Abstract
The retina is an intricately organized neural tissue built on cone and rod pathways for color and night vision. Genetic mutations that disrupt the proper function of the rod circuit contribute to blinding diseases including retinitis pigmentosa and congenital stationary night blindness (CSNB). Down Syndrome cell adhesion molecule like 1 (Dscaml1) is expressed by rods, rod bipolar cells (RBCs), and sub-populations of amacrine cells, and has been linked to a middle age onset of CSNB in humans. However, how Dscaml1 contributes to this visual deficit remains unexplored. Here, we probed Dscaml1’s role in the maintenance of the rod-to-RBC synapse using a loss of function mouse model. We used immunohistochemistry to investigate the anatomical formation and maintenance of the rod-to-RBC synapse in the young, adult, and aging retina. We generated 3D reconstructions, using serial electron micrographs, of rod spherules and RBCs to measure the number of invaginating neurites, RBC dendritic tip number, and RBC mitochondrial morphology. We find that while rod-to-RBC synapses form and are maintained, similar to wildtype, that there is an increase in the number of invaginating neurites in rod spherules, a reduction in RBC dendritic tips, and reduced mitochondrial volume and complexity in the Dscaml1 mutant retina compared to controls. We also observed precocious sprouting of RBC dendrites into the outer nuclear layer (ONL) of the Dscaml1 mutant retina compared to controls. These results contribute to our knowledge of Dscaml1’s role in rod circuit development and maintenance and give additional insight into possible genetic therapy targets for blinding diseases and disorders like CSNB.
Introduction
The neural retina is composed of two connected circuits: one for color vision, and the other for vision in dim light, or night vision. Some of the common causes of night vision loss (nyctalopia), including vitamin A deficiencies and cataracts, are readily treatable. Nyctalopia resulting from genetic mutations, including those that cause retinitis pigmentosa (RP) and congenital stationary night blindness (CSNB), have been historically more difficult to treat but are current targets for successful gene therapy approaches to restore the target gene function and prevent blindness [1–8]. Examples include X-linked CSNB and Leber congenital amaurosis (LCA), caused by mutations in NYX and GUCY2D (respectively), where patients suffer a decreased ability to see in dimly lit environments that is usually present at birth or develops during adolescence [9–11]. The success of gene therapy approaches in treating genetic causes of vision loss has highlighted the need to identify and better understand the activity of genes linked to blinding diseases in order to expand the pool of candidates for gene therapy trials.
Dscaml1 has been identified as a candidate gene in humans for a middle age of onset of CSNB [12]. Dscaml1 is expressed by and required for normal organization of several cell types in the rod pathway including rod bipolar cells (RBCs) and All amacrine cells. However, ERG studies, gross morphology and synaptic markers suggest that the rod circuit remains functional in a Dscaml1 loss of function mouse model [13]. Studies in mice have shown that wildtype cells and synapses of the rod circuit undergo significant age-related changes including denervation at the rod synapse and ectopic sprouting of horizontal and bipolar cell dendrites [14–16]. We hypothesize that the middle age of onset of CSNB associated with a mutation of Dscaml1 could reflect increased sensitivity to normal aging of the rod circuit, and here test if signs of premature or exaggerated aging are observed in Dscaml1 mutant mice. We therefore used a Dscaml1 loss of function mouse model to test the role of Dscaml1 in establishing precise circuitry of the rod pathway and in maintaining the rod circuit in the aging mouse.
Dscam and Dscaml1 are neural cell adhesion molecules in the immunoglobulin superfamily [17]. Dscams work alongside other cell adhesion molecules to pattern the retina, which is largely organized by differential expression of transcription factors and the adhesion molecules these regulate, as opposed to the neural activity that plays a prominent role in organizing neurons in other regions of the brain [18–21]. Mammalian Dscams play important roles in the spatial arrangement of neural soma and dendrites in bipolar, amacrine and retinal ganglion cells, and also function as a pro-growth factor in the axon pathfinding of retinal ganglion cells into the brain [22–25]. Dscaml1 is expressed by rods, RBCs and sub-populations of amacrine cells, including AII amacrine cells, which convey visual information from RBCs to retinal ganglion cells. These cells make up the core of the rod pathway, which initiates when rods detect photons and send visual information to RBCs at the rod-to-RBC synapse. While Dscaml1 regulates dendritic arborization and soma spacing of RBCs and AII amacrine cells, the rod-to-RBC synapse itself is histologically and functionally intact in the Dscaml1 loss of function mouse model, as measured by immunohistochemistry, electron microscopy and ERG [13].
Here we build on previous work by utilizing a loss of function allele of Dscaml1 to measure the requirement of Dscaml1 for maintenance of the rod-to-rod bipolar cell synapse. We used immunohistochemistry to investigate the development and maintenance of this synapse. We observed similar patterns of retinal maturation in the wildtype and Dscaml1 mutant retina. RBC dendrite sprouting into the outer nuclear layer (ONL), a correlation of aging and synaptic dysfunction, was increased at earlier ages in the Dscaml1 mutant retina compared to wildtype, but similar at advanced ages. Electron micrograph studies revealed an increase in the number of neurite tips invaginating into Dscaml1 mutant rods, a decrease in the number of Dscaml1 RBC dendritic tips and an increase in the number of RBC dendrites that did not contact rod spherules. In summary, we report that anatomical changes were observed comparing organization of RBC dendrites of the wildtype and Dscaml1 mutant retina, alongside subtle signs of precocious aging.
Methods
Animals/Housing
Mice were housed in the University of Idaho Laboratory Animal and Research Facility (LARF). Mice were fed ad libitum and on a 12-hour light:dark cycle. Dscaml1 mutant mice were derived from ES cell line CC0772 [13]. Dscaml1 mutant mice carry a LacZ gene trap in the third exon of Dscaml1 that intercepts and truncates the Dscaml1 transcript early in the N-terminus of the protein [13]. Bax null mice were obtained from the Jackson Laboratory [26]. Mice were maintained on a mixed genetic background including C57BL/6J and 129/SvJ. Wild type litter mate controls were used for EM studies and in Fig 1. In Fig 2 we utilized both Dscaml1 mutant heterozygous litter mate controls and inbred C57BL/6J mice wild type controls, the latter because our aging wild type mice succumbed before the time of study. The mice are broken out into separate pools and no difference was detected comparing litter mate heterozygous controls and C57BL/6J controls. Wild type C57BL/6 mice at 12 and 18-month of age were obtained from the NIH aged rodent center and were from a C57BL/6JN genetic background, which are C57BL/6J mice acquired, bred and periodically refreshed by the NIH from stock acquired from The Jackson Laboratory. These mice differ from the NIH strain C57BL/6N, which carries the rd8 retinal degeneration mutation. Younger wild type mice were bred in house derived from C57BL/6J mice acquired from the Jackson Laboratory. All protocols were approved by the University of Idaho Institutional Care and Use Committee.
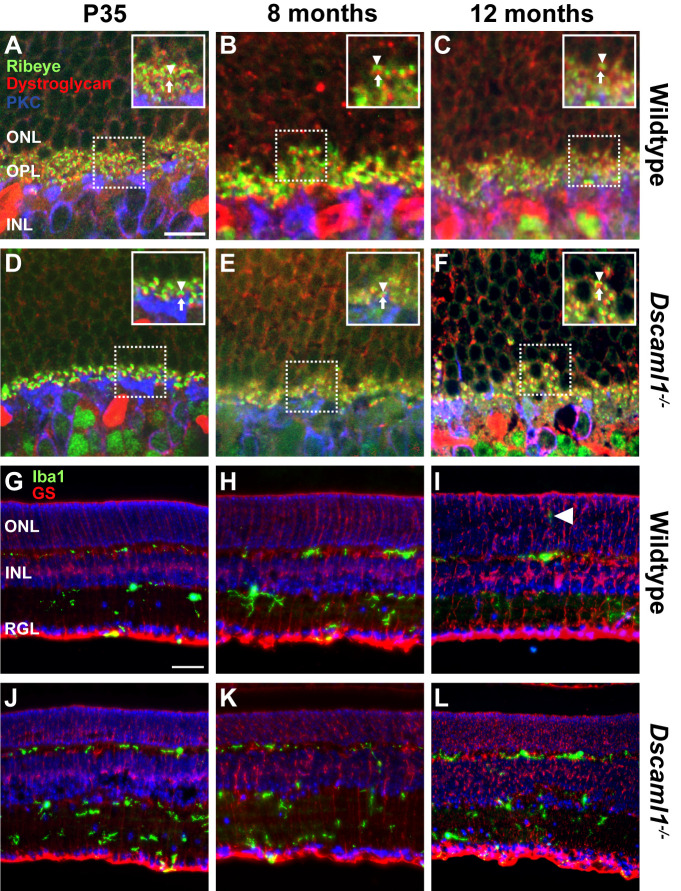
Fig 1. The rod-to-RBC synapse across the mouse lifespan.
