Abstract
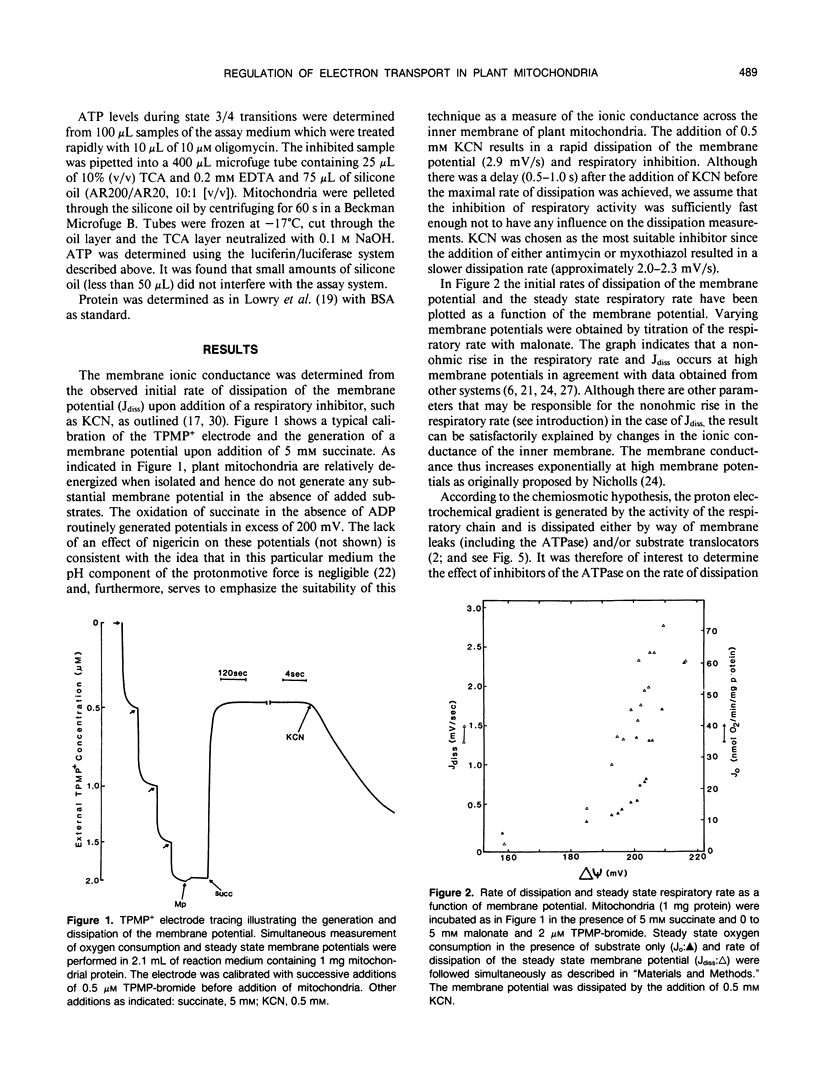
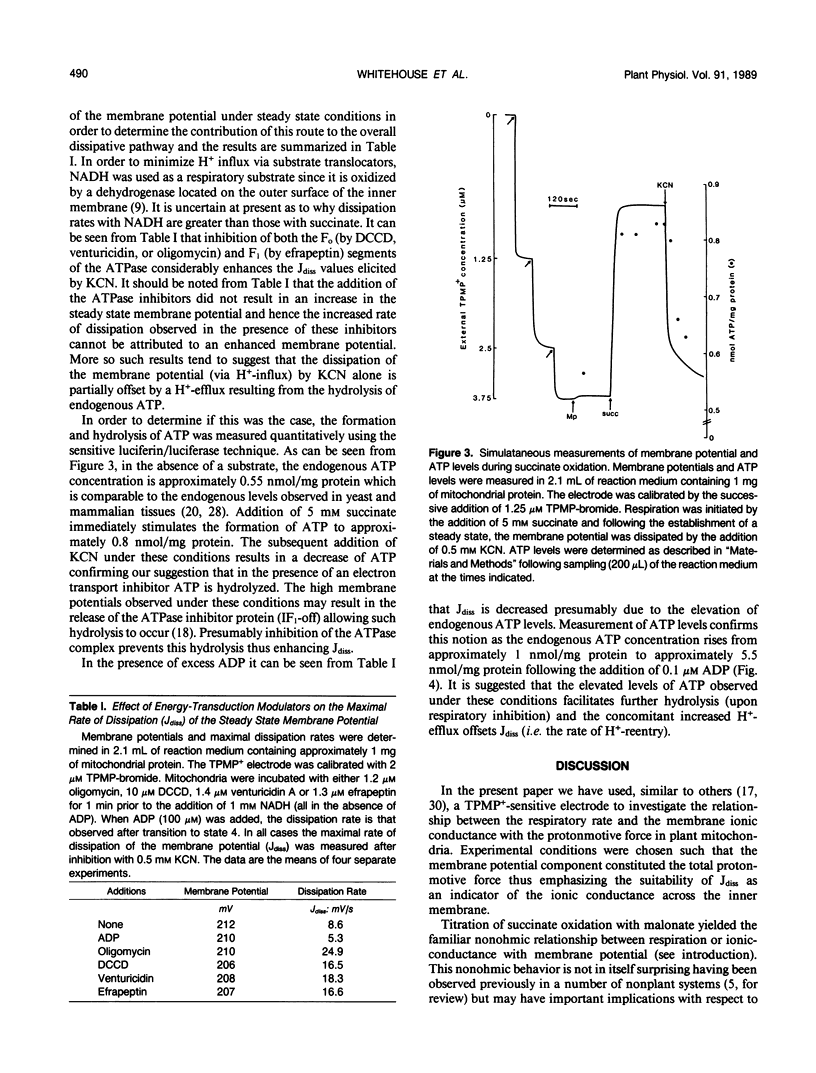
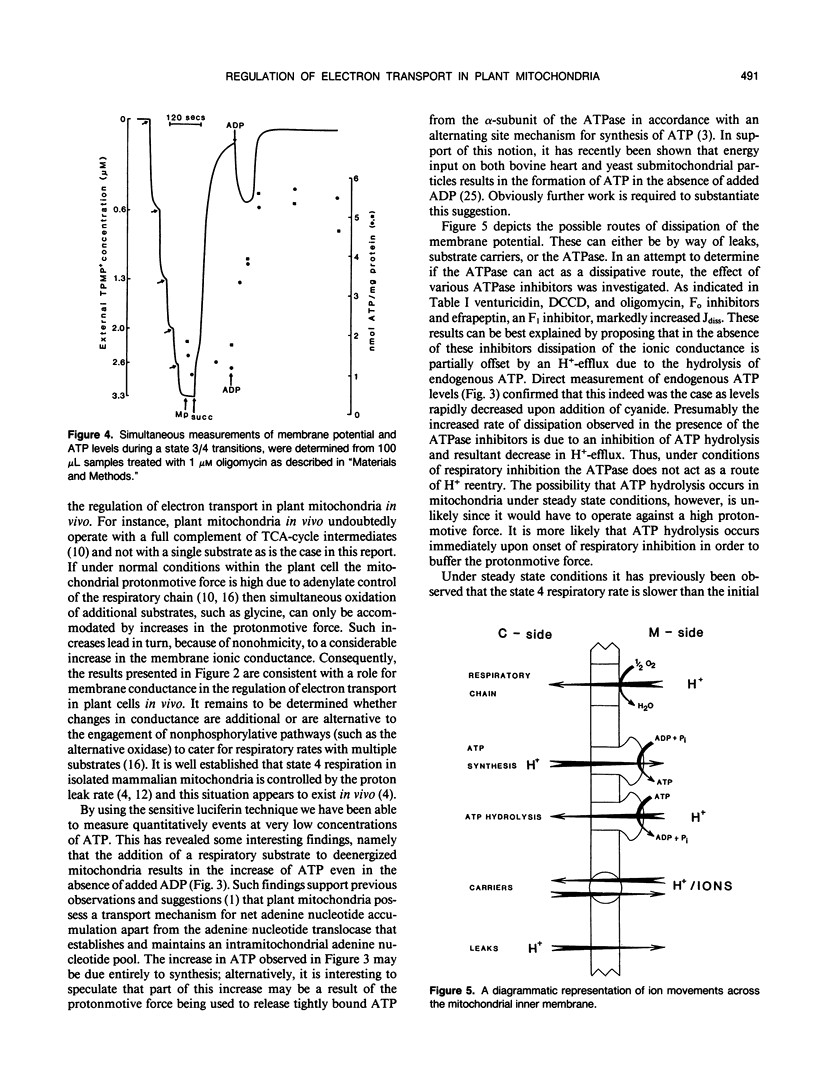
The relationship between the respiratory rate and the membrane ionic current on the protonmotive force has been investigated in percoll purified potato mitochondria. The dependence of the membrane ionic current on the membrane potential was monitored using a methyltriphenylphosphonium-sensitive electrode and determining the maximal net rate of depolarization following the addition of a respiratory inhibitor. We have confirmed that a nonohmic relationship exists between the ionic conductance and membrane potential. Addition of ATPase inhibitors markedly increased the initial rate of dissipation suggesting that in their absence the dissipation rate induced by respiratory inhibitors is partially offset by H+-efflux due to the hydrolysis of endogenous ATP. This was corroborated by direct measurement of endogenous ATP levels which decreased significantly following dissipation of the membrane potential. Results are discussed in terms of the regulation of electron transport in plant mitochondria in vivo.
