Abstract
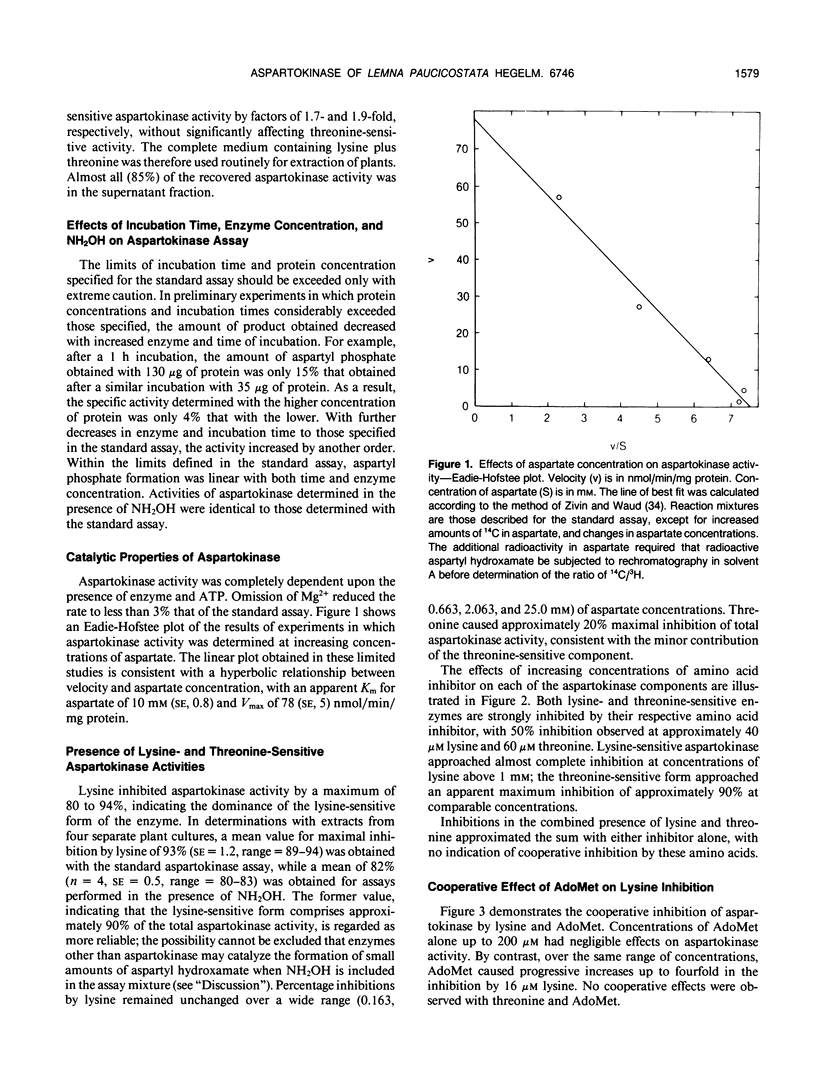
A sensitive and specific method was developed for assay of aspartokinase (EC 2.7.2.4) in crude extracts of Lemna paucicostata. Lysine inhibited approximately 93%, and threonine approximately 6%; together, these amino acids inhibited 99%. Inhibition by lysine was synergistically increased by S-adenosylmethionine, which by itself had no effect on activity. Essentially complete inhibition of threonine-resistant activity was obtained with lysine, and of lysine-resistant activity with threonine. Inhibition by lysine and threonine was additive, with no indication of concerted inhibition. Aspartate concentration had no effect on the relative proportions of lysine- and threonine-sensitive activities. Aspartokinase activity was in large excess of that reported by other workers, the maximum capacity (Vmax) far exceeding the in vivo requirements. Estimations of rates of aspartokinase in vivo suggest that the step catalyzed by this enzyme may not be the overall `rate-limiting' one for entry of 4-carbon units into the aspartate family of amino acids, and that feedback inhibition of this enzyme by lysine and threonine may not be a major factor in regulating flux through this step.