A) Representative image of rod-to-RBC synapses formed in the wildtype retina at P35. B) Rod-to-RBC synapses were maintained in the wildtype retina at 8 months. C) Rod-to-RBC synapses remain intact in the wildtype retina at one year. D) Representative image of synapses between rod and RBCs in the Dscaml1 mutant retina at P35. E) Rod-to-RBC synapses were maintained in the Dscaml1 mutant retina at 8 months. F) Rod-to-RBC synapses remain intact in the Dscaml1 mutant retina at one year. No differences were detected comparing wildtype and Dscaml1 mutant retinas across all the ages examined (P14, P21, P35, 3 months, 8 months, 12 months, 18-months). G) Representative image of Müller glia and microglia in the wildtype retina at P35. H) Representative image of Müller glia and microglia in the wildtype retina at 8 months. I) Representative image of Müller glia and microglia in the wildtype retina at one year demonstrating occasional microglia projecting into the ONL (arrowhead). J) Representative image of Müller glia and microglia in the Dscaml1 mutant retina at P35. K) Müller glia and microglia in representative image of the Dscaml1 mutant retina at 8 months. L) Representative image of Müller glia and microglia in the Dscaml1 mutant retina at one year. No differences were observed comparing wildtype and Dscaml1 mutant retinas. Arrowheads (A-F) point to ribeye horseshoe. Arrows (A-F) point to dystroglycan puncta. PKC = rod bipolar cells, dystroglycan = synaptic cleft, ribeye = presynaptic, GS = Müller glia, Iba1 = microglia. Abbreviations: RBC = rod bipolar cell, ONL = outer nuclear layer, OPL = outer plexiform layer, INL = inner nuclear layer. Sample size = 12 to 20 images, across at least 3 mice per genotype at all time points. Scale bar = 20 μm (A-F) and 50 μm (G-L).
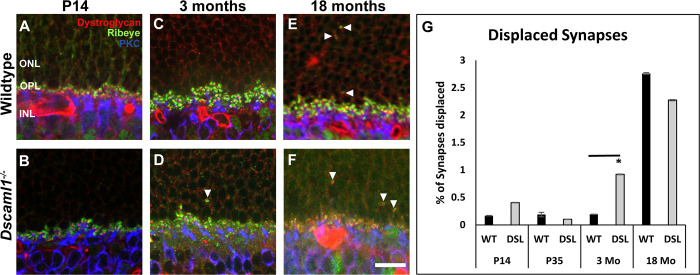
Fig 2. Displaced rod-to-RBC synapses during development and aging.
A) Representative image of rod-to-RBC synapses in the wildtype mouse retina at P14. Synapses are restricted to the OPL. B) Rod-to-RBC synapses in the Dscaml1 mutant retina are also restricted to the OPL at P14. C) Rod-to-RBC synapses in the wildtype mouse retina at 3 months. Synapses are mostly located within the OPL. D) An increase in the number of rod synapses was observed in the ONL of the Dscaml1 mutant retina at 3 months of age. E) An increase in the number of rod synapses was observed in the ONL of the wildtype retina at 18-months. F) An increase in the number of displaced rod synapses was also observed in the Dscaml1 mutant retina at 18-months. G) No significant difference in the number of displaced synapses was detected comparing the wildtype, heterozygous mutants, and Dscaml1 mutant retina at P14, P35, and 18-months (P14, p = 0.22; P35, p = 0.39; 18-months, p = 0.35). An increase in displaced synapses was detected at 3 months of age comparing the homozygous Dscaml1 mutant retina to heterozygous mutant and wild type retina (p = 0.001 and p = 0.002, respectively). PKC = RBCs, dystroglycan = postsynaptic, ribeye = presynaptic. Abbreviations: RBC = rod bipolar cell, PKC = protein kinase C, ONL = outer nuclear layer, OPL = outer plexiform layer, INL = inner nuclear layer, WT = wildtype, DSL = Dscaml1 mutant. Sample sizes = between 10 and 15 images, collected from 3 mice per genotype at all time points, except at 18-month heterozygous mutant, which only had 6 images across 2 mice. Total synapses counted: wildtype = 14,417 synapses, littermate control = 9,907 synapses, Dscaml1 mutant = 10,879 synapses. Error bars = standard deviation. Scale bar = 10 μm.
Tissue preparation
Mice taken for immunohistochemistry studies were anesthetized with tri-bromoethanol, and transcardially perfused with 1X phosphate buffered saline (PBS) (10X PBS: distilled H2O, 80g NaCl, 2.0g KCL, 26.8g Na2HPO4 7H2O, 2.4g KH2PO4, pH 7.4). Eyes were removed and hemisected in 1XPBS. The posterior eye cup was fixed in 4% paraformaldehyde (PFA) for thirty minutes at room temperature followed by three 10-minute washes in 1XPBS. Fixed tissue was sunk in 30% sucrose overnight followed by a 50/50 mixture of 30% sucrose and optimal cutting temperature (OCT; Sakura® Finetek Inc.) and frozen in liquid nitrogen. Eye cups were sectioned on a Leica GM 1510 S cryostat in 10 μm sections and stored in at -20°C for immunohistochemistry. All procedures were approved by the University of Idaho Animal Care and Use Committee. Mice were taken for study at post-natal days (P)14, P21, P35, 3 months, 8 months, 12 months, and 18-months.
Serial election micrographs
Serial block-face electron micrograph (EM) images were generated by Renovo neural EM services (Cleveland, Ohio) using a Zeiss Sigma VP scanning electron microscope. Renovo protocols were followed for tissue processing. Two wildtype, two Bax null, and three Dscaml1 mutant samples from two mice were used to generate electron micrograph volumes. Mice were taken for study at three months of age. Mice were perfused with cacodylate buffer and retinas were fixed for four days in cacodylate buffer before processing. Retina pieces were collected mid-range between central and peripheral retina. Sections were imaged at resolutions between 6.5–7 nm across images with 60–65 nm steps in between slices, depending on the individual data set.
3D electron micrograph reconstruction rendering/Data collection
The National Institutes of Health software, FIJI/ImageJ, was used for developing serial images into 3D stacks of retina tissue, using TrakEM plugin software as previously described [26]. Individual rod spherules, RBCs, and mitochondria were traced on individual area lists to enable isolated analysis. Data sets include wildtype, Bax null, and Dscaml1 mutant retinas. For rod spherules, the number of invaginations were counted for each observed invagination of single neurites from interneurons (bipolar and horizontal cells). For RBC dendrite tip data collection, entire RBCs were traced on a single area list and each dendrite tip was then manually followed until it either terminated or contacted/invaginated into a rod spherule. The number of RBC dendrite tips that contacted a rod spherule was divided by the total number of dendrite tips to determine the percentage of tips that contacted rod spherules for comparison across genotypes. Mitochondria data was collected in the same manner as RBC data. Individual whole mitochondria were manually traced on individual area lists within already identified and traced RBCs. If mitochondria were not able to be traced completely due to going outside of the image stack, or were more than fifty percent contained in the axon, they were not included in the data.
Immunohistochemistry
Retina sections were rehydrated in 1XPBS for 10 minutes, followed by incubation in blocking solution: PBS, 5% normal donkey serum, 0.1% triton for 20 minutes at room temperature. Primary antibodies were added to 0.1% block and incubated on sections overnight at 4°C. Sections were washed 3 x 10 minutes, followed by adding secondary antibodies diluted in 0.1% blocking solution and incubated for two hours at room temperature. Finally, a 1:100,000 dilution of DAPI 10 mg/ml in DMSO was added to the second of three final washes in 1XPBS, followed by coverslip mounting in 80% glycerol. Primary antibodies used: protein kinase C alpha (PKCα; anti-mouse, 1:100, Santa Cruz Biotechnology, MC5: sc-80), ribeye (anti-rabbit, 1:500, Synaptic Systems, #192 003), dystroglycan (Mouse IgG1, Developmental Studies Hybridoma Bank, mandag2 clone 7D11), glutamine synthase (Mouse IgG2a, 1:2000, Millipore, #MAB302), ionized calcium binding adapter molecule 1 (Iba1; rabbit, 1:500, WAKO Pure Chemical Industries, Ltd., #019–19741). All secondary antibodies were purchased from Jackson ImmunoResearch and used at 1:1000 dilutions.
