C3-BEP and combined-BEP reflect subtle distinctions in each assay’s tumor cell PD-L1 identification. Despite the current study using a subgroup of patients who were enrolled in the IMpower010 study, the combined-BEP and 22C3-BEP are generally representative of the ITT population and have similar clinical outcomes to the PD-L1 subgroups of the ITT population.6 In contrast to the 22C3-BEP and combined-BEP, there was a smaller difference in DFS treatment effect between the PD-L1-high and PD-L1-low subgroups in the non-22C3-BEP (online supplemental figure 2A,B). The treatment effect of the PD-L1-low subgroup in the non-22C3-BEP was numerically greater than that in the 22C3-BEP or combined-BEP, while the treatment effect in the PD-L1-high subgroup of the non-22C3-BEP was numerically lower than that in the 22C3-BEP or combined-BEP. These results are likely to be driven by the chance selection of patients in the non-22C3-BEP and variation of HR estimation in subgroups of moderate sizes (online supplemental figure 2A,B). However, the key finding in this analysis is that the SP263 and 22C3 assays have a high degree of patient-level concordance, and PD-L1 subgroups selected by both assays have highly comparable clinical efficacy.
Nivolumab in combination with chemotherapy is currently the only US Food and Drug Administration-approved neoadjuvant immunotherapy for early-stage NSCLC.25 Although a PD-L1 efficacy dependence was observed, a trend for event-free survival improvement was noted for the PD-L1-negative subgroup, and nivolumab plus chemotherapy is approved in the US regardless of PD-L1 expression level.26 Further, for patients with stage IB–IIIA NSCLC, pembrolizumab improved DFS compared with placebo as adjuvant therapy following resection, regardless of PD-L1 expression.27 Despite not reaching statistical significance, treatment with pembrolizumab resulted in an 18% reduction in the risk of disease recurrence or death in the PD-L1-high population, highlighting the need for a better understanding of the clinical data across PD-L1 expression levels using currently available assays in the early lung cancer setting.27
This analysis of the IMpower010 study offers the first evidence of assay concordance in patients with early-stage NSCLC, a population for whom correctly defining tumor PD-L1 expression may influence treatment decisions. Both the SP26310 and 22C317 assays are approved to assess PD-L1 expression on tumor cells and are routinely used in clinical practice. The comparable clinical efficacy of atezolizumab over BSC across PD-L1 expression subgroups further supports the use of either assay to identify patients with early-stage NSCLC who are most likely to experience benefit from adjuvant atezolizumab.
Acknowledgments
We thank the patients and their families, Dr Masao Harada, and Vy Ma and Ivette Estay from the biomarker operation team. Medical writing assistance for this manuscript was provided by Marcia Gamboa, PhD, of Health Interactions and funded by F. Hoffmann-La Roche Ltd.
Footnotes
CZ and MKS contributed equally.
MDT and SN contributed equally.
Contributors: CZ: conceptualization, methodology, validation, investigation, resources, writing—reviewing and editing, project administration. CZ is responsible for the overall content and accepts full responsibility for the finished work and/or the conduct of the study, had access to the data, and controlled the decision to publish. MKS: formal analysis, writing—original draft, writing—reviewing and editing, visualization. HX: validation, formal analysis, writing—reviewing and editing. EF: conceptualization, resources, writing—reviewing and editing, supervision. HW: resources, writing—reviewing and editing, supervision. NA: writing—reviewing and editing. MR: conceptualization, resources, writing—reviewing and editing. RL: writing—reviewing and editing. AK: writing—reviewing and editing. SO: resources, writing—reviewing and editing. HT: writing—reviewing and editing. JH: investigation and writing—reviewing and editing. SLM: writing—reviewing and editing. EB: conceptualization, investigation, data curation, writing—reviewing and editing. BG: writing—reviewing and editing. VM: writing—reviewing and editing. MB: conceptualization, writing—original draft, and writing—reviewing and editing, supervision. MM: conceptualization, methodology, investigation, writing—reviewing and editing, supervision. WZ: software, formal analysis, writing—reviewing and editing, visualization. MDT: conceptualization, methodology, validation, formal analysis, investigation, data curation, writing—original draft, writing—reviewing and editing, visualization, supervision, project administration, funding acquisition. SN: resources, and writing—reviewing and editing.
Funding: This work was supported by F. Hoffmann-La Roche Ltd/Genentech, Inc, a member of the Roche Group. Editorial support, funded by the sponsor, was provided by an independent medical writer under the guidance of the authors.