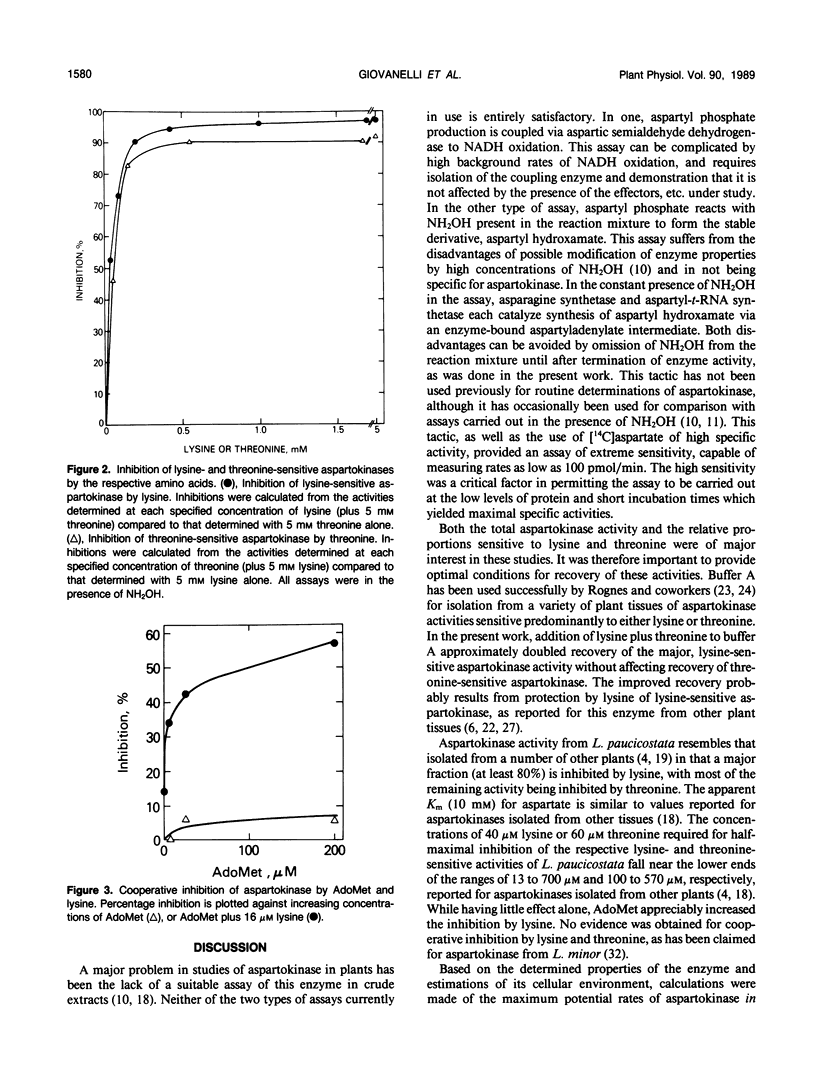
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bright S. W., Leggatt M. M., Miflin B. J. Amino Acid levels in carrot cell suspension culture: no correlation with aspartate kinase isoenzyme levels. Plant Physiol. 1979 Mar;63(3):586–588. doi: 10.1104/pp.63.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman D. J., Leech R. M. Changes in Pool Sizes of Free Amino Acids and Amides in Leaves and Plastids of Zea mays during Leaf Development. Plant Physiol. 1979 Mar;63(3):567–572. doi: 10.1104/pp.63.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H., Giovanelli J. Lemna paucicostata Hegelm. 6746: DEVELOPMENT OF STANDARDIZED GROWTH CONDITIONS SUITABLE FOR BIOCHEMICAL EXPERIMENTATION. Plant Physiol. 1980 May;65(5):906–912. doi: 10.1104/pp.65.5.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H. Methionine biosynthesis in lemna: inhibitor studies. Plant Physiol. 1982 May;69(5):1070–1076. doi: 10.1104/pp.69.5.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H. Responses of Sulfur-Containing Compounds in Lemna paucicostata Hegelm. 6746 to Changes in Availability of Sulfur Sources. Plant Physiol. 1984 Jun;75(2):474–479. doi: 10.1104/pp.75.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta P., Prakash L. Aspartokinase of Rhodopseudomonas spheroides. Regulation of enzyme activity by aspartate beta-semialdehyde. J Biol Chem. 1966 Dec 25;241(24):5827–5835. [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H., Datko A. H. In Vivo Regulation of Threonine and Isoleucine Biosynthesis in Lemna paucicostata Hegelm. 6746. Plant Physiol. 1988 Feb;86(2):369–377. doi: 10.1104/pp.86.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H., Datko A. H. In vivo regulation of de novo methionine biosynthesis in a higher plant (lemna). Plant Physiol. 1985 Feb;77(2):450–455. doi: 10.1104/pp.77.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H., Datko A. H. Regulatory Structure of the Biosynthetic Pathway for the Aspartate Family of Amino Acids in Lemna paucicostata Hegelm. 6746, with Special Reference to the Role of Aspartokinase. Plant Physiol. 1989 Aug;90(4):1584–1599. doi: 10.1104/pp.90.4.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H., Datko A. H., Thompson G. A. Effects of Orthophosphate and Adenosine 5'-Phosphate on Threonine Synthase and Cystathionine gamma-Synthate of Lemna paucicostata Hegelm. 6746. Plant Physiol. 1986 Jun;81(2):577–583. doi: 10.1104/pp.81.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea P. J., Mills W. R., Miflin B. J. The isolation of a lysine-sensitive aspartate kinase from pea leaves and its involvement in homoserine biosynthesis in isolated chloroplasts. FEBS Lett. 1979 Feb 1;98(1):165–168. doi: 10.1016/0014-5793(79)80175-1. