Abstract
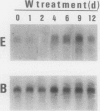
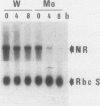
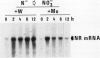
Nitrate reductase (NR, EC 1.6.6.1) from higher plants is a homodimeric enzyme carrying a molybdenum cofactor at the catalytic site. Tungsten can be substituted for molybdenum in the cofactor structure, resulting in an inactive enzyme. When nitratefed Nicotiana tabacum plants were grown on a nutrient solution in which tungstate was substituted for molybdate, NR activity in the leaves decreased to a very low level within 24 hours while NR protein accumulated progressively to a level severalfold higher than the control after 6 days. NR mRNA level in molybdate-grown plants exhibited a considerable day-night fluctuation. However, when plants were treated with tungstate, NR mRNA level remained very high. NR activity and protein increased over a 24-hour period when nitrate was added back to N-starved molybdate-grown plants. NR mRNA level increased markedly during the first 2 hours and then decreased. In the presence of tungstate, however, the induction of NR activity by nitrate was totally abolished while high levels of NR protein and mRNA were both induced, and the high level of NR mRNA was maintained over a 10-hour period. These results suggest that the substitution of tungsten for molybdenum in NR complex leads to an overexpression of the NR structural gene. Possible mechanisms involved in this deregulation are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aslam M., Oaks A. Comparative studies on the induction and inactivation of nitrate reductase in corn roots and leaves. Plant Physiol. 1976 Apr;57(4):572–576. doi: 10.1104/pp.57.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Calza R, Huttner E, Vincentz M, Rouzé P, Galangau F, Vaucheret H, Chérel I, Meyer C, Kronenberger J, Caboche M. Cloning of DNA fragments complementary to tobacco nitrate reductase mRNA and encoding epitopes common to the nitrate reductases from higher plants. Mol Gen Genet. 1987 Oct;209(3):552–562. doi: 10.1007/BF00331162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C. L., Dewdney J., Kleinhofs A., Goodman H. M. Cloning and nitrate induction of nitrate reductase mRNA. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6825–6828. doi: 10.1073/pnas.83.18.6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherel I., Grosclaude J., Rouze P. Monoclonal antibodies identify multiple epitopes on maize leaf nitrate reductase. Biochem Biophys Res Commun. 1985 Jun 28;129(3):686–693. doi: 10.1016/0006-291X(85)91946-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherel I., Marion-Poll A., Meyer C., Rouze P. Immunological comparisons of nitrate reductase of different plant species using monoclonal antibodies. Plant Physiol. 1986 Jun;81(2):376–378. doi: 10.1104/pp.81.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Filner P. Regulation of nitrate reductase in cultured tobacco cells. Biochim Biophys Acta. 1966 May 5;118(2):299–310. doi: 10.1016/s0926-6593(66)80038-3. [DOI] [PubMed] [Google Scholar]