Competing interests: CZ reports consulting fees from Innovent Biologics, Qilu, Hengrui, and TopAlliance Biosciences Inc; and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Eli Lilly China, Sanofi, Boehringer Ingelheim, Roche, MSD, Qilu, Hengrui, Innovent Biologics, C-Stone LUYE Pharma, TopAlliance Biosciences Inc, Amoy Diagnostics, and AnHeart. MS reports employment by Genentech and stockholding in Roche. HX reports stockholding in Roche as a former Roche employee. EF reports grants or contracts from Merck Healthcare KGaA and Fundación Merck Salud; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Amgen, AstraZeneca, Bristol Myers Squibb, Eli Lilly, F. Hoffman-La Roche, Janssen, Medical Trends, Medscape, Merck Serono, Merck Sharp & Dohme, PeerVoice, Pfizer, Sanofi, Takeda, and Touch Oncology; and is an independent member of the board for Grífols. HW reports grants or contracts from ACEA Biosciences, Arrys Therapeutics, AstraZeneca/Medimmune, Bristol Myers Squibb, Clovis Oncology, Genentech/Roche, Merck, Novartis, SeaGen, Xcovery, and Helsinn; participation on a data safety monitoring board or advisory board for AstraZeneca, Janssen, Daiichi Sankyo, Blueprint, Mirati, Merck, and Genentech/Roche; and leadership or fiduciary role in other board, society, committee, or advocacy group (paid or unpaid) for International Association for the Study of Lung Cancer (IASLC) and ECOG-ARIN. MR reports consulting fees from Amgen, AstraZeneca, BeiGene, Bristol Myers Squibb, Boehringer-Ingelheim, Daiichi Sankyo, GSK, Mirati, Merck, MSD, Novartis, Pfizer, Sanofi, and Roche; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Amgen, AstraZeneca, BeiGene, Bristol Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, GSK, Mirati, Merck, MSD, Novartis, Pfizer, Sanofi, and Roche; support for attending meetings and/or travel from Amgen, AstraZeneca, BeiGene, Bristol Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, GSK, Mirati, Merck, MSD, Novartis, Pfizer, Sanofi, and Roche; and participation on a data safety monitoring board or advisory board for Sanofi and Daiichi Sankyo. SO reports grants or contracts from AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Chugai Pharmaceuticals, Daiichi Sankyo, Ono Pharmaceutical, Pfizer, MSD, Sanofi, Taiho, and Takeda. Hiroshi Tanaka reports grants or contracts from Chugai Pharmaceuticals, AstraZeneca, MSD, Ono Pharmaceutical, and Bristol Myers Squibb; and honoraria for Chugai Pharmaceuticals, AstraZeneca, MSD, Ono Pharmaceutical, and Bristol Myers Squibb. SLM reports support from Genentech as a research site for this trial. EB reports employment by Genentech and stockholding in Roche. BG reports employment by Genentech and stockholding in Roche. VM reports employment by Roche and stockholding in Roche. MB reports employment by Genentech and stockholding in Roche. MM reports being a former employee of Roche/Genentech. WZ reports employment by Roche and stockholding in Roche. MDT reports stock or stock options as a former employee of Roche/Genentech. SN reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AstraZeneca, Amgen, Boehringer Ingelheim, Roche, Pfizer, Takeda, Novartis, Sanofi, Janssen, and GSK; and participation on a data safety monitoring board or advisory board for AstraZeneca, Amgen, Boehringer Ingelheim, Roche, Pfizer, Takeda, Novartis, Sanofi, Janssen, and GSK. NA, RL, AK and JH declare no conflicts of interest.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was conducted in accordance with the guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki. The protocol was approved by an institutional review board or an independent ethics committee at each participating site. IRB#16-001589-AM-00021, reference/ID number: IORG0000105. Participants gave informed consent to participate in the study before taking part.
