Abstract
OBJECTIVE
To examine whether the effect of conventional lifestyle interventions on type 2 diabetes incidence differs by glucose-defined prediabetes phenotype.
RESEARCH DESIGN AND METHODS
We searched multiple databases until 1 April 2023 for randomized controlled trials that recruited people with isolated impaired fasting glucose (i-IFG), isolated impaired glucose tolerance (i-IGT), and impaired fasting glucose plus impaired glucose tolerance (IFG+IGT). Individual participant data were pooled from relevant trials and analyzed through random-effects models with use of the within-trial interactions approach.
RESULTS
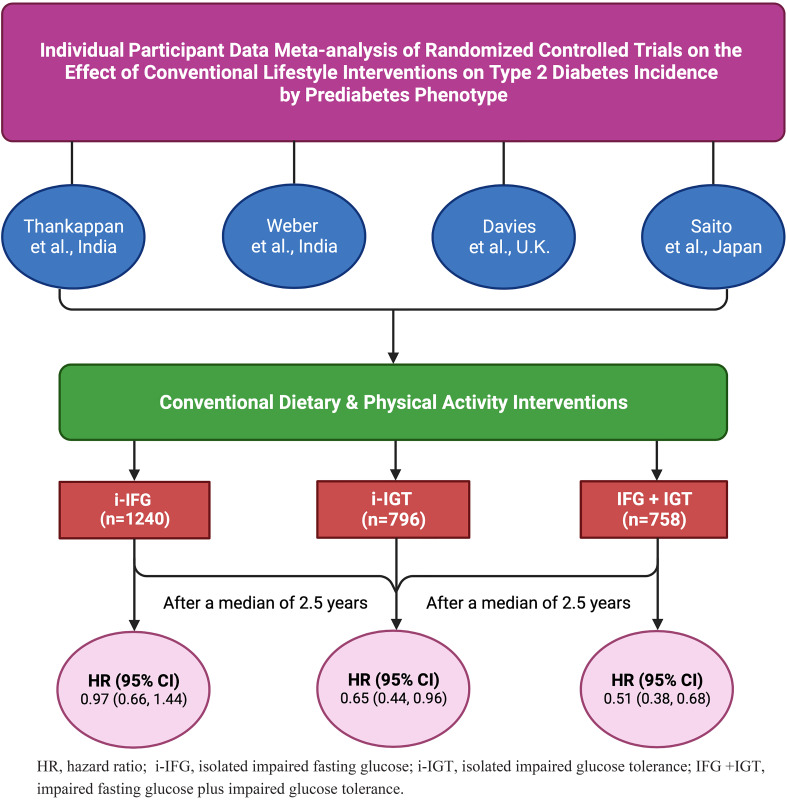
Four trials with 2,794 participants (mean age 53.0 years, 60.7% men) were included: 1,240 (44.4%), 796 (28.5%), and 758 (27.1%) had i-IFG, i-IGT, and IFG+IGT, respectively. After a median of 2.5 years, the pooled hazard ratio for diabetes incidence in i-IFG was 0.97 (95% CI 0.66, 1.44), i-IGT 0.65 (0.44, 0.96), and IFG+IGT 0.51 (0.38, 0.68; Pinteraction = 0.01).
CONCLUSIONS
Conventional lifestyle interventions reduced diabetes incidence in people with IGT (with or without IFG) but not in those with i-IFG.
Graphical Abstract
Introduction
Conventional lifestyle interventions incorporating behavioral counseling to change diet and physical activity reduce type 2 diabetes incidence in people with prediabetes (1). It remains unclear, however, whether they are effective in all three glucose-defined prediabetes phenotypes, including isolated impaired fasting glucose (i-IFG), isolated impaired glucose tolerance (i-IGT), and impaired fasting glucose plus impaired glucose tolerance (IFG+IGT) (2). In this systematic review and individual participant data (IPD) meta-analysis, we examined whether the effect of conventional lifestyle interventions on diabetes incidence differs by glucose-defined prediabetes phenotype.
Research Design and Methods
We followed standard guidelines for the conduct and reporting of this study (Supplementary Table 1) (3,4), which is registered with International prospective register of systematic reviews (PROSPERO) (no. CRD42020197356).