- Fu Y. H., Marzluf G. A. Metabolic control and autogenous regulation of nit-3, the nitrate reductase structural gene of Neurospora crassa. J Bacteriol. 1988 Feb;170(2):657–661. doi: 10.1128/jb.170.2.657-661.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galangau F., Daniel-Vedele F., Moureaux T., Dorbe M. F., Leydecker M. T., Caboche M. Expression of leaf nitrate reductase genes from tomato and tobacco in relation to light-dark regimes and nitrate supply. Plant Physiol. 1988 Oct;88(2):383–388. doi: 10.1104/pp.88.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer Y. M., Wray J. L., Filner P. The effect of tungstate on nitrate assimilation in higher plant tissues. Plant Physiol. 1969 Aug;44(8):1197–1199. doi: 10.1104/pp.44.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlburt B. K., Garrett R. H. Nitrate assimilation in Neurospora crassa: enzymatic and immunoblot analysis of wild-type and nit mutant protein products in nitrate-induced and glutamine-repressed cultures. Mol Gen Genet. 1988 Jan;211(1):35–40. doi: 10.1007/BF00338390. [DOI] [PubMed] [Google Scholar]
- Notton B. A., Hewitt E. J. The role of tungsten in the inhibition of nitrate reductase activity in spinach (spinacea oleracea L.) leaves. Biochem Biophys Res Commun. 1971 Aug 6;44(3):702–710. doi: 10.1016/s0006-291x(71)80140-7. [DOI] [PubMed] [Google Scholar]
- Oaks A., Aslam M., Boesel I. Ammonium and amino acids as regulators of nitrate reductase in corn roots. Plant Physiol. 1977 Mar;59(3):391–394. doi: 10.1104/pp.59.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinck M., Guilley E., Durr A., Hoff M., Pinck L., Fleck J. Complete sequence of one of the mRNAs coding for the small subunit of ribulose bisphosphate carboxylase of Nicotiana sylvestris. Biochimie. 1984 Jul-Aug;66(7-8):539–545. doi: 10.1016/0300-9084(84)90148-2. [DOI] [PubMed] [Google Scholar]
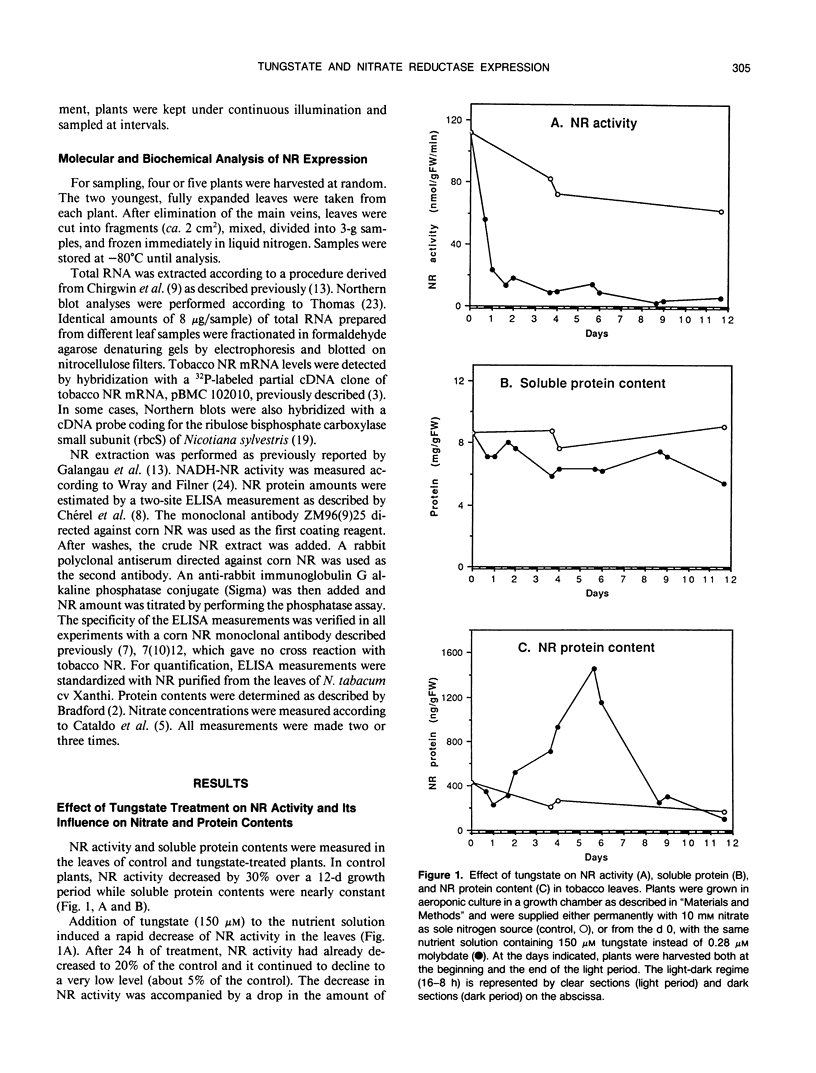
- Somers D. A., Kuo T. M., Kleinhofs A., Warner R. L., Oaks A. Synthesis and degradation of barley nitrate reductase. Plant Physiol. 1983 Aug;72(4):949–952. doi: 10.1104/pp.72.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray J. L., Filner P. Structural and functional relationships of enzyme activities induced by nitrate in barley. Biochem J. 1970 Oct;119(4):715–725. doi: 10.1042/bj1190715. [DOI] [PMC free article] [PubMed] [Google Scholar]