Imaging/Analysis
Image z-stacks of retina sections for rod-to-RBC synapse analysis were obtained from a Nikon spinning disc microscope at the University of Idaho Imaging Core. Image adjustments were made uniformly across whole images. For displaced synapse data collection, all synapses co-labelled with ribeye and dystroglycan antibodies were counted by hand, in a single in focus z-plane, including one image on either side of z-stack to accommodate for tissue not lying flat. Synapses were determined to be displaced if they were inset beyond one rod spherule into the outer nuclear layer. The number of displaced synapses was divided by the number of total synapses to determine the percent of total synapses that are displaced for each image and data presented are the means.
Statistical analysis
Kruskal Wallis and Dunn’s post hoc test was used for comparisons of displaced synapses across ages and comparing genotypes within ages. One-way ANOVA and Tukey Kramer post hoc tests were used for comparisons of total dendrite number and mitochondrial volume. Kruskal Wallis and Dunn’s post hoc tests were used for comparison of the percent of dendrites that terminate at a spherule, mitochondrial complexity, and for percent of total mitochondria that fall over a certain volume across genotypes.
Results
Preserved integrity of the rod-to-RBC synapse in Dscaml1 mutant retina in the young, adult, and aging retina
We first investigated the integrity of the rod-to-RBC synapse from post-natal development to advanced age of the retina (18-months) in wildtype and Dscaml1 mutant mice. Different species have different age-related sensitivities to aging of the rod circuit. For example, humans exhibit loss of rods and RBCs with aging [27–30]. Denervation of the rod synapses and sprouting of RBC and horizontal cell neurites is observed in both mouse and human [15, 16, 31], while neither of these changes is observed in marmoset [32]. Several factors contributing to rod denervation associated with aging have been identified including increases in AMPK signaling and microglial activation [33, 34]. We hypothesized that loss of Dscaml1 could increase sensitivity of the rod circuit to aging. We therefore imaged integrity of the rod synapse and associated cells, RBC dendrite sprouting and glial activation in wildtype and dscaml1 mutant mice at time point between P14 and 18-months.
We labeled the rod-to-RBC synapse by staining retina sections with antibodies to ribeye and dystroglycan, markers of the ribbon synapse and perisynaptic space, respectively (Fig 1A) [35, 36]. We imaged sections in the central retina approximately halfway between the optic nerve and peripheral retina. Synapses formed and were anatomically intact as evidenced by the presence of the ribbon protein ribeye and synaptic cleft protein dystroglycan localized to the dendritic tips of RBCs, identified with PKC staining by postnatal day 14 in both wildtype and Dscaml1 mutant retinas (not shown). Pairing was observed at RBC dendritic tips at all ages measured with no qualitative differences detected comparing wildtype and Dscaml1 mutant retina between postnatal days 14 to 18-months of age (P35, 240, and 365 shown) (Fig 1A–1F). Müller glia and microglia play important roles in responding to damage in the retina and their activation and remodeling before during and after degeneration of rods [37–39]. No differences were observed in Müller glia, labeled with glutamine synthase, or microglia, labeled with antibodies to Iba1 (Fig 1G–1L), with occasional microglial projections into the outer nuclear layer (ONL) observed in both wildtype and Dscaml1 mutant retina sections (Fig 1G arrowhead).
Precocious increase in displaced rod-to-RBC synapses in the Dscaml1 mutant retina
Previous studies have demonstrated an increase in the number of rod-to-RBC synapses in the outer nuclear layer during the aging of the neural retina [14–16]. The increase in the number of displaced synapses has also been observed in models of synaptic dysfunction and involves sprouting of RBC and horizontal cell neurites into the ONL to form synapses with denervated rod cells [16, 40–43]. We measured the number of displaced synapses in Dscaml1 homozygous mutant, Dscaml1 heterozygous mutant and wildtype retinas at P14, P35, 3 months, and 18-months of age compared this to the number of synapses in the outer plexiform layer (Fig 2A–2F). We observed an increase in the number of displaced synapses at 18-months of age in all genotypes compared to P14, P35, and 3 months. The percent of displaced synapses increases from 0.167 percent of all synapses (P14) to 2.67 percent (18-months) in wildtype, from 0.23 percent (P14) to 3.75 percent (18-months) in heterozygous mutant mice, and from 0.40 percent (P14) to 2.21 percent (18-months) in the Dscaml1 mutant retina (p < 0.001 for all) (Fig 2G). We did not observe a significant difference in the number of displaced synapses comparing wildtype, heterozygous mutant, and Dscaml1 mutant retinas at early and late stages (P14: p = 0.22, P35: p = 0.39, 18-months: p = 0.35). We did observe an increase in the percent of displaced synapses in the Dscaml1 mutant retina compared to wildtype and heterozygous mutant controls at 3 months of age (3 months: p = 0.002 and p = 0.001, respectively) (Fig 2G).
Dscaml1 mutant rod spherules contain an increased number of invaginating neurites
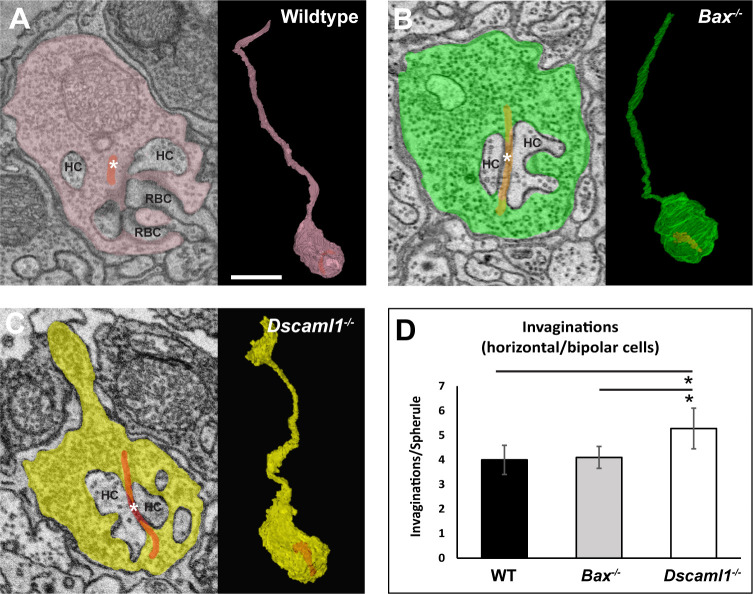
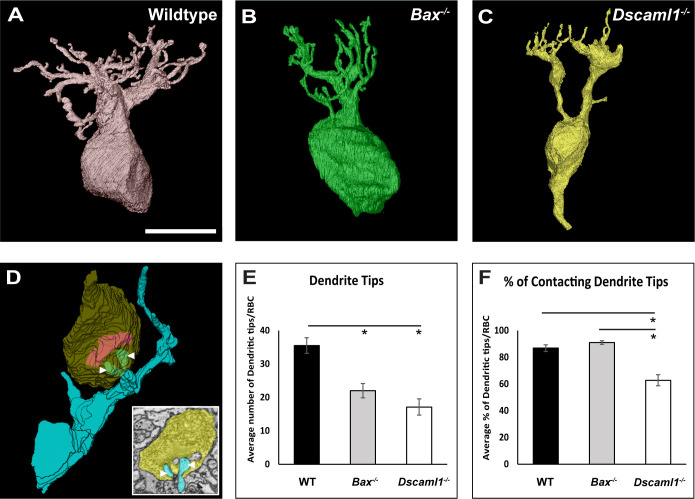
The rod spherules, or axon terminals of rod photoreceptors, are the synaptic junction for rods, horizontal cell axon tips, and RBC and OFF bipolar cell dendritic tips [44–50]. We generated 3-D reconstructions of rod spherule electron micrographs to study the ultrastructure of Dscaml1 mutant and control synapses. We included Bax null retinas as a control for RBC cell density. Bax null mice have an increase in the number of RBCs, but not rods, like that reported in the Dscaml1 mutant retina [13, 51, 52]. After reconstructing the rod spherules, we compared the number of invaginations into rod spherules in wildtype, Bax null, and Dscaml1 mutant retinas (Fig 3A–3C). Wildtype and Bax null spherules had no difference in the number of invaginations with an average of 4.0 and 4.1 invaginations per spherule, respectfully. Dscaml1 spherules had an average of 5.29 invaginations per spherule compared to wildtype and Bax null retinas (p < 0.001 for both) (Fig 3D).
Fig 3. 3D reconstruction of rod spherules comparing neurite invagination number.