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B. Theoretical principles in the approaches to control of metabolic pathways and their application to glycolysis in muscle. J Mol Cell Cardiol. 1979 Sep;11(9):839–856. doi: 10.1016/0022-2828(79)90480-2. [DOI] [PubMed] [Google Scholar]
- Rognes S. E., Lea P. J., Miflin B. J. S-adenosylmethionine--a novel regulator of aspartate kinase. Nature. 1980 Sep 25;287(5780):357–359. doi: 10.1038/287357a0. [DOI] [PubMed] [Google Scholar]
- Shames S. L., Ash D. E., Wedler F. C., Villafranca J. J. Interaction of aspartate and aspartate-derived antimetabolites with the enzymes of the threonine biosynthetic pathway of Escherichia coli. J Biol Chem. 1984 Dec 25;259(24):15331–15339. [PubMed] [Google Scholar]
- Shewry P. R., Miflin B. J. Properties and Regulation of Aspartate Kinase from Barley Seedlings (Hordeum vulgare L.). Plant Physiol. 1977 Jan;59(1):69–73. doi: 10.1104/pp.59.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson G. A., Datko A. H., Mudd S. H., Giovanelli J. Methionine Biosynthesis in Lemna: STUDIES ON THE REGULATION OF CYSTATHIONINE gamma-SYNTHASE, O-PHOSPHOHOMOSERINE SULFHYDRYLASE, AND O-ACETYLSERINE SULFHYDRYLASE. Plant Physiol. 1982 May;69(5):1077–1083. doi: 10.1104/pp.69.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahnbaeck-Spencer R., Mills W. R., Henke R. R., Burdge E. L., Wilson K. G. Intracellular localization of beta-aspartate kinase in spinach (Spinacea oleracea). FEBS Lett. 1979 Aug 15;104(2):303–308. doi: 10.1016/0014-5793(79)80839-x. [DOI] [PubMed] [Google Scholar]
- Wallsgrove R. M., Lea P. J., Miflin B. J. Intracellular localization of aspartate kinase and the enzymes of threonine and methionine biosynthesis in green leaves. Plant Physiol. 1983 Apr;71(4):780–784. doi: 10.1104/pp.71.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. F., Dennis D. T. Aspartokinase from wheat germ: isolation, characterization, and regulation. Plant Physiol. 1973 Feb;51(2):322–326. doi: 10.1104/pp.51.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. F., Dennis D. T. Aspartokinase in Lemna minor L: Studies on the in Vivo and in Vitro Regulation of the Enzyme. Plant Physiol. 1973 Feb;51(2):327–331. doi: 10.1104/pp.51.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivin J. A., Waud D. R. How to analyze binding, enzyme and uptake data: the simplest case, a single phase. Life Sci. 1982 Apr 26;30(17):1407–1422. doi: 10.1016/0024-3205(82)90554-9. [DOI] [PubMed] [Google Scholar]


