Abstract
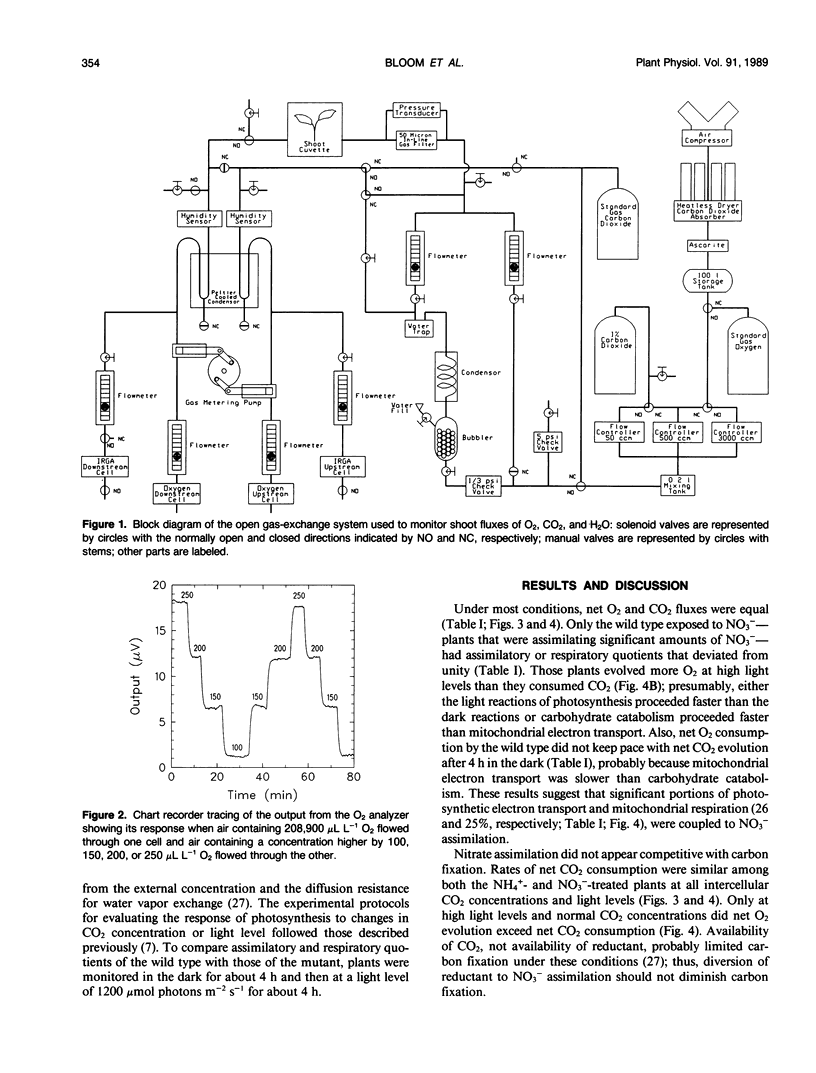
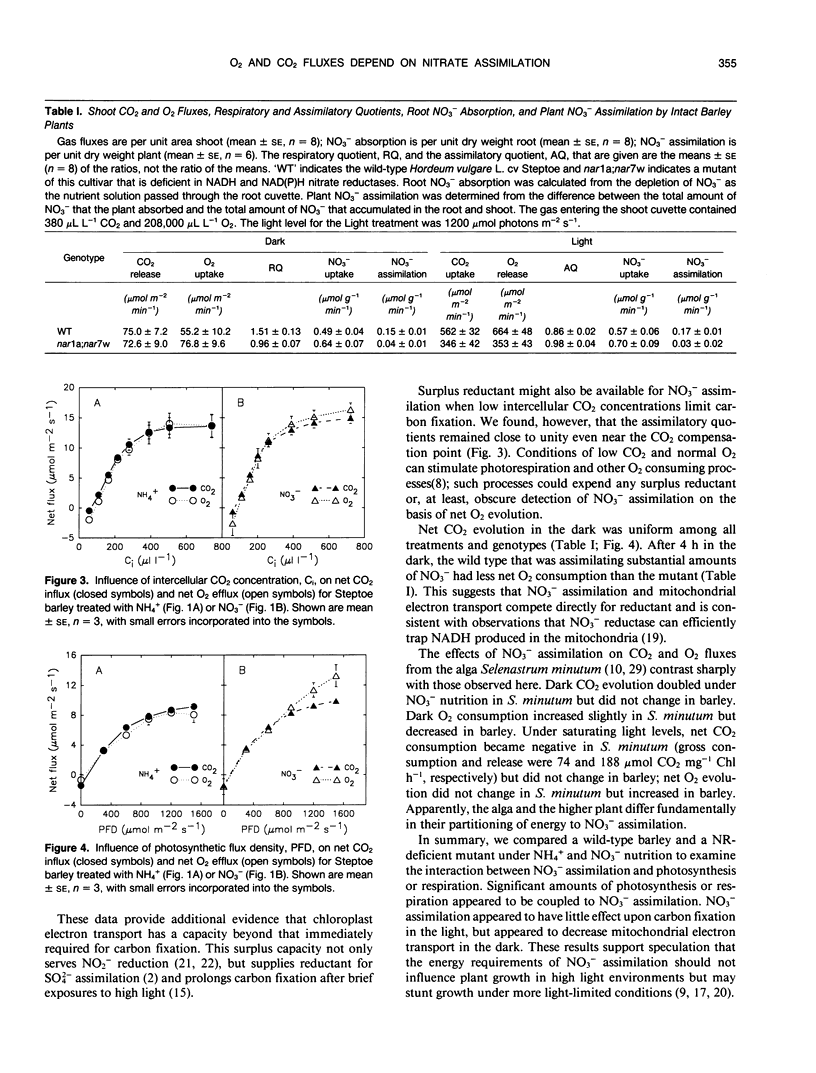
A custom oxygen analyzer in conjunction with an infrared carbon dioxide analyzer and humidity sensors permitted simultaneous measurements of oxygen, carbon dioxide, and water vapor fluxes from the shoots of intact barley plants (Hordeum vulgare L. cv Steptoe). The oxygen analyzer is based on a calciazirconium sensor and can resolve concentration differences to within 2 microliters per liter against the normal background of 210,000 microliters per liter. In wild-type plants receiving ammonium as their sole nitrogen source or in nitrate reductase-deficient mutants, photosynthetic and respiratory fluxes of oxygen equaled those of carbon dioxide. By contrast, wild-type plants exposed to nitrate had unequal oxygen and carbon dioxide fluxes: oxygen evolution at high light exceeded carbon dioxide consumption by 26% and carbon dioxide evolution in the dark exceeded oxygen consumption by 25%. These results indicate that a substantial portion of photosynthetic electron transport or respiration generates reductant for nitrate assimilation rather than for carbon fixation or mitochondrial electron transport.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aslam M., Huffaker R. C., Rains D. W. Early effects of salinity on nitrate assimilation in barley seedlings. Plant Physiol. 1984 Oct;76(2):321–325. doi: 10.1104/pp.76.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom A. J., Caldwell R. M. Root excision decreases nutrient absorption and gas fluxes. Plant Physiol. 1988 Aug;87(4):794–796. doi: 10.1104/pp.87.4.