Full text
PDF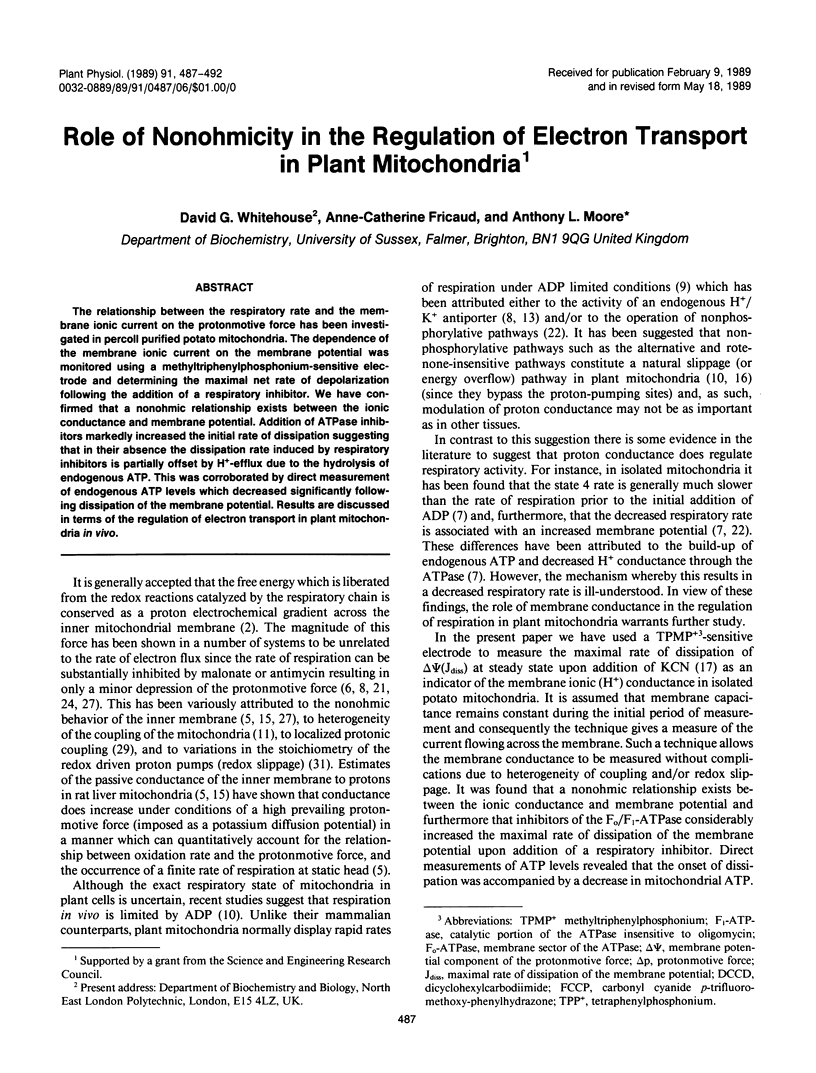


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer P. D., Kohlbrenner W. E., McIntosh D. B., Smith L. T., O'Neal C. C. ATP and ADP modulations of catalysis by F1 and Ca2+, Mg2+-ATPases. Ann N Y Acad Sci. 1982;402:65–83. doi: 10.1111/j.1749-6632.1982.tb25732.x. [DOI] [PubMed] [Google Scholar]
- Brand M. D., Murphy M. P. Control of electron flux through the respiratory chain in mitochondria and cells. Biol Rev Camb Philos Soc. 1987 May;62(2):141–193. doi: 10.1111/j.1469-185x.1987.tb01265.x. [DOI] [PubMed] [Google Scholar]
- Brown G. C., Brand M. D. Changes in permeability to protons and other cations at high proton motive force in rat liver mitochondria. Biochem J. 1986 Feb 15;234(1):75–81. doi: 10.1042/bj2340075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton N. P., Clark A. J., Jackson J. B. Changes in membrane ionic conductance, but not changes in slip, can account for the non-linear dependence of the electrochemical proton gradient upon the electron-transport rate in chromatophores. Eur J Biochem. 1984 Jul 2;142(1):193–198. doi: 10.1111/j.1432-1033.1984.tb08269.x. [DOI] [PubMed] [Google Scholar]
- Duszyński J., Wojtczak L. The apparent non-linearity of the relationship between the rate of respiration and the protonmotive force of mitochondria can be explained by heterogeneity of mitochondrial preparations. FEBS Lett. 1985 Mar 25;182(2):243–248. doi: 10.1016/0014-5793(85)80307-0. [DOI] [PubMed] [Google Scholar]
- Groen A. K., Wanders R. J., Westerhoff H. V., van der Meer R., Tager J. M. Quantification of the contribution of various steps to the control of mitochondrial respiration. J Biol Chem. 1982 Mar 25;257(6):2754–2757. [PubMed] [Google Scholar]
- Hensley J. R., Hanson J. B. The action of valinomycin in uncoupling corn mitochondria. Plant Physiol. 1975 Jul;56(1):13–18. doi: 10.1104/pp.56.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamo N., Muratsugu M., Hongoh R., Kobatake Y. Membrane potential of mitochondria measured with an electrode sensitive to tetraphenyl phosphonium and relationship between proton electrochemical potential and phosphorylation potential in steady state. J Membr Biol. 1979 Aug;49(2):105–121. doi: 10.1007/BF01868720. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy G., Hinkle P. C. Non-ohmic proton conductance of mitochondria and liposomes. Biochemistry. 1984 Apr 10;23(8):1640–1645. doi: 10.1021/bi00303a009. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lemasters J. J., Hackenbrock C. R. The energized state of rat liver mitochondria. ATP equivalence, uncoupler sensitivity, and decay kinetics. J Biol Chem. 1980 Jun 25;255(12):5674–5680. [PubMed] [Google Scholar]