Search Strategies and Eligibility Criteria
We searched MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), Embase, Scopus, and ClinicalTrials.gov from inception to 1 April 2023 using the search strategies given in Supplementary Table 2. No language restrictions were applied. We considered randomized controlled trials (RCTs) satisfying the eligibility criteria: 1) recruiting of adults (≥18 years) with i-IFG, with i-IGT, and with IFG+IGT, defined based on the American Diabetes Association (ADA) (5) or World Health Organization (WHO) (6) criteria, and 2) evaluation of the effect of conventional dietary or physical activity interventions on diabetes incidence (fasting plasma glucose ≥126 mg/dL, 2-h plasma glucose ≥200 mg/dL, or taking antidiabetes medications) (5) in comparison with a control group (usual care or minimal intervention). Conventional lifestyle interventions are similar to or based on the interventions tested in landmark lifestyle RCTs for diabetes prevention (1,7). We excluded studies reporting exclusively pharmaceutical or surgical interventions.
Data Sharing
We contacted principal investigators (PIs) of eligible studies to obtain IPD that are relevant for this study. The PIs had ethics approval to share their study data. After signing data-sharing agreements, de-identified IPD were obtained and checked for accuracy, consistency, and completeness.
Risk of Bias and Certainty of the Evidence Assessment
The Cochrane risk-of-bias tool, version 2 (RoB 2), was used to assess the bias in each study (8), and the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework was used to determine the certainty of the evidence (9).
Two reviewers (T.S. and R.B) independently screened study titles, abstracts, and full texts; extracted study-level data from published articles; and performed the risk of bias and GRADE assessments, with disagreements resolved by discussion or by a third author (R.J.T).
Statistical Analyses
Analyses were done per the intention-to-treat principle (3). We pooled the incidence rates of diabetes (per 1,000 person-years) across studies using the random-effects DerSimonian-Laird models (3). Cox regression was used to estimate hazard ratios (HRs) (and 95% CIs) for diabetes incidence in individually randomized trials, and shared frailty models (10) were used in cluster-randomized trials to account for the correlation of observations within clusters. We conducted a two-stage IPD meta-analysis (3). Firstly, we analyzed the IPD of each study separately to obtain relevant aggregate data (HRs and 95% CIs). If no IPD were available for a study, we used the effect estimates from the published article. Secondly, we pooled these aggregate estimates using random-effects models (3). Effect modification by prediabetes phenotype was assessed with addition of an interaction term between the phenotype and the treatment group in each study separately. If only aggregate data were available for a study, we used the HRs (and 95% CIs) from the published article to estimate the interaction HR (and 95% CI) using the equation developed by Riley and Fisher (11) (Supplementary Table 3). The interaction estimates were then pooled with use of random-effects models (3). The proportion of variability in effect estimates due to between-study heterogeneity was quantified with I2 (3). We did not assess publication bias, as the number of included studies was <10 (3). We conducted sensitivity analyses to assess the robustness of our results. In the Diabetes Community Lifestyle Improvement Program (D-CLIP), 72.2%, 48.2%, and 75.5% of intervention participants with i-IFG, with i-IGT, and with IFG+IGT, respectively, required metformin (500 mg twice daily), in addition to undergoing lifestyle interventions, at 4 months or later (12). So, in D-CLIP, we adjusted for metformin use (yes or no) in Cox models. In addition, we imputed missing outcome data (varied from 0 to 9.1% across studies) using multiple imputation (13) (Supplementary Table 4). Analyses were performed in Stata software.
Data and Resource Availability
Data-sharing agreements with PIs of the individual studies restrict further dissemination of data to third parties.
Results
A total of 3,678 articles were identified through our systematic search, among which four studies met our eligibility criteria and were included in this meta-analysis (Supplementary Fig. 1).
Table 1 shows the characteristics of included studies. We obtained the IPD of three studies: Kerala Diabetes Prevention Program (K-DPP) (14) and D-CLIP (12) from India and Let’s Prevent Diabetes from the U.K. (15). IPD of the Zensharen Study for Prevention of Lifestyle Diseases (ZSPLD) from Japan (16) were unavailable because the organization that conducted this study no longer exists. K-DPP and D-CLIP were conducted in the community (12,14), whereas Let’s Prevent Diabetes and ZSPLD were done in clinical settings (15,16). In all four studies behavior change counseling was implemented for achievement of diet and physical activity modification, lasting 0.5–3.0 years.
Table 1.
