Abstract
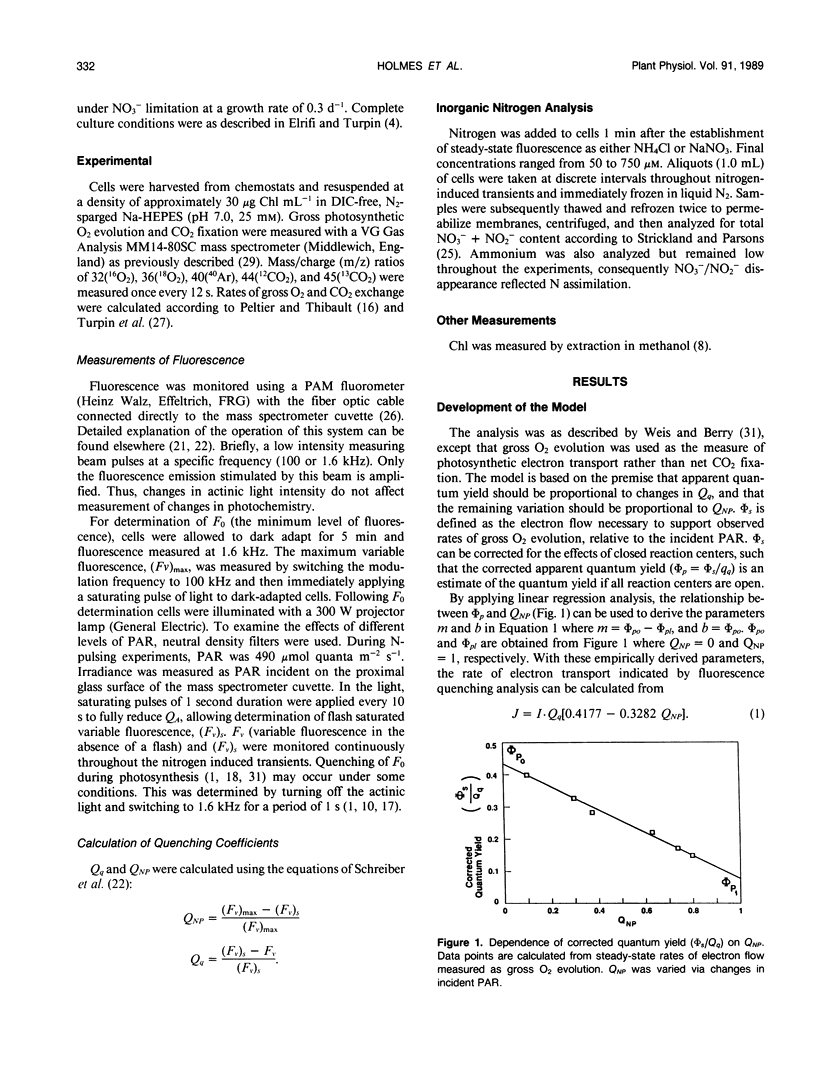
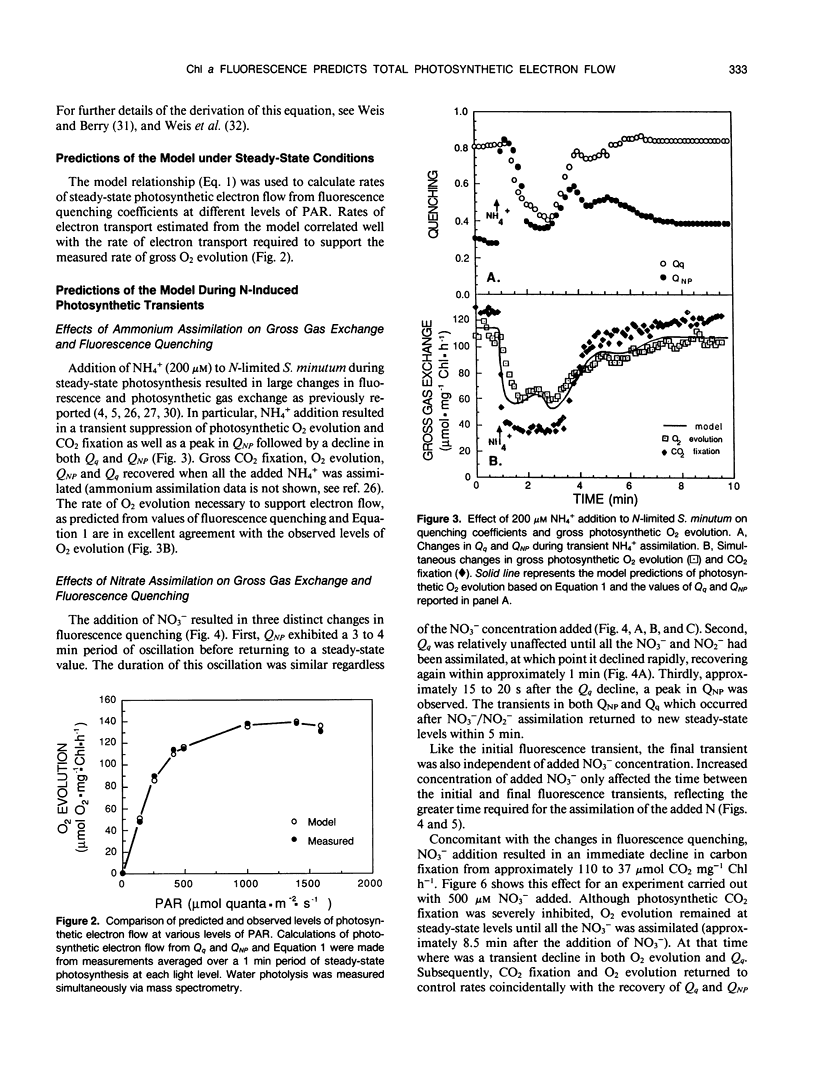
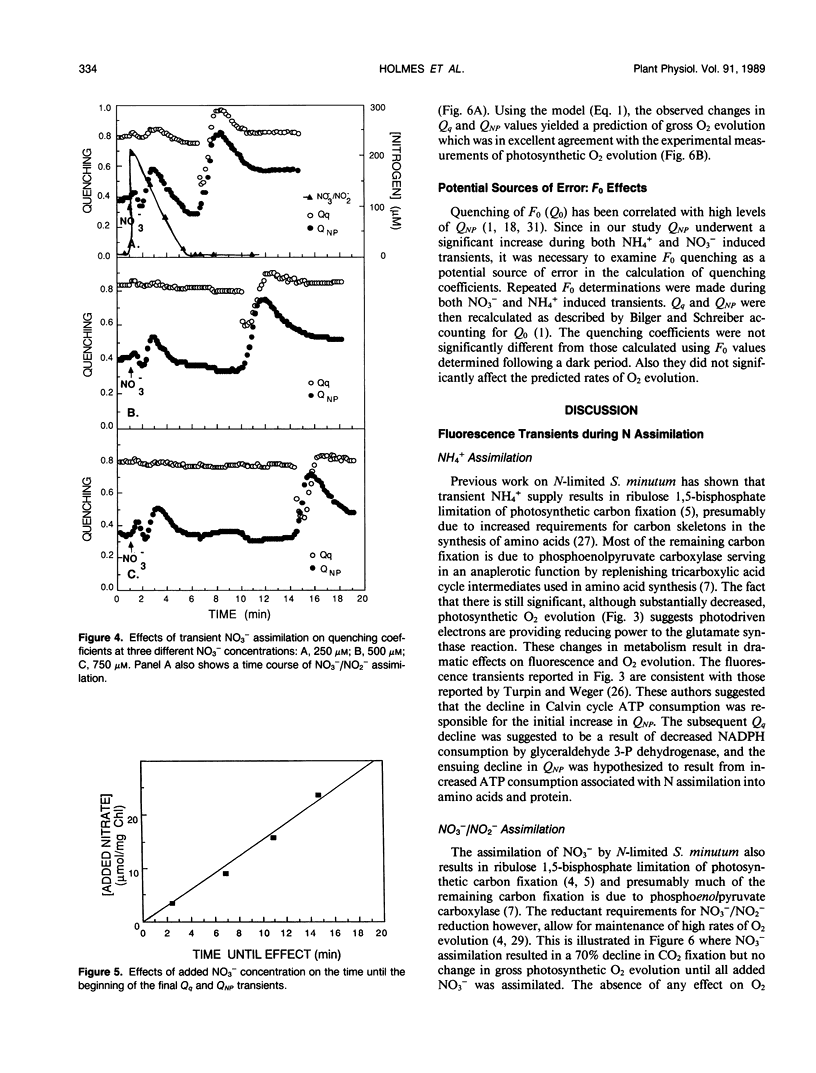
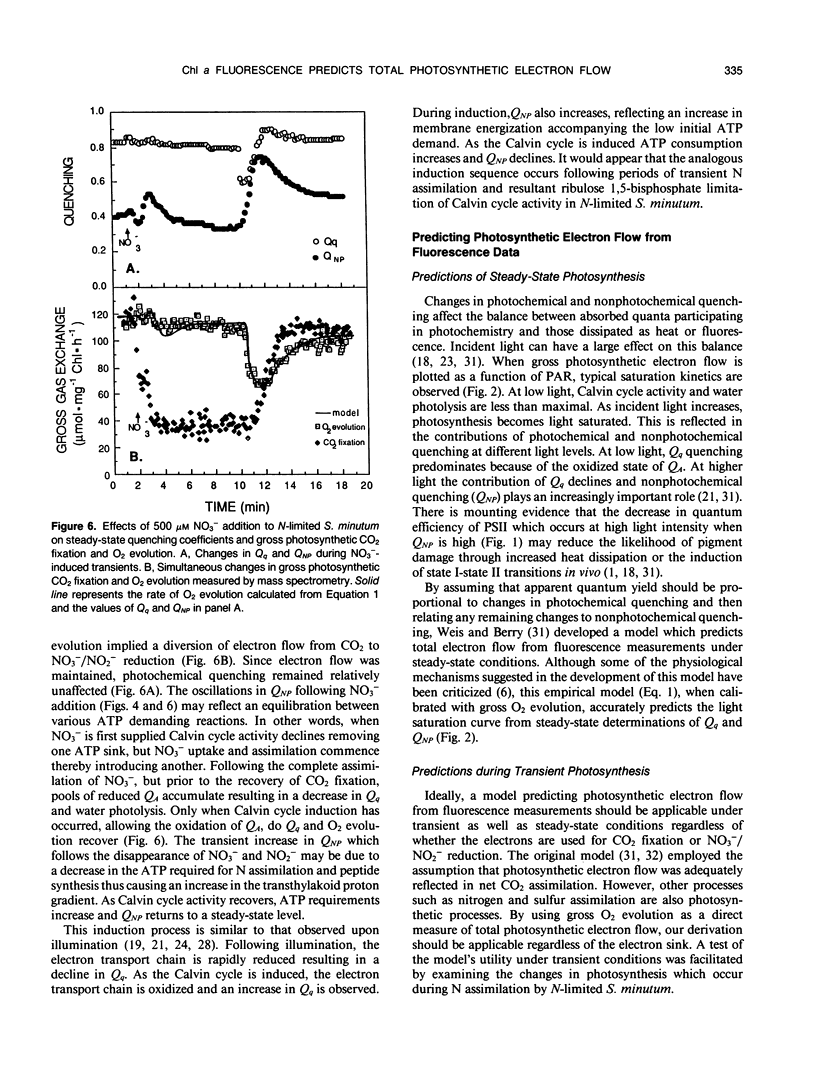
A model which predicts total photosynthetic electron flow from a linear regression of the relationship between corrected steady-state quantum yield and nonphotochemical quenching (E Weis, JA Berry [1987] Biochem Biophys Acta 894: 198-208) was formulated for N-limited cells of the green alga Selenastrum minutum. Unlike other models based on net CO2 fixation, our model is based on total photosynthetic electron flow measured as gross O2 evolution. This allowed for the prediction of total photosynthetic electron flow from water to both CO2 fixation and NO3−/NO2− reduction. The linear regression equation predicting electron flow is of the form: J = I · Qq[0.4777-0.3282 QNP] (where J = gross photosynthetic electron flow, I = incident PAR, Qq = photochemical quenching, QNP = nonphotochemical quenching). During steady-state photosynthesis, over a range of irradiance, the model predicted a photosynthetic light saturation curve which was well correlated with that observed. Although developed under steady-state conditions, the model was tested during nonsteady-state