- Lippe G., Sorgato M. C., Harris D. A. The binding and release of the inhibitor protein are governed independently by ATP and membrane potential in ox-heart submitochondrial vesicles. Biochim Biophys Acta. 1988 Mar 30;933(1):12–21. doi: 10.1016/0005-2728(88)90051-5. [DOI] [PubMed] [Google Scholar]
- Lundin M., Pereira da Silva L., Baltscheffsky H. Energy-dependent formation of free ATP in yeast submitochondrial particles, and its stimulation by oligomycin. Biochim Biophys Acta. 1987 Mar 4;890(3):279–285. doi: 10.1016/0005-2728(87)90154-x. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. The effective proton conductance of the inner membrane of mitochondria from brown adipose tissue. Dependency on proton electrochemical potential gradient. Eur J Biochem. 1977 Jul 15;77(2):349–356. doi: 10.1111/j.1432-1033.1977.tb11674.x. [DOI] [PubMed] [Google Scholar]
- Penefsky H. S. Energy-dependent dissociation of ATP from high affinity catalytic sites of beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1985 Nov 5;260(25):13735–13741. [PubMed] [Google Scholar]
- Proudlove M. O., Beechey R. B., Moore A. L. Pyruvate transport by thermogenic-tissue mitochondria. Biochem J. 1987 Oct 15;247(2):441–447. doi: 10.1042/bj2470441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater E. C. Mechanism of oxidative phosphorylation. Annu Rev Biochem. 1977;46:1015–1026. doi: 10.1146/annurev.bi.46.070177.005055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgato M. C., Ferguson S. J. Variable proton conductance of submitochondrial particles. Biochemistry. 1979 Dec 11;18(25):5737–5742. doi: 10.1021/bi00592a034. [DOI] [PubMed] [Google Scholar]
- Vàdineanu A., Berden J. A., Slater E. C. Proteins required for the binding of mitrochondrial ATPase to the mitochondrial inner membrane. Biochim Biophys Acta. 1976 Dec 6;449(3):468–479. doi: 10.1016/0005-2728(76)90156-0. [DOI] [PubMed] [Google Scholar]
- Westerhoff H. V., Melandri B. A., Venturoli G., Azzone G. F., Kell D. B. A minimal hypothesis for membrane-linked free-energy transduction. The role of independent, small coupling units. Biochim Biophys Acta. 1984 Dec 17;768(3-4):257–292. doi: 10.1016/0304-4173(84)90019-3. [DOI] [PubMed] [Google Scholar]
- Wojtczak L., Zókiewska A., Duszyński J. Energy-storage capacity of the mitochondrial proton-motive force. Biochim Biophys Acta. 1986 Sep 10;851(2):313–321. doi: 10.1016/0005-2728(86)90138-6. [DOI] [PubMed] [Google Scholar]
- Zoratti M., Favaron M., Pietrobon D., Azzone G. F. Intrinsic uncoupling of mitochondrial proton pumps. 1. Non-ohmic conductance cannot account for the nonlinear dependence of static head respiration on delta microH. Biochemistry. 1986 Feb 25;25(4):760–767. doi: 10.1021/bi00352a004. [DOI] [PubMed] [Google Scholar]