A) Electron micrograph and 3D rod spherule reconstruction in wildtype retina. B) Electron micrograph and 3D rod spherule reconstruction in Bax null retina. C) Electron micrograph and 3D rod spherule reconstruction in Dscaml1 mutant retina. D) An increase in the number of neurite invaginations in Dscaml1 mutant rod spherule was observed compared to both the wildtype and Bax null retinas (p < 0.001). Abbreviations: WT = wildtype, Asterisks on images = synaptic ribbon, HC = horizontal cell. RBC = rod bipolar cell. Asterisks on graph indicate significance. Spherule sample sizes = WT: 18, Bax: 20, Dscaml1: 18. Error bars = standard error of the mean. Scale bar = 5 μm.
Intermingling of RBC dendritic arbors in the wildtype and Dscaml1 mutant retina
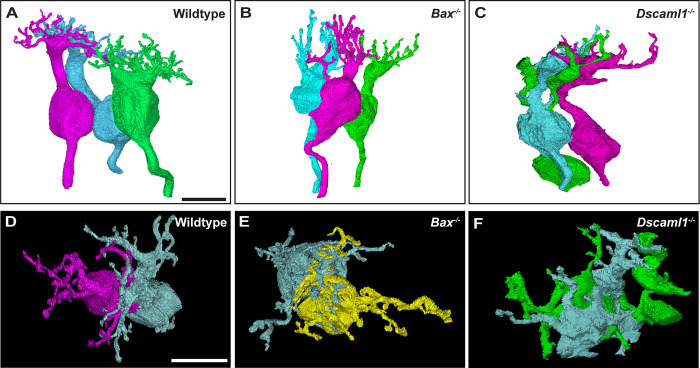
RBC dendrites do not tile, and the dendritic arbors of adjacent cells normally overlap with each other in the mouse retina [53, 54]. We reconstructed multiple RBCs to compare the RBC dendritic arbors in wildtype, Bax null and Dscaml1 mutant retina (Fig 4A–4C). We utilized reconstructed RBCs with adjacent cell soma to compare overlap of dendritic arbors in the wildtype, Bax null, and Dscaml1 mutant retina. RBC dendritic arbors in the wildtype, Bax null, and Dscaml1 mutant RBCs interdigitate extensively, with distinct arbors of Dscaml1 mutant RBC dendrites observed, compared to extensive fasciculation observed in other Dscam or Dscaml1 deficient retinal cell types (Fig 4D–4E).
Fig 4. Dendritic overlap of 3D reconstructed rod bipolar cells.
A) Representative 3D reconstructions of three neighboring RBCs’ spatial arrangement in wildtype retina. B) Representative 3D reconstructions of three neighboring RBCs’ spatial arrangement in the Bax null retina. C) Representative 3D reconstructions of three neighboring RBCs’ spatial arrangement in the Dscaml1 mutant retina. D) 3D RBC reconstruction comparing enface dendritic area with neighboring RBC in wildtype retina. E) 3D RBC reconstruction comparing enface dendritic area with neighboring RBC in Bax null retina. F) 3D RBC reconstruction comparing enface dendritic area with neighboring RBC in Dscaml1 mutant retina. Scale bars = 5 μm.
Dscaml1 mutant RBCs have a reduced number of dendritic tips and a reduced percentage of tips that terminate at rod spherules
We next counted the number of dendritic tips of individual RBC dendritic arbors in wildtype, Bax null, and Dscaml1 mutant retinas (Fig 5A–5C). We simultaneously counted the number of dendritic tips that terminated within the rod spherule. We observed several examples of a single rod being innervated by multiple RBC dendrite tips originating from the same cell in the Dscaml1 mutant retina, something we did not observe in other genotypes (Fig 5D). We found a reduction in the number of total RBC dendritic tips in both the Bax null and Dscaml1 mutant retina compared to wildtype, and no difference between the number of dendritic tips formed by Dscaml1 mutant RBCs when compared to the number of Bax null RBC dendritic tips (WT:Bax, p = 0.019; WT:Dscaml1, p < 0.001; Bax:Dscaml1, p = 0.37) (Fig 5E). We divided the number of dendritic tips that invaginated into rod spherules by the total number of dendritic tips to compare the percentage of total dendritic tips that contacted rod spherules across the wildtype, Bax null, and Dscaml1 mutant retina. We traced wildtype and Bax null RBC dendritic tips to the rod spherule 86.9% and 91.0% (respectfully) but were able to trace only 63.5% of Dscaml1 mutant dendritic tips to the rod spherule (WT:Bax, p = 0.350; WT:Dscaml1, p = 0.010; Bax:Dscaml1, p < 0.001) (Fig 5F).
Fig 5. 3D RBC reconstructions and dendritic comparisons.
A) 3D RBC reconstruction in the wildtype retina. B) 3D RBC reconstruction in the Bax null retina. C) 3D RBC reconstruction in the Dscaml1 mutant retina. D) 3D RBC dendritic tip reconstruction in Dscaml1 mutant retina with two dendritic tips (arrowheads) terminating at the reconstructed rod spherule. Inset in D is a single slice within the serial electron micrograph stack demonstrating two RBC dendritic tips that split from a single RBC tip at point of invagination (yellow = spherule, blue = RBC dendrite tips). E) A decrease in the average total number of dendrites per RBC was observed in both Bax null and Dscaml1 mutant retinas when compared to the wildtype retina. (WT:Bax, p = 0.019; WT:Dscaml1, p < 0.001; Bax:Dscaml1, p = 0.37). F) A decrease the percent of dendritic tips per RBC that terminate at the rod spherules in Dscaml1 mutant retinas was observed compared to wildtype and Bax null retinas (WT:Bax, p = 0.350; WT:Dscaml1, p = 0.010; Bax:Dscaml1, p < 0.001). Abbreviations: WT = wildtype, RBC = rod bipolar cell. Asterisks indicate significance. Arrowheads = dendritic tips. Sample sizes = WT: 8 RBC, Bax: 6 RBC, Dscaml1: 6 RBC. Error bars = standard error of the mean. Scale bar = 5 μm.
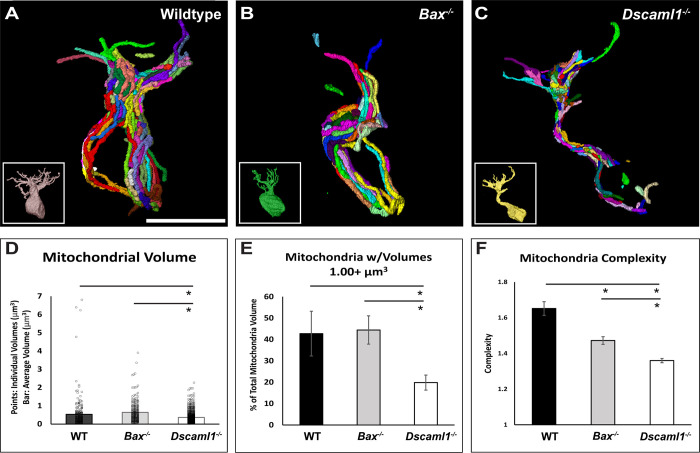
Mitochondrial volume and complexity are reduced in Dscaml1 mutant retina compared to controls
We measured the size, volume, and complexity of mitochondria in RBCs. Dscam genes regulate developmental cell death through Bax-dependent mitochondrial developmental cell death, and we were curious if there were ultrastructural differences at the mitochondria [13, 23, 55]. We compared individual volumes of mitochondria within RBCs of wildtype, Bax null, and Dscaml1 mutant retinas (Fig 6A–6C). We observed a reduced average mitochondrion volume in Dscaml1 mutant RBCs when compared to both wildtype and Bax null RBCs (p = 0.002 and p < 0.001, respectfully) (Fig 6D). We also noticed that the percentage of larger mitochondria was reduced in the Dscaml1 mutant retina compared to controls (Fig 6E). Mitochondria with volumes greater than 1.00 μm3 contributed to 42.8% and 44.5% of the total mitochondrial volume within wildtype and Bax null RBCs (respectfully), compared to only 19.3% of total mitochondrial volume in Dscaml1 mutant RBCs (WT:Bax, p = 0.910; WT:Dscaml1, p = 0.043; Bax:Dscaml1, p = 0.031) (Fig 6E). Increased mitochondrial function can be correlated with increased complexity and fusion of the mitochondria within a cell [56, 57]. We therefore also calculated the complexity of each mitochondrion using a surface area to volume ratio that allowed us to determine how far a mitochondrion strayed from a perfect sphere with a ratio of 1:1. We found that mitochondria in both Bax null and Dscaml1 mutant retinas had reduced complexity when compared to wildtype mitochondria (p = 0.006 and p < 0.001) (respectfully), with Dscaml1 mutant mitochondria showing even less complexity than Bax null RBC mitochondria (p < 0.001) (Fig 6F).
