Abstract
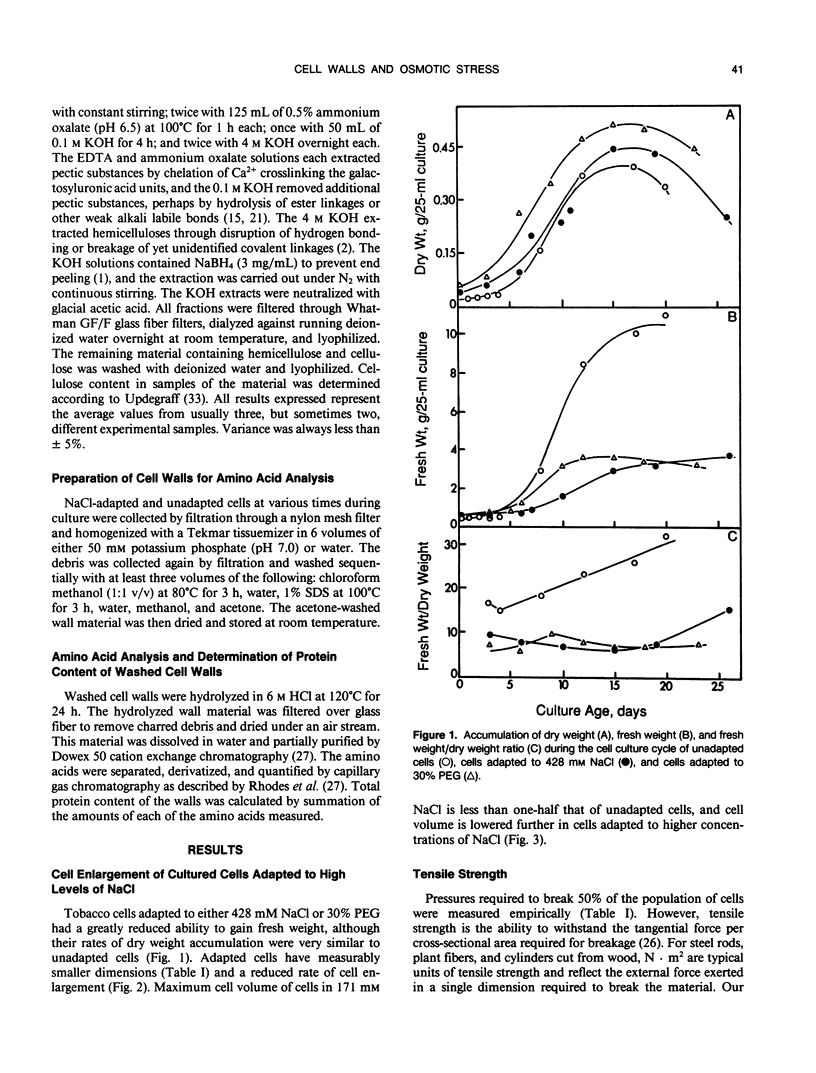
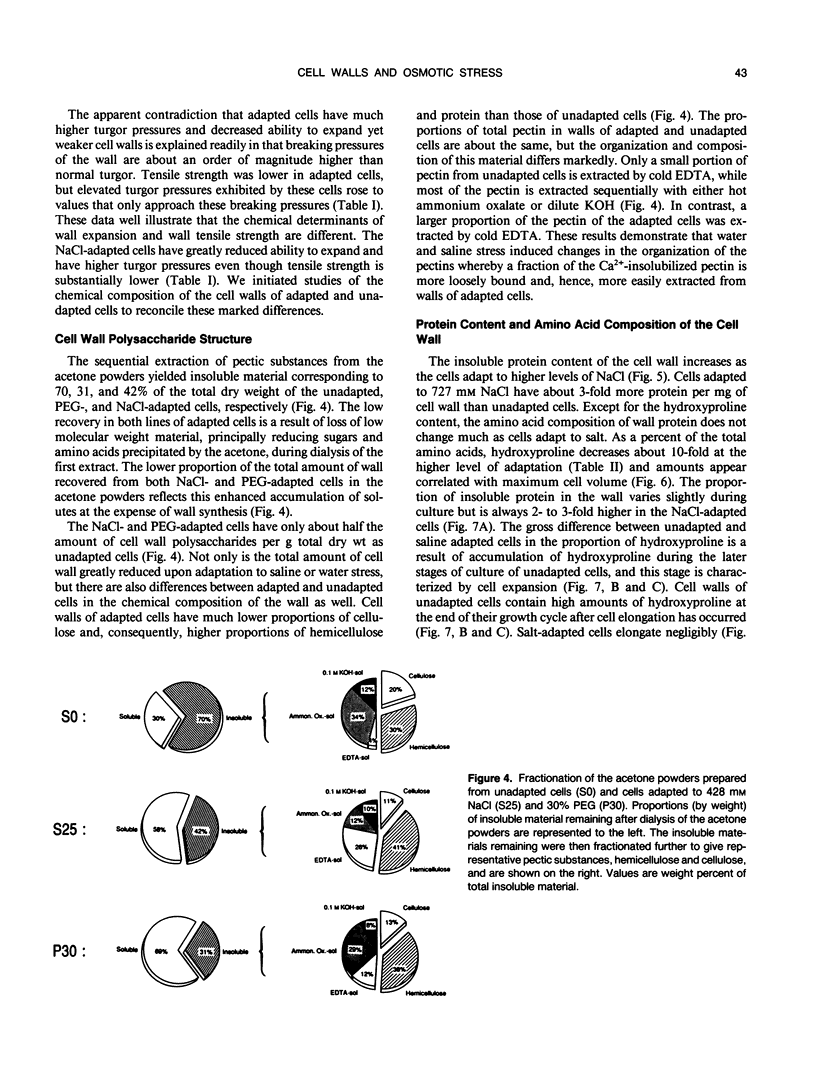
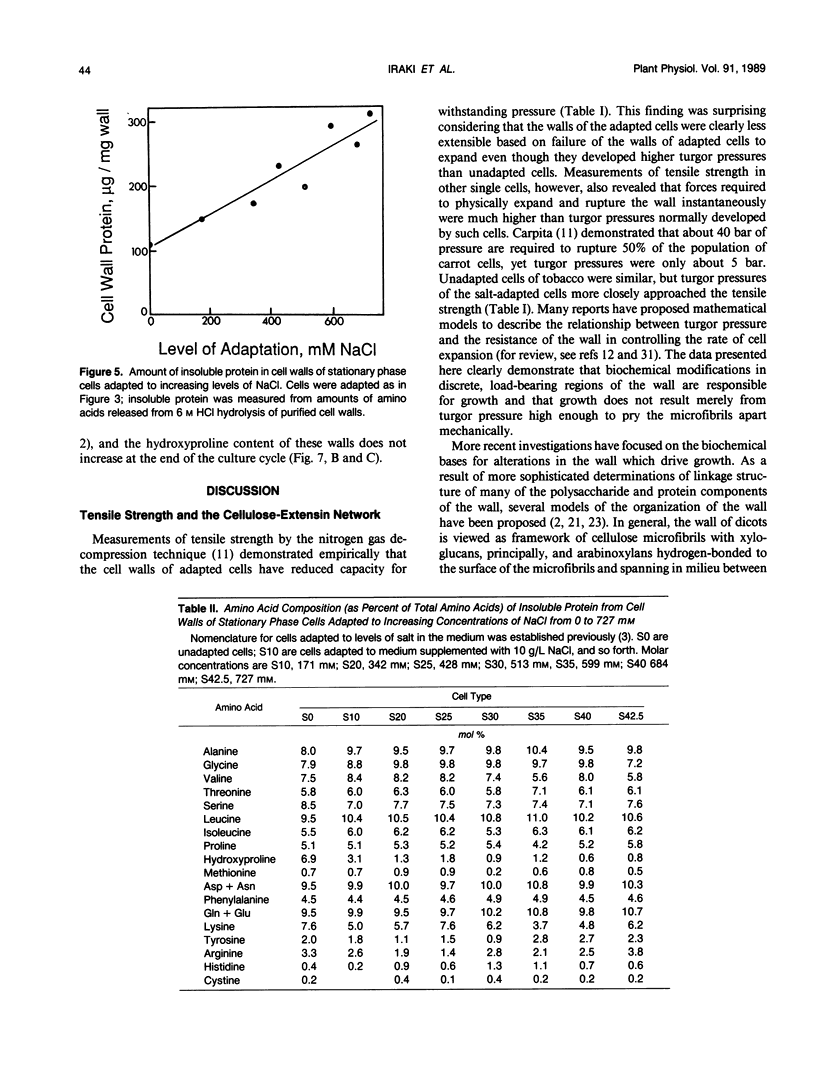
Cells of tobacco (Nicotiana tabacum L.) adapted to grow in severe osmotic stress of 428 millimolar NaCl (−23 bar) or 30% polyethylene glycol 8000 (−28 bar) exhibit a drastically altered growth physiology that results in slower cell expansion and fully expanded cells with volumes only one-fifth to one-eighth those of unadapted cells. This reduced cell volume occurs despite maintenance of turgor pressures sometimes severalfold higher than those of unadapted cells. This report and others (NM Iraki et al [1989] Plant Physiol 90: 000-000 and 000-000) document physical and biochemical alterations of the cell walls which might explain how adapted cells decrease the ability of the wall to expand despite diversion of carbon used for osmotic adjustment away from synthesis of cell wall polysaccharides. Tensile strength measured by a gas decompression technique showed empirically that walls of NaCl-adapted cells are much weaker than those of unadapted cells. Correlated with this weakening was a substantial decrease in the proportion of crystalline cellulose in the primary cell wall. Even though the amount of insoluble protein associated with the wall was increased relative to other wall components, the amount of hydroxyproline in the insoluble protein of the wall was only about 10% that of unadapted cells. These results indicate that a cellulosic-extensin framework is a primary determinant of absolute wall tensile strength, but complete formation of this framework apparently is sacrificed to divert carbon to substances needed for osmotic adjustment. We propose that the absolute mass of this framework is not a principal determinant of the ability of the cell wall to extend.