Abstract
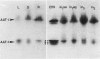
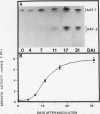
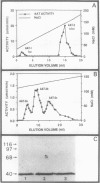
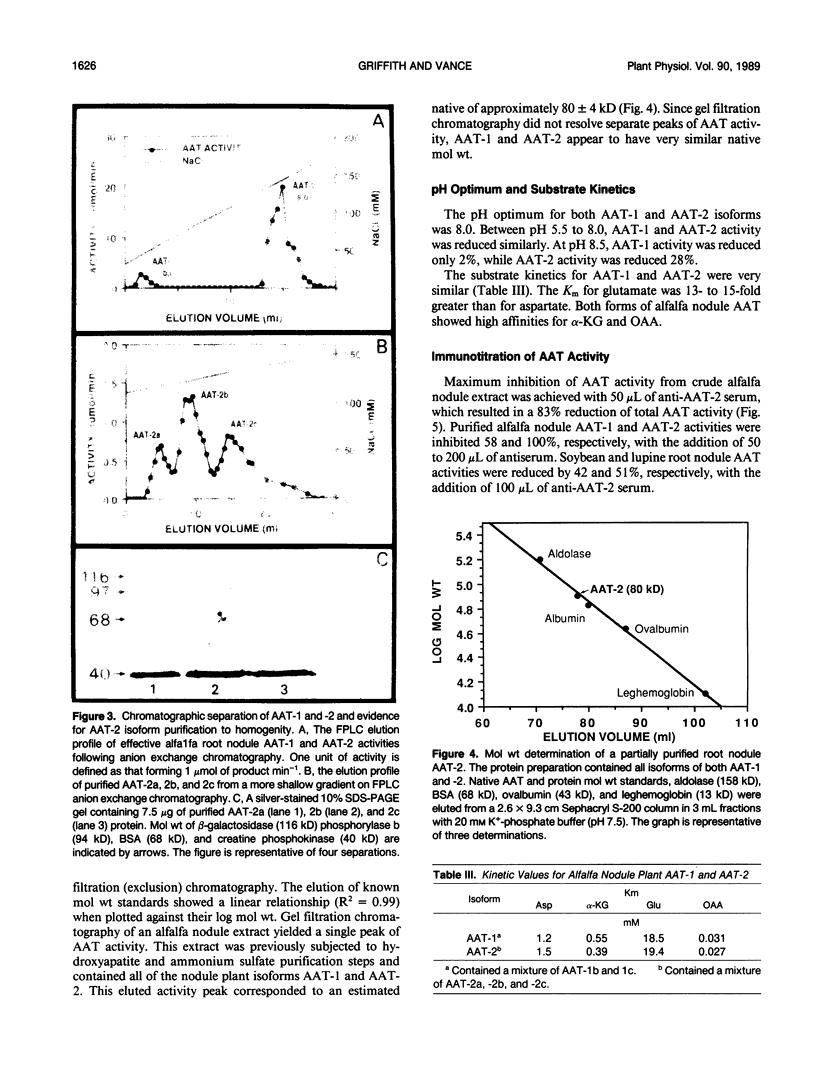
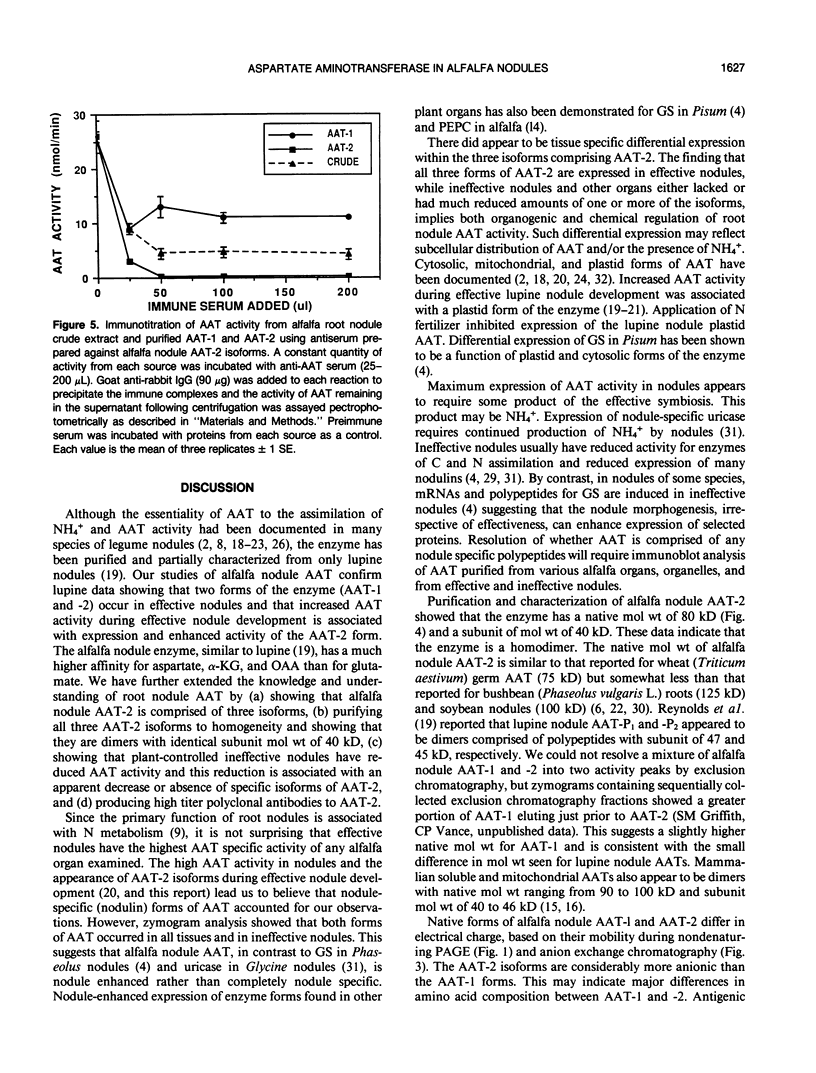
Aspartate aminotransferase (l-aspartate:2-oxoglutarate aminotransferase, EC 2.6.1.1 [AAT]), a key enzyme in the assimilation of C and N compounds, was purified from the cytosol of alfalfa (Medicago sativa L.) root nodules. Isoforms that increased during nodule development, AAT-2a, AAT-2b, and AAT-2c, were purified greater than 447-fold to apparent homogeneity, and high titer polyclonal antibodies were produced. The native molecular weight of the AAT-2 isoforms was approximately 80 kilodatons with a subunit molecular weight of 40 kilodatons, indicating that the holoenzymes are dimers. The AAT-2 isoforms comprised approximately 0.4% of the total soluble nodule protein. The AAT specific activity was measured in leaf, stem, root, and nodule organs, and zymograms of each were compared. Enzyme activity was 4- to 37-fold greater in effective (nitrogen fixing) nodules than in leaves, stems, and roots. Effective nodule AAT-specific activity was 3- to 8-fold greater than that of plant-controlled ineffective nodules. No differences in Km were observed between AAT-1 and AAT-2. Antibodies raised against AAT-2 were more selective against AAT-2 than AAT-1. Evidence obtained from zymograms suggests that the expression of alfalfa nodule AAT is controlled at two different gene loci, AAT-1 and AAT-2, resulting in different dimeric isoforms.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CAMMARATA P. S., COHEN P. P. Spectrophotometric measurement of transamination reactions. J Biol Chem. 1951 Nov;193(1):45–52. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Forest J. C., Wightman F. Amino acid metabolism in plants. 3. Purification and some properties of a multispecific aminotransferase isolated from bushbean seedlings (Phaseolus vulgaris L.). Can J Biochem. 1972 Jul;50(7):813–829. doi: 10.1139/o72-113. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MORINO Y., KAGAMIYAMA H., WADA H. IMMUNOCHEMICAL DISTINCTION BETWEEN GLUTAMIC-OXALOACETIC TRANSAMINASES FROM THE SOLUBLE AND MITOCHONDRIAL FRACTIONS OF MAMMALIAN TISSUES. J Biol Chem. 1964 Mar;239:943–944. [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Miller S. S., Boylan K. L., Vance C. P. Alfalfa Root Nodule Carbon Dioxide Fixation : III. Immunological Studies of Nodule Phosphoenolpyruvate Carboxylase. Plant Physiol. 