Fig 6. 3D reduced mitochondria size and complexity in the Dscaml1 mutant retina.
A) 3D reconstruction of mitochondria in RBC of the wildtype retina. B) 3D reconstruction of mitochondria in RBC of the Bax null retina. C) 3D reconstruction of mitochondria in RBC of the Dscaml1 mutant retina. D) A decrease in the average mitochondrial volume was observed in the Dscaml1 mutant RBC soma and dendrites when compared to both wildtype and Bax null retinas. (p = 0.002 and p < 0.001, respectfully). E) A decrease in the percent of total mitochondrial volume that mitochondria over 1.00 um3 make up in Dscaml1 mutant retina was observed compared to wildtype and Bax null retinas (p = 0.043 and p = 0.031, respectfully). F) A decrease in mitochondrial complexity was observed in both the Bax null and Dscaml1 mutant RBC when compared to wildtype retina (p = 0.006 and p < 0.001), with a larger decrease in complexity in Dscaml1 mutant compared to Bax null retina. (p < 0.001). Each color represents individual mitochondria. Abbreviations: WT = wildtype. Asterisks indicate significance. Mitochondria sample sizes (for all data) = WT: 234, across 3 RBC; Bax: 235, across 3 RBC; Dscaml1: 684, across 6 RBC. Error bars = standard error of the mean. Scale bar = 5μm.
Discussion
In this study, we measured anatomical formation and maintenance of the rod synapse in wildtype and Dscaml1 mutant mice. A mutation in Dscaml1 has been linked to middle age onset of congenital stationary night blindness. Dscaml1 is expressed in cells of the rod circuit of the mouse retina, consistent with this finding. We used a loss of function mouse model to measure formation and anatomical connectivity of the synapse during the mouse life span to test if Dscaml1 is required to maintain the rod synapse. We identified changes in connectivity, aging and organization of the synapse.
The rod circuit is susceptible to different degrees of age-related degeneration and changes in different species. Loss of rods, RBCs and sprouting of RBC dendrites associated with aging have been reported in humans, while other animals, such as marmosets do not appear to undergo age related changes to the rod circuit, or even zebrafish, which continuously produce rods during the lifespan [27–32, 58]. Mice represent an intermediate between these examples with evidence of rod denervation and RBC dendrite sprouting but not significant cell loss. We observed a similar gross organization pattern to the wildtype in the aging Dscaml1 mutant mouse retina. RBC and horizontal cell neurite sprouting into the outer nuclear layer is associated with both aging in the mouse retina and synaptic dysfunction [15, 16, 31]. We did not observe excess sprouting and synapse formation during early developmental stages or advanced age (1 year and 18-months) but did observe an increase in sprouting at an intermediate time point in the Dscaml1 mutant retina, suggesting that normal processes observed in the aging retina may be advanced in the absence of Dscaml1. An increase in RBC sprouting has been observed in mutant mouse models lacking ribbon and other synaptic proteins, and while Dscaml1 is not required for the formation of functional synapses [13, 14, 16, 40–43], its absence did result in precocious sprouting in this study, suggesting a role at the rod synapse.
Next, we measured the ultrastructure of the rod synapse and bipolar cell dendritic fields using serial electron micrograph reconstructions. We found that the number of invaginations was increased in Dscaml1 mutant retinas compared to controls (Fig 3). We observed overlap of RBC dendritic arbors in all genotypes, consistent with the known lack of RBC dendritic arbor tiling. We did not observe significant clumping or fasciculation of RBC dendrites with each other like has been observed for other cell types, but this observation may have been limited by the number of cells traced per field and the density of RBCs. Cone bipolar cells exhibit a form of plasticity after loss of Dscam that involves sprouting of dendrites [24]. We observed a reduced number of dendrite tips in the Dscaml1 mutant retina and an increase in the number of tips that do not make anatomical connections with rod spherules, which would be consistent with dendritic sprouting observed in OFF bipolar cells in the Dscam mutant retina. In the wildtype retina, RBCs occasionally make synaptic connections at cone pedicles. Compared to other studies, we observed a higher percentage of wildtype RBC dendrite tips that did not terminate at the rod spherules and speculate that this may be due to the slice thickness of the serial electron micrograph stacks used in this study compared to other studies in which less tissue was removed between serial image acquisition. We also observed an increase in the number of invaginating neurites in rod spherules, despite a decrease in the number of dendritic tips per RBC, compared to wildtype and Bax null retinas. Contacts between cone bipolar cells and rod spherules have been observed in other mutant genetic backgrounds and may be occurring here also, but thorough tracing of stacks will be required to determine if additional invaginations observed at rod spherules, despite the decrease in RBC tips, can be explained by other cell types contacting RBCs in the Dscaml1 mutant retina [59]. Different Dscam1 isoforms are known to interact weakly in Drosophila and related sidekick proteins also interact weakly with each other [25, 60, 61]. Similar weak interactions between Dscam and Dscaml1 could contribute to rod-cone pathway specificity. Additional volume generation and tracing can be performed to test this. We also observed several instances in which RBC dendrite tips branched immediately before both tips invaginated into the same rod spherule in the Dscaml1 mutant retina (Fig 5D) but not in other genotypes, suggesting an iso-neuronal role for Dscaml1 in RBC, similar to iso-neuronal avoidance mediated by Drosophila [62, 63]. Other studies have reported a change to the rod ribbon ultrastructure in aging mice [64]. EM studies at more advanced ages could help to determine if similar changes occur in Dscaml1 mutant mice.
We also measured mitochondria in wildtype, Bax null, and Dscaml1 mutant mouse RBCs and observed a decrease in both the individual volume and complexity of the mitochondria in Dscaml1 mutant RBCs compared to controls. Mitochondria in neurons are involved in many intracellular processes beyond ATP production and are essential to proper signal transduction through nuclear gene regulation, metabolism, synaptic vesicles, reactive oxygen species production, and ion regulation [65–69]. Mitochondria accomplish this through their ability to undergo morphological changes: fusion and fission [70]. Larger mitochondria increase ATP yields and calcium homeostasis, while smaller mitochondria are involved in intracellular transportation, observed at apoptosis initiation, and often are targeted to undergo mitophagy to improve cellular stability [56, 57, 71–75]. However, fragmentation and decline in mitochondrial quality has also been associated with mitochondrial dysfunction, contributing to the severity of neurological diseases, disorders, and aging [76–81]. These findings demonstrate that intracellular factors may contribute to individual RBC function that is not detectable by a measure of bulk output like electroretinogram, especially in cases where there is an increase in RBC number that can disguise ERG results. However, it is unclear if changes in the mitochondria are primary or secondary to changes in dendritic organization in RBCs. A further limitation is that while a large number of mitochondria were reconstructed, these were sampled from a small number of RBCs. Additional tracing will establish the degree of variability of mitochondria within RBCs in addition to across genotypes.
In conclusion, we surveyed the rod-to-RBC synapse in Dscaml1 mutant mouse retina from postnatal development through aging and observed that synapses are formed and maintained like what is observed in wildtype mouse retina. We measured the number of neurite invaginations at the rod spherule and found an increase in the number of invaginations in Dscaml1 mutant retina compared to controls. We quantified the number of dendritic tips in wildtype, Bax null, and Dscaml1 mutant retina. Both the total dendritic tip number and percent of dendritic tips that contacted spherules was reduced in Dscaml1 mutant retina compared to controls. Finally, we measured individual mitochondrial volume and complexity in RBCs of wildtype, Bax null, and Dscaml1 mutant retina and report a decrease in both the mitochondrial volume and complexity when compared to controls. The rod circuit differs significantly in mouse and human. Rods represent the vast majority of photoreceptors in mice but have a sparse distribution in humans. It is possible that changes shown here are sufficient to further degrade the minimal human rod pathway. Mutations observed in humans are missense compared to the loss of function mutation used here and this is a known limitation of this study. However, these results contribute knowledge of Dscaml1’s role in rod circuit development and maintenance and give insight into possible genetic therapy targets for blinding diseases and disorders like CSNB.