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binzel M. L., Hasegawa P. M., Handa A. K., Bressan R. A. Adaptation of Tobacco Cells to NaCl. Plant Physiol. 1985 Sep;79(1):118–125. doi: 10.1104/pp.79.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binzel M. L., Hasegawa P. M., Rhodes D., Handa S., Handa A. K., Bressan R. A. Solute Accumulation in Tobacco Cells Adapted to NaCl. Plant Physiol. 1987 Aug;84(4):1408–1415. doi: 10.1104/pp.84.4.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binzel M. L., Hess F. D., Bressan R. A., Hasegawa P. M. Intracellular compartmentation of ions in salt adapted tobacco cells. Plant Physiol. 1988 Feb;86(2):607–614. doi: 10.1104/pp.86.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozarth C. S., Mullet J. E., Boyer J. S. Cell wall proteins at low water potentials. Plant Physiol. 1987 Sep;85(1):261–267. doi: 10.1104/pp.85.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressan R. A., Handa A. K., Handa S., Hasegawa P. M. Growth and water relations of cultured tomato cells after adjustment to low external water potentials. Plant Physiol. 1982 Nov;70(5):1303–1309. doi: 10.1104/pp.70.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita N. C. Tensile strength of cell walls of living cells. Plant Physiol. 1985 Oct;79(2):485–488. doi: 10.1104/pp.79.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa A. K., Bressan R. A., Handa S., Hasegawa P. M. Characteristics of cultured tomato cells after prolonged exposure to medium containing polyethylene glycol. Plant Physiol. 1982 Feb;69(2):514–521. doi: 10.1104/pp.69.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa S., Bressan R. A., Handa A. K., Carpita N. C., Hasegawa P. M. Solutes contributing to osmotic adjustment in cultured plant cells adapted to water stress. Plant Physiol. 