References
- 1. Kim JM, Chen DS. Immune escape to PD-L1/PD-1 blockade: seven steps to success (or failure). Ann Oncol 2016;27:1492–504. 10.1093/annonc/mdw217 [DOI] [PubMed] [Google Scholar]
- 2. Owen DH, Singh N, Ismaila N, et al. Therapy for stage IV non-small-cell lung cancer without driver alterations: ASCO living guideline, version 2022.2. J Clin Oncol 2023;41:e10–20. 10.1200/JCO.22.02124 [DOI] [PubMed] [Google Scholar]
- 3. Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv192–237. 10.1093/annonc/mdy275 [DOI] [PubMed] [Google Scholar]
- 4. Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely Resected non–small-cell lung cancer. N Engl J Med 2004;350:351–60. 10.1056/NEJMoa031644 [DOI] [PubMed] [Google Scholar]
- 5. Pignon J-P, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE collaborative group. J Clin Oncol 2008;26:3552–9. 10.1200/JCO.2007.13.9030 [DOI] [PubMed] [Google Scholar]
- 6. Felip E, Altorki N, Zhou C, et al. Adjuvant Atezolizumab after adjuvant chemotherapy in Resected stage IB-IIIA non-small-cell lung cancer (Impower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 2021;398:1344–57. 10.1016/S0140-6736(21)02098-5 [DOI] [PubMed] [Google Scholar]
- 7. Highlights of Prescribing Information, Available: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761034s042lbl.pdf
- 8. Tecentriq Product Monograph, Available: https://www.rochecanada.com/PMs/Tecentriq/Tecentriq_PM_E.pdf
- 9. Tecentriq . Summary of product characteristics. 2022. Available: https://www.ema.europa.eu/en/documents/product-information/tecentriq-epar-product-information_en.pdf
- 10. FDA Approval Letter: VENTANA PD-L1 (SP263) Assay, Available: https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160046S010A.pdf
- 11. Antonia SJ, Balmanoukian A, Brahmer J, et al. Clinical activity, tolerability, and long-term follow-up of durvalumab in patients with advanced NSCLC. J Thorac Oncol 2019;14:1794–806. 10.1016/j.jtho.2019.06.010 [DOI] [PubMed] [Google Scholar]
- 12. Herbst RS, Baas P, Kim D-W, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540–50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 13. Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a Multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837–46. 10.1016/S0140-6736(16)00587-0 [DOI] [PubMed] [Google Scholar]
- 14. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, Multicentre randomised controlled trial. Lancet 2017;389:255–65. 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med 2015;373:1627–39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non–small-cell lung cancer. N Engl J Med 2015;372:2018–28. 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 17. PD-L1 IHC 22C3 PharmDx Premarket Approval, Available: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P150013S014
- 18. Gadgeel S, Hirsch FR, Kerr K, et al. Comparison of Sp142 and 22C3 immunohistochemistry PD-L1 assays for clinical efficacy of atezolizumab in non-small cell lung cancer: results from the randomized OAK trial. Clin Lung Cancer 2022;23:21–33. 10.1016/j.cllc.2021.05.007 [DOI] [PubMed] [Google Scholar]
- 19. Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol 2017;12:208–22. 10.1016/j.jtho.2016.11.2228 [DOI] [PubMed] [Google Scholar]
- 20. Tsao MS, Kerr KM, Kockx M, et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of blueprint phase 2 project. Journal of Thoracic Oncology 2018;13:1302–11. 10.1016/j.jtho.2018.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ratcliffe MJ, Sharpe A, Midha A, et al. Agreement between programmed cell death Ligand-1 diagnostic assays across multiple protein expression cutoffs in non–small cell lung cancer. Clin Cancer Res 2017;23:3585–91. 10.1158/1078-0432.CCR-16-2375 [DOI] [PubMed] [Google Scholar]
- 22. Ventana Medical Systems Inc . VENTANA PD-L1 (Sp263) assay staining of non-small cell lung cancer. 2019. Available: https://www.rochebiomarkers.be/content/media/Files/PD-L1_SP263_interpretation_guide_NSCLC.pdf
- 23. Agilent Dako . PD-L1 IHC 22C3 pharmDx interpretation manual - NSCLC. 2021. Available: https://www.agilent.com/cs/library/usermanuals/public/29158_pd-l1-ihc-22C3-pharmdx-nsclc-interpretation-manual.pdf
- 24. Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med 2020;383:1328–39. 10.1056/NEJMoa1917346 [DOI] [PubMed] [Google Scholar]
- 25. FDA approves neoadjuvant nivolumab and platinum-doublet chemotherapy for early-stage non-small cell lung cancer, Available: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-neoadjuvant-nivolumab-and-platinum-doublet-chemotherapy-early-stage-non-small-cell-lung
- 26. Forde PM, Spicer J, Girard N. Neoadjuvant nivolumab plus chemotherapy in lung cancer. Reply. N Engl J Med 2022;387:572–3. 10.1056/NEJMc2208133 [DOI] [PubMed] [Google Scholar]
- 27. Paz-Ares L, O’Brien MER, Mauer M, et al. Vp3-2022: Pembrolizumab (Pembro) versus placebo for early-stage non-small cell lung cancer (NSCLC) following complete resection and adjuvant chemotherapy (Chemo) when indicated: randomized, triple-blind, phase III EORTC-1416-LCG/ETOP. Ann Oncol 2022;33:451–3. 10.1016/j.annonc.2022.02.224 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2023-007047supp001.pdf (201.7KB, pdf)
jitc-2023-007047supp002.pdf (1.3MB, pdf)
Data Availability Statement
Data are available upon reasonable request. Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).