Supporting information
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
This project funded in part by the National Eye Institute: National Institute of Health Grant #R21EY028297 (PGF), National Science Foundation REU site Award #1757826, UI Honors Program Grant for Undergraduate Research and Creative Scholarship (RHD), UI Office for Undergraduate Research and Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health Idaho INBRE Grant #P20GM103408 (PGF), and the National Science Foundation Graduate Research Fellowship under Award No. 22-35197 (MRC). The funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dryja TP, McGee TL, Reichel E, Hahn LB, Cowley GS, Yandell DW, et al. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. 1990;343(6256):364–6. Epub 1990/01/25. doi: 10.1038/343364a0 . [DOI] [PubMed] [Google Scholar]
- 2.Kim AH, Liu PK, Chang YH, Kang EY, Wang HH, Chen N, et al. Congenital Stationary Night Blindness: Clinical and Genetic Features. Int J Mol Sci. 2022;23(23). Epub 2022/12/12. doi: 10.3390/ijms232314965 ; PubMed Central PMCID: PMC9740538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acland GM, Aguirre GD, Bennett J, Aleman TS, Cideciyan AV, Bennicelli J, et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther. 2005;12(6):1072–82. Epub 2005/10/18. doi: 10.1016/j.ymthe.2005.08.008 ; PubMed Central PMCID: PMC3647373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cideciyan AV, Jacobson SG, Beltran WA, Sumaroka A, Swider M, Iwabe S, et al. Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc Natl Acad Sci U S A. 2013;110(6):E517–25. Epub 2013/01/24. doi: 10.1073/pnas.1218933110 ; PubMed Central PMCID: PMC3568385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gardiner KL, Cideciyan AV, Swider M, Dufour VL, Sumaroka A, Komaromy AM, et al. Long-Term Structural Outcomes of Late-Stage RPE65 Gene Therapy. Mol Ther. 2020;28(1):266–78. Epub 2019/10/13. doi: 10.1016/j.ymthe.2019.08.013 ; PubMed Central PMCID: PMC6951840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright AF. Long-term effects of retinal gene therapy in childhood blindness. N Engl J Med. 2015;372(20):1954–5. Epub 2015/05/06. doi: 10.1056/NEJMe1503419 . [DOI] [PubMed] [Google Scholar]
- 7.Bainbridge JW, Mehat MS, Sundaram V, Robbie SJ, Barker SE, Ripamonti C, et al. Long-term effect of gene therapy on Leber’s congenital amaurosis. N Engl J Med. 2015;372(20):1887–97. Epub 2015/05/06. doi: 10.1056/NEJMoa1414221 ; PubMed Central PMCID: PMC4497809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gene Therapy Trial for the Treatment of X-linked Retinitis Pigmentosa Associated with Variants in the RPGR Gene [Internet]. National Library of Medicine. Feb 29, 2000. [cited January 16, 2023]. Available from: https://clinicaltrials.gov/study/NCT04671433. [Google Scholar]
- 9.Boycott KM, Pearce WG, Musarella MA, Weleber RG, Maybaum TA, Birch DG, et al. Evidence for genetic heterogeneity in X-linked congenital stationary night blindness. Am J Hum Genet. 1998;62(4):865–75. Epub 1998/06/13. doi: 10.1086/301781 ; PubMed Central PMCID: PMC1377021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobson SG, Cideciyan AV, Ratnakaram R, Heon E, Schwartz SB, Roman AJ, et al. Gene therapy for leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Arch Ophthalmol. 2012;130(1):9–24. Epub 2011/09/14. doi: 10.1001/archophthalmol.2011.298 ; PubMed Central PMCID: PMC3600816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobson SG, Cideciyan AV, Ho AC, Roman AJ, Wu V, Garafalo AV, et al. Night vision restored in days after decades of congenital blindness. iScience. 2022;25(10):105274. Epub 2022/10/25. doi: 10.1016/j.isci.2022.105274 ; PubMed Central PMCID: PMC9579015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Astuti GDN, van den Born LI, Khan MI, Hamel CP, Bocquet B, Manes G, et al. Identification of Inherited Retinal Disease-Associated Genetic Variants in 11 Candidate Genes. Genes (Basel). 2018;9(1). Epub 2018/01/11. doi: 10.3390/genes9010021 ; PubMed Central PMCID: PMC5793174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuerst PG, Bruce F, Tian M, Wei W, Elstrott J, Feller MB, et al. DSCAM and DSCAML1 function in self-avoidance in multiple cell types in the developing mouse retina. Neuron: NIH Public Access; 2009. p. 484–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samuel MA, Zhang Y, Meister M, Sanes JR. Age-related alterations in neurons of the mouse retina. J Neurosci. 2011;31(44):16033–44. Epub 2011/11/04. doi: 10.1523/JNEUROSCI.3580-11.2011 ; PubMed Central PMCID: PMC3238393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terzibasi E, Calamusa M, Novelli E, Domenici L, Strettoi E, Cellerino A. Age-dependent remodelling of retinal circuitry. Neurobiol Aging. 2009;30(5):819–28. Epub 2007/10/09. doi: 10.1016/j.neurobiolaging.2007.08.017 . [DOI] [PubMed] [Google Scholar]
- 16.Liets LC, Eliasieh K, van der List DA, Chalupa LM. Dendrites of rod bipolar cells sprout in normal aging retina. Proc Natl Acad Sci U S A. 2006;103(32):12156–60. Epub 2006/08/02. doi: 10.1073/pnas.0605211103 ; PubMed Central PMCID: PMC1524926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwala KL, Ganesh S, Tsutsumi Y, Suzuki T, Amano K, Yamakawa K. Cloning and functional characterization of DSCAML1, a novel DSCAM-like cell adhesion molecule that mediates homophilic intercellular adhesion. Biochem Biophys Res Commun. 2001;285(3):760–72. Epub 2001/07/17. doi: 10.1006/bbrc.2001.5214 . [DOI] [PubMed] [Google Scholar]
- 18.Tsai TY, Garner RM, Megason SG. Adhesion-Based Self-Organization in Tissue Patterning. Annu Rev Cell Dev Biol. 2022;38:349–74. Epub 2022/05/15. doi: 10.1146/annurev-cellbio-120420-100215 ; PubMed Central PMCID: PMC9547846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livesey FJ, Cepko CL. Vertebrate neural cell-fate determination: lessons from the retina. Nat Rev Neurosci. 2001;2(2):109–18. Epub 2001/03/17. doi: 10.1038/35053522 . [DOI] [PubMed] [Google Scholar]
- 20.Badea TC, Cahill H, Ecker J, Hattar S, Nathans J. Distinct roles of transcription factors brn3a and brn3b in controlling the development, morphology, and function of retinal ganglion cells. Neuron. 2009;61(6):852–64. Epub 2009/03/28. doi: 10.1016/j.neuron.2009.01.020 ; PubMed Central PMCID: PMC2679215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng YR, Shekhar K, Yan W, Herrmann D, Sappington A, Bryman GS, et al. Molecular Classification and Comparative Taxonomics of Foveal and Peripheral Cells in Primate Retina. Cell. 2019;176(5):1222–37 e22. Epub 2019/02/05. doi: 10.1016/j.cell.2019.01.004 ; PubMed Central PMCID: PMC6424338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruce FM, Brown S, Smith JN, Fuerst PG, Erskine L. DSCAM promotes axon fasciculation and growth in the developing optic pathway. Proc Natl Acad Sci U S A. 2017;114(7):1702–7. Epub 2017/02/01. doi: 10.1073/pnas.1618606114 ; PubMed Central PMCID: PMC5321013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li S, Sukeena JM, Simmons AB, Hansen EJ, Nuhn RE, Samuels IS, et al. DSCAM promotes refinement in the mouse retina through cell death and restriction of exploring dendrites. J Neurosci. 2015;35(14):5640–54. Epub 2015/04/10. doi: 10.1523/JNEUROSCI.2202-14.2015 ; PubMed Central PMCID: PMC4388924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simmons AB, Bloomsburg SJ, Sukeena JM, Miller CJ, Ortega-Burgos Y, Borghuis BG, et al. DSCAM-mediated control of dendritic and axonal arbor outgrowth enforces tiling and inhibits synaptic plasticity. Proc Natl Acad Sci U S A. 2017;114(47):E10224–E33. Epub 2017/11/09. doi: 10.1073/pnas.1713548114 ; PubMed Central PMCID: PMC5703318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamagata M, Sanes JR. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature. 2008;451(7177):465–9. Epub 2008/01/25. doi: 10.1038/nature06469 . [DOI] [PubMed] [Google Scholar]