1983 Nov;73(3):834–843. doi: 10.1104/pp.73.3.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labavitch J. M., Ray P. M. Relationship between Promotion of Xyloglucan Metabolism and Induction of Elongation by Indoleacetic Acid. Plant Physiol. 1974 Oct;54(4):499–502. doi: 10.1104/pp.54.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D., Handa S., Bressan R. A. Metabolic changes associated with adaptation of plant cells to water stress. Plant Physiol. 1986 Dec;82(4):890–903. doi: 10.1104/pp.82.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadava D., Chrispeels M. J. Hydroxyproline-rich cell wall protein (extensin): role in the cessation of elongation in excised pea epicotyls. Dev Biol. 1973 Jan;30(1):49–55. doi: 10.1016/0012-1606(73)90047-x. [DOI] [PubMed] [Google Scholar]
- Stafstrom J. P., Staehelin L. A. Cross-linking patterns in salt-extractable extensin from carrot cell walls. Plant Physiol. 1986 May;81(1):234–241. doi: 10.1104/pp.81.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry M. E., Jones R. L. Soluble Cell Wall Polysaccharides Released from Pea Stems by Centrifugation : I. EFFECT OF AUXIN. Plant Physiol. 1981 Sep;68(3):531–537. doi: 10.1104/pp.68.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updegraff D. M. Semimicro determination of cellulose in biological materials. Anal Biochem. 1969 Dec;32(3):420–424. doi: 10.1016/s0003-2697(69)80009-6. [DOI] [PubMed] [Google Scholar]
- WARDROP A. B. Cell wall organization and the properties of the xylem, I. Cell wall organization and the variation of breaking load in tension of the xylem in conifer stems. Aust J Sci Res B. 1951 Nov;4(4):391–414. [PubMed] [Google Scholar]