1987 Jun;84(2):501–508. doi: 10.1104/pp.84.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaru K., Nomiyama H., Shimada K., Nagashima F., Morino Y. Cloning and sequence analysis of mRNA for mouse aspartate aminotransferase isoenzymes. J Biol Chem. 1986 Dec 25;261(36):16976–16983. [PubMed] [Google Scholar]
- Reynolds P. H., Boland M. J., Blevins D. G., Schubert K. R., Randall D. D. Enzymes of amide and ureide biogenesis in developing soybean nodules. Plant Physiol. 1982 Jun;69(6):1334–1338. doi: 10.1104/pp.69.6.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P. H., Boland M. J., Farnden K. J. Enzymes of nitrogen metabolism in legume nodules: partial purification and properties of the aspartate aminotransferases from lupine nodules. Arch Biochem Biophys. 1981 Jul;209(2):524–533. doi: 10.1016/0003-9861(81)90310-6. [DOI] [PubMed] [Google Scholar]
- Scandalios J. G., Sorenson J. C., Ott L. A. Genetic control and intracellular localization of glutamate oxaloacetic transaminase in maize. Biochem Genet. 1975 Dec;13(11-12):759–769. doi: 10.1007/BF00484407. [DOI] [PubMed] [Google Scholar]
- Shaw C. R., Prasad R. Starch gel electrophoresis of enzymes--a compilation of recipes. Biochem Genet. 1970 Apr;4(2):297–320. doi: 10.1007/BF00485780. [DOI] [PubMed] [Google Scholar]
- Shelp B. J., Atkins C. A., Storer P. J., Canvin D. T. Cellular and subcellular organization of pathways of ammonia assimilation and ureide synthesis in nodules of cowpea (Vigna unguiculata L. Walp.). Arch Biochem Biophys. 1983 Jul 15;224(2):429–441. doi: 10.1016/0003-9861(83)90229-1. [DOI] [PubMed] [Google Scholar]
- Snapp S. S., Vance C. P. Asparagine Biosynthesis in Alfalfa (Medicago sativa L.) Root Nodules. Plant Physiol. 1986 Oct;82(2):390–395. doi: 10.1104/pp.82.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta T. C., Faris M. A., Macdowall F. D. Pathways of Nitrogen Metabolism in Nodules of Alfalfa (Medicago sativa L.). Plant Physiol. 1986 Apr;80(4):1002–1005. doi: 10.1104/pp.80.4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verjee Z. H., Evered D. F. Purification and some properties of aspartate aminotransferase from wheat germ. Biochim Biophys Acta. 1969 Jul 8;185(1):103–110. doi: 10.1016/0005-2744(69)90286-1. [DOI] [PubMed] [Google Scholar]