- 26.Li S, Mitchell J, Briggs DJ, Young JK, Long SS, Fuerst PG. Morphological Diversity of the Rod Spherule: A Study of Serially Reconstructed Electron Micrographs. PLoS One. 2016;11(3):e0150024. Epub 2016/03/02. doi: 10.1371/journal.pone.0150024 ; PubMed Central PMCID: PMC4773090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aggarwal P, Nag TC, Wadhwa S. Age-related decrease in rod bipolar cell density of the human retina: an immunohistochemical study. J Biosci. 2007;32(2):293–8. Epub 2007/04/17. doi: 10.1007/s12038-007-0029-9 . [DOI] [PubMed] [Google Scholar]
- 28.Gartner S, Henkind P. Aging and degeneration of the human macula. 1. Outer nuclear layer and photoreceptors. Br J Ophthalmol. 1981;65(1):23–8. Epub 1981/01/01. doi: 10.1136/bjo.65.1.23 ; PubMed Central PMCID: PMC1039407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao H, Hollyfield JG. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1992;33(1):1–17. Epub 1992/01/01. . [PubMed] [Google Scholar]
- 30.Curcio CA, Millican CL, Allen KA, Kalina RE. Aging of the human photoreceptor mosaic: evidence for selective vulnerability of rods in central retina. Invest Ophthalmol Vis Sci. 1993;34(12):3278–96. Epub 1993/11/01. . [PubMed] [Google Scholar]
- 31.Eliasieh K, Liets LC, Chalupa LM. Cellular reorganization in the human retina during normal aging. Invest Ophthalmol Vis Sci. 2007;48(6):2824–30. Epub 2007/05/26. doi: 10.1167/iovs.06-1228 . [DOI] [PubMed] [Google Scholar]
- 32.Shareck F, Roy C, Yaguchi M, Morosoli R, Kluepfel D. Sequences of three genes specifying xylanases in Streptomyces lividans. Gene. 1991;107(1):75–82. Epub 1991/10/30. doi: 10.1016/0378-1119(91)90299-q . [DOI] [PubMed] [Google Scholar]
- 33.Li R, Liang Y, Lin B. Accumulation of systematic TPM1 mediates inflammation and neuronal remodeling by phosphorylating PKA and regulating the FABP5/NF-kappaB signaling pathway in the retina of aged mice. Aging Cell. 2022;21(3):e13566. Epub 2022/02/12. doi: 10.1111/acel.13566 ; PubMed Central PMCID: PMC8920455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samuel MA, Voinescu PE, Lilley BN, de Cabo R, Foretz M, Viollet B, et al. LKB1 and AMPK regulate synaptic remodeling in old age. Nat Neurosci. 2014;17(9):1190–7. Epub 2014/08/05. doi: 10.1038/nn.3772 ; PubMed Central PMCID: PMC5369022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmitz F, Konigstorfer A, Sudhof TC. RIBEYE, a component of synaptic ribbons: a protein’s journey through evolution provides insight into synaptic ribbon function. Neuron. 2000;28(3):857–72. Epub 2001/02/13. doi: 10.1016/s0896-6273(00)00159-8 . [DOI] [PubMed] [Google Scholar]
- 36.Drenckhahn D, Holbach M, Ness W, Schmitz F, Anderson LV. Dystrophin and the dystrophin-associated glycoprotein, beta-dystroglycan, co-localize in photoreceptor synaptic complexes of the human retina. Neuroscience. 1996;73(2):605–12. Epub 1996/07/01. doi: 10.1016/0306-4522(96)00069-3 . [DOI] [PubMed] [Google Scholar]
- 37.Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, et al. Muller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25(4):397–424. Epub 2006/07/15. doi: 10.1016/j.preteyeres.2006.05.003 . [DOI] [PubMed] [Google Scholar]
- 38.Reynisson H, Kalloniatis M, Fletcher EL, Shivdasani MN, Nivison-Smith L. Loss of Muller cell glutamine synthetase immunoreactivity is associated with neuronal changes in late-stage retinal degeneration. Front Neuroanat. 2023;17:997722. Epub 2023/03/25. doi: 10.3389/fnana.2023.997722 ; PubMed Central PMCID: PMC10029270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blank T, Goldmann T, Koch M, Amann L, Schon C, Bonin M, et al. Early Microglia Activation Precedes Photoreceptor Degeneration in a Mouse Model of CNGB1-Linked Retinitis Pigmentosa. Front Immunol. 2017;8:1930. Epub 2018/01/23. doi: 10.3389/fimmu.2017.01930 ; PubMed Central PMCID: PMC5760536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michalakis S, Schaferhoff K, Spiwoks-Becker I, Zabouri N, Koch S, Koch F, et al. Characterization of neurite outgrowth and ectopic synaptogenesis in response to photoreceptor dysfunction. Cell Mol Life Sci. 2013;70(10):1831–47. Epub 2012/12/28. doi: 10.1007/s00018-012-1230-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lewis GP, Linberg KA, Fisher SK. Neurite outgrowth from bipolar and horizontal cells after experimental retinal detachment. Invest Ophthalmol Vis Sci. 1998;39(2):424–34. Epub 1998/03/07. . [PubMed] [Google Scholar]
- 42.Dick O, tom Dieck S, Altrock WD, Ammermuller J, Weiler R, Garner CC, et al. The presynaptic active zone protein bassoon is essential for photoreceptor ribbon synapse formation in the retina. Neuron. 2003;37(5):775–86. Epub 2003/03/12. doi: 10.1016/s0896-6273(03)00086-2 . [DOI] [PubMed] [Google Scholar]
- 43.Huttl S, Michalakis S, Seeliger M, Luo DG, Acar N, Geiger H, et al. Impaired channel targeting and retinal degeneration in mice lacking the cyclic nucleotide-gated channel subunit CNGB1. J Neurosci. 2005;25(1):130–8. Epub 2005/01/07. doi: 10.1523/JNEUROSCI.3764-04.2005 ; PubMed Central PMCID: PMC2885903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolb H. Organization of the outer plexiform layer of the primate retina: electron microscopy of Golgi-impregnated cells. Philos Trans R Soc Lond B Biol Sci. 1970;258(823):261–83. Epub 1970/05/07. doi: 10.1098/rstb.1970.0036 . [DOI] [PubMed] [Google Scholar]
- 45.Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J Comp Neurol. 1979;188(2):245–62. Epub 1979/11/15. doi: 10.1002/cne.901880204 [DOI] [PubMed] [Google Scholar]
- 46.Dowling JE, Boycott BB. Organization of the primate retina: electron microscopy. Proc R Soc Lond B Biol Sci. 1966;166(1002):80–111. Epub 1966/11/15. doi: 10.1098/rspb.1966.0086 . [DOI] [PubMed] [Google Scholar]
- 47.Rogerson LE, Behrens C, Euler T, Berens P, Schubert T. Connectomics of synaptic microcircuits: lessons from the outer retina. J Physiol. 2017;595(16):5517–24. Epub 2017/03/16. doi: 10.1113/JP273671 ; PubMed Central PMCID: PMC5556146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haverkamp S, Specht D, Majumdar S, Zaidi NF, Brandstatter JH, Wasco W, et al. Type 4 OFF cone bipolar cells of the mouse retina express calsenilin and contact cones as well as rods. J Comp Neurol. 2008;507(1):1087–101. Epub 2007/12/21. doi: 10.1002/cne.21612 . [DOI] [PubMed] [Google Scholar]
- 49.Mataruga A, Kremmer E, Muller F. Type 3a and type 3b OFF cone bipolar cells provide for the alternative rod pathway in the mouse retina. J Comp Neurol. 2007;502(6):1123–37. Epub 2007/04/21. doi: 10.1002/cne.21367 . [DOI] [PubMed] [Google Scholar]
- 50.Tsukamoto Y, Omi N. Some OFF bipolar cell types make contact with both rods and cones in macaque and mouse retinas. Front Neuroanat. 2014;8:105. Epub 2014/10/14. doi: 10.3389/fnana.2014.00105 ; PubMed Central PMCID: PMC4176460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pequignot MO, Provost AC, Salle S, Taupin P, Sainton KM, Marchant D, et al. Major role of BAX in apoptosis during retinal development and in establishment of a functional postnatal retina. Dev Dyn. 2003;228(2):231–8. Epub 2003/10/01. doi: 10.1002/dvdy.10376 . [DOI] [PubMed] [Google Scholar]