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canvin D. T., Berry J. A., Badger M. R., Fock H., Osmond C. B. Oxygen exchange in leaves in the light. Plant Physiol. 1980 Aug;66(2):302–307. doi: 10.1104/pp.66.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrifi I. R., Turpin D. H. Nitrate and Ammonium Induced Photosynthetic Suppression in N-Limited Selenastrum minutum. Plant Physiol. 1986 May;81(1):273–279. doi: 10.1104/pp.81.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. M. Carbon dioxide and nitrite photoassimilatory processes do not intercompete for reducing equivalents in spinach and soybean leaf chloroplasts. Plant Physiol. 1986 Mar;80(3):676–684. doi: 10.1104/pp.80.3.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. M. Spinach Leaf Chloroplast CO(2) and NO(2) Photoassimilations Do Not Compete for Photogenerated Reductant: Manipulation of Reductant Levels by Quantum Flux Density Titrations. Plant Physiol. 1988 Dec;88(4):1373–1380. doi: 10.1104/pp.88.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze E. D., Bloom A. J. Relationship between Mineral Nitrogen Influx and Transpiration in Radish and Tomato. Plant Physiol. 1984 Nov;76(3):827–828. doi: 10.1104/pp.76.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weger H. G., Turpin D. H. Mitochondrial Respiration Can Support NO(3) and NO(2) Reduction during Photosynthesis : Interactions between Photosynthesis, Respiration, and N Assimilation in the N-Limited Green Alga Selenastrum minutum. Plant Physiol. 1989 Feb;89(2):409–415. doi: 10.1104/pp.89.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]