photosynthesis induced by transient nitrogen assimilation. The model predicted transient rates of gross O2 evolution which were in excellent agreement with the rates observed under a variety of conditions regardless of whether CO2 or NO3−/NO2− served as the physiological electron acceptor. The fluorescence transients resulting from ammonium and nitrate assimilation are discussed with respect to metabolic demands for reductant and ATP.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradbury M., Baker N. R. Analysis of the slow phases of the in vivo chlorophyll fluorescence induction curve. Changes in the redox state of photosystem II electron acceptors and fluorescence emission from photosystems I and II. Biochim Biophys Acta. 1981 May 13;635(3):542–551. doi: 10.1016/0005-2728(81)90113-4. [DOI] [PubMed] [Google Scholar]
- Elrifi I. R., Holmes J. J., Weger H. G., Mayo W. P., Turpin D. H. RuBP Limitation of Photosynthetic Carbon Fixation during NH(3) Assimilation : Interactions between Photosynthesis, Respiration, and Ammonium Assimilation in N-Limited Green Algae. Plant Physiol. 1988 Jun;87(2):395–401. doi: 10.1104/pp.87.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrifi I. R., Turpin D. H. Nitrate and Ammonium Induced Photosynthetic Suppression in N-Limited Selenastrum minutum. Plant Physiol. 1986 May;81(1):273–279. doi: 10.1104/pp.81.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy R. D., Vanlerberghe G. C., Turpin D. H. Significance of Phosphoenolpyruvate Carboxylase during Ammonium Assimilation: Carbon Isotope Discrimination in Photosynthesis and Respiration by the N-Limited Green Alga Selenastrum minutum. Plant Physiol. 1989 Apr;89(4):1150–1157. doi: 10.1104/pp.89.4.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier G., Thibault P. O(2) uptake in the light in chlamydomonas: evidence for persistent mitochondrial respiration. Plant Physiol. 1985 Sep;79(1):225–230. doi: 10.1104/pp.79.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. B., Sivak M. N., Walker D. A. Carbon Dioxide-Induced Oscillations in Fluorescence and Photosynthesis: Role of Thylakoid Membrane Energization in Regulation of Photosystem II Activity. Plant Physiol. 1988 Dec;88(4):1125–1130. doi: 10.1104/pp.88.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. B., Sivak M. N., Walker D. A. Relationship between Steady-State Fluorescence Yield and Photosynthetic Efficiency in Spinach Leaf Tissue. Plant Physiol. 1988 Sep;88(1):158–163. doi: 10.1104/pp.88.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpin D. H., Weger H. G. Steady-State Chlorophyll a Fluorescence Transients during Ammonium Assimilation by the N-Limited Green Alga Selenastrum minutum. Plant Physiol. 1988 Sep;88(1):97–101. doi: 10.1104/pp.88.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. A., Sivak M. N., Prinsley R. T., Cheesbrough J. K. Simultaneous measurement of oscillations in oxygen evolution and chlorophyll a fluorescence in leaf pieces. Plant Physiol. 1983 Nov;73(3):542–549. doi: 10.1104/pp.73.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weger H. G., Birch D. G., Elrifi I. R., Turpin D. H. Ammonium Assimilation Requires Mitochondrial Respiration in the Light : A Study with the Green Alga Selenastrum minutum. Plant Physiol. 1988 Mar;86(3):688–692. doi: 10.1104/pp.86.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weger H. G., Turpin D. H. Mitochondrial Respiration Can Support NO(3) and NO(2) Reduction during Photosynthesis : Interactions between Photosynthesis, Respiration, and N Assimilation in the N-Limited Green Alga Selenastrum minutum. Plant Physiol. 1989 Feb;89(2):409–415. doi: 10.1104/pp.89.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]