- 52.Keeley PW, Patel SS, Reese BE. Cell numbers, cell ratios, and developmental plasticity in the rod pathway of the mouse retina. J Anat. 2022. Epub 2022/03/17. doi: 10.1111/joa.13653 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Behrens C, Schubert T, Haverkamp S, Euler T, Berens P. Connectivity map of bipolar cells and photoreceptors in the mouse retina. Elife. 2016;5. Epub 2016/11/26. doi: 10.7554/eLife.20041 ; PubMed Central PMCID: PMC5148610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anastassov IA, Wang W, Dunn FA. Synaptogenesis and synaptic protein localization in the postnatal development of rod bipolar cell dendrites in mouse retina. J Comp Neurol. 2019;527(1):52–66. Epub 2017/05/27. doi: 10.1002/cne.24251 ; PubMed Central PMCID: PMC5745277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fuerst PG, Koizumi A, Masland RH, Burgess RW. Neurite arborization and mosaic spacing in the mouse retina require DSCAM. Nature. 2008;451(7177):470–4. Epub 2008/01/25. doi: 10.1038/nature06514 ; PubMed Central PMCID: PMC2259282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kowaltowski AJ, Menezes-Filho SL, Assali EA, Goncalves IG, Cabral-Costa JV, Abreu P, et al. Mitochondrial morphology regulates organellar Ca(2+) uptake and changes cellular Ca(2+) homeostasis. FASEB J. 2019;33(12):13176–88. Epub 2019/09/05. doi: 10.1096/fj.201901136R ; PubMed Central PMCID: PMC9272750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gomes LC, Benedetto GD, Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nature Cell Biology 2011. p. 589–98. doi: 10.1038/ncb2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J Neurosci. 2007;27(26):7028–40. Epub 2007/06/29. doi: 10.1523/JNEUROSCI.1624-07.2007 ; PubMed Central PMCID: PMC6672216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsukamoto Y, Morigiwa K, Ishii M, Takao M, Iwatsuki K, Nakanishi S, et al. A novel connection between rods and ON cone bipolar cells revealed by ectopic metabotropic glutamate receptor 7 (mGluR7) in mGluR6-deficient mouse retinas. J Neurosci. 2007;27(23):6261–7. Epub 2007/06/08. doi: 10.1523/JNEUROSCI.5646-06.2007 ; PubMed Central PMCID: PMC6672139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu W, Ahlsen G, Baker D, Shapiro L, Zipursky SL. Complementary chimeric isoforms reveal Dscam1 binding specificity in vivo. Neuron. 2012;74(2):261–8. Epub 2012/05/01. doi: 10.1016/j.neuron.2012.02.029 ; PubMed Central PMCID: PMC3429342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wojtowicz WM, Flanagan JJ, Millard SS, Zipursky SL, Clemens JC. Alternative splicing of Drosophila Dscam generates axon guidance receptors that exhibit isoform-specific homophilic binding. Cell. 2004;118(5):619–33. Epub 2004/09/02. doi: 10.1016/j.cell.2004.08.021 ; PubMed Central PMCID: PMC2691713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hughes ME, Bortnick R, Tsubouchi A, Baumer P, Kondo M, Uemura T, et al. Homophilic Dscam interactions control complex dendrite morphogenesis. Neuron. 2007;54(3):417–27. Epub 2007/05/08. doi: 10.1016/j.neuron.2007.04.013 ; PubMed Central PMCID: PMC1963440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matthews BJ, Kim ME, Flanagan JJ, Hattori D, Clemens JC, Zipursky SL, et al. Dendrite self-avoidance is controlled by Dscam. Cell. 2007;129(3):593–604. Epub 2007/05/08. doi: 10.1016/j.cell.2007.04.013 . [DOI] [PubMed] [Google Scholar]
- 64.Fuchs M, Scholz M, Sendelbeck A, Atorf J, Schlegel C, Enz R, et al. Rod photoreceptor ribbon synapses in DBA/2J mice show progressive age-related structural changes. PLoS One. 2012;7(9):e44645. Epub 2012/09/08. doi: 10.1371/journal.pone.0044645 ; PubMed Central PMCID: PMC3434146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Janssen JJE, Grefte S, Keijer J, de Boer VCJ. Mito-Nuclear Communication by Mitochondrial Metabolites and Its Regulation by B-Vitamins. Front Physiol. 2019;10:78. Epub 2019/02/28. doi: 10.3389/fphys.2019.00078 ; PubMed Central PMCID: PMC6379835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pathak D, Shields LY, Mendelsohn BA, Haddad D, Lin W, Gerencser AA, et al. The role of mitochondrially derived ATP in synaptic vesicle recycling. J Biol Chem. 2015;290(37):22325–36. Epub 2015/07/02. doi: 10.1074/jbc.M115.656405 ; PubMed Central PMCID: PMC4566209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hirabayashi Y, Kwon SK, Paek H, Pernice WM, Paul MA, Lee J, et al. ER-mitochondria tethering by PDZD8 regulates Ca(2+) dynamics in mammalian neurons. Science. 2017;358(6363):623–30. Epub 2017/11/04. doi: 10.1126/science.aan6009 ; PubMed Central PMCID: PMC5818999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Datta S, Jaiswal M. Mitochondrial calcium at the synapse. Mitochondrion. 2021;59:135–53. Epub 2021/04/26. doi: 10.1016/j.mito.2021.04.006 . [DOI] [PubMed] [Google Scholar]
- 69.Han Y, Chu X, Cui L, Fu S, Gao C, Li Y, et al. Neuronal mitochondria-targeted therapy for Alzheimer’s disease by systemic delivery of resveratrol using dual-modified novel biomimetic nanosystems. Drug Deliv. 2020;27(1):502–18. Epub 2020/04/02. doi: 10.1080/10717544.2020.1745328 ; PubMed Central PMCID: PMC7170363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee H, Yoon Y. Mitochondrial fission and fusion. Biochem Soc Trans. 2016;44(6):1725–35. Epub 2016/12/04. doi: 10.1042/BST20160129 . [DOI] [PubMed] [Google Scholar]
- 71.Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22(12):1577–90. Epub 2008/06/19. doi: 10.1101/gad.1658508 ; PubMed Central PMCID: PMC2732420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen H, Chan DC. Mitochondrial dynamics—fusion, fission, movement, and mitophagy—in neurodegenerative diseases. Hum Mol Genet. 2009;18(R2):R169–76. Epub 2009/10/08. doi: 10.1093/hmg/ddp326 ; PubMed Central PMCID: PMC2758711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20(1):31–42. Epub 2012/06/30. doi: 10.1038/cdd.2012.81 ; PubMed Central PMCID: PMC3524633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin MY, Sheng ZH. Regulation of mitochondrial transport in neurons. Exp Cell Res. 2015;334(1):35–44. Epub 2015/01/24. doi: 10.1016/j.yexcr.2015.01.004 ; PubMed Central PMCID: PMC4433773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen H, Chan DC. Mitochondrial dynamics in mammals. Curr Top Dev Biol. 2004;59:119–44. Epub 2004/02/21. doi: 10.1016/S0070-2153(04)59005-1 . [DOI] [PubMed] [Google Scholar]
- 76.Carmo C, Naia L, Lopes C, Rego AC. Mitochondrial Dysfunction in Huntington’s Disease. Adv Exp Med Biol. 2018;1049:59–83. Epub 2018/02/11. doi: 10.1007/978-3-319-71779-1_3 . [DOI] [PubMed] [Google Scholar]
- 77.Folbergrova J, Kunz WS. Mitochondrial dysfunction in epilepsy. Mitochondrion. 2012;12(1):35–40. Epub 2011/05/03. doi: 10.1016/j.mito.2011.04.004 . [DOI] [PubMed] [Google Scholar]
- 78.Trigo D, Avelar C, Fernandes M, Sa J, da Cruz ESO. Mitochondria, energy, and metabolism in neuronal health and disease. FEBS Lett. 2022;596(9):1095–110. Epub 2022/01/29. doi: 10.1002/1873-3468.14298 . [DOI] [PubMed] [Google Scholar]
- 79.Ashleigh T, Swerdlow RH, Beal MF. The role of mitochondrial dysfunction in Alzheimer’s disease pathogenesis. Alzheimers Dement. 2023;19(1):333–42. Epub 2022/05/07. doi: 10.1002/alz.12683 . [DOI] [PubMed] [Google Scholar]
- 80.Rose S, Niyazov DM, Rossignol DA, Goldenthal M, Kahler SG, Frye RE. Clinical and Molecular Characteristics of Mitochondrial Dysfunction in Autism Spectrum Disorder. Mol Diagn Ther. 2018;22(5):571–93. Epub 2018/07/25. doi: 10.1007/s40291-018-0352-x ; PubMed Central PMCID: PMC6132446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jang JY, Blum A, Liu J, Finkel T. The role of mitochondria in aging. J Clin Invest. 2018;128(9):3662–70. Epub 2018/07/31. doi: 10.1172/JCI120842 ; PubMed Central PMCID: PMC6118639 exists. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.