Characteristics of studies and participants included in the meta-analysis
| First author and year (study), country (ref. no.) | Study design | Study setting | Type of data | Criteria for prediabetes | N | Age in years | % Male | Components of lifestyle intervention programs | Control group | |
|---|---|---|---|---|---|---|---|---|---|---|
| Diet | Physical activity | |||||||||
| Thankappan 2018 (K-DPP), India (14) | Cluster RCT | Community (clusters are polling areas*) | IPD | ADA | 695 | 46.4 (7.3) | 55.0 | Increase fruit and vegetable intake and reduce portion size of rice and intake of fried foods and refined sugars | Walking groups and yoga sessions | Received a booklet on healthy lifestyle advice |
| Weber 2016 (D-CLIP), India (12) | RCT | Community | IPD | ADA | 578 | 44.4 (9.3) | 63.2 | Reduce daily calorie intake and portion sizes and increase fiber intake | ≥150 min of moderate-intensity exercise weekly | One-on-one visits with a clinician, dietitian, and fitness trainer on a single day and one group class on diabetes prevention |
| Davies 2016 (LPD), UK (15) | Cluster RCT | General practices (clusters) | IPD | WHO | 880 | 63.9 (7.8) | 63.6 | Restrict total fat and saturated fat intake to 30% and 10% of daily total energy intake, respectively, and increase fiber intake | Participants were provided with a pedometer to help increase daily physical activity | Received an information booklet on lifestyle change |
| Saito 2011 (ZSPLD), Japan (16) | RCT | Clinics and hospitals | Aggregate data | ADA | 641 | N/A | N/A | Limit fat and carbohydrate intake to 20–25% and 55–60% of total energy intake, respectively | Advised to walk to achieve an energy expenditure of 200 kcal/day. Sedentary individuals were encouraged to increase daily physical activity | Received individual instructions on lifestyle modification from the medical staff four times at 12-month intervals for 3 years |
Data for age are means (SD). K-DPP, Kerala Diabetes Prevention Program; D-CLIP, Diabetes Community Lifestyle Improvement Program; LPD, Let’s Prevent Diabetes; ZSPLD, Zensharen Study for Prevention of Lifestyle Diseases; N/A, not available; RCT, randomized controlled trial; ADA, American Diabetes Association; WHO, World Health Organization.
Polling areas are well-defined and identifiable locations demarcated with landmarks such as hills, rivers, roads, etc. by the Election Commission of India.
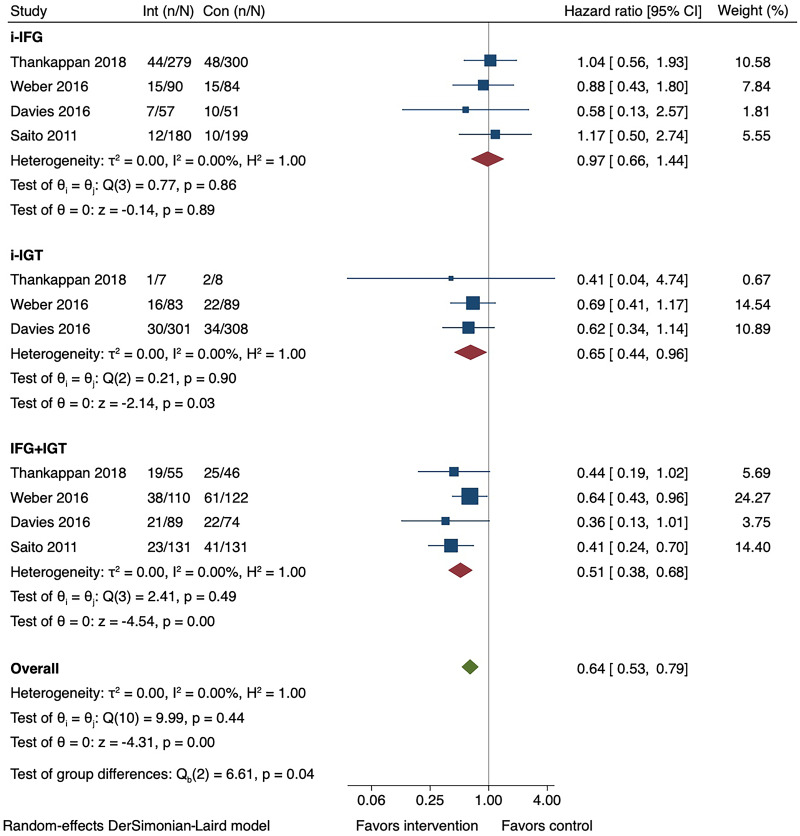
A total of 2,794 participants (mean age 53.0 years, 60.7% men) were included in the meta-analysis: 1,240 (44.4%), 796 (28.5%), and 758 (27.1%) had i-IFG, i-IGT, and IFG+IGT, respectively. The overall pooled incidence rate of diabetes was highest in the IFG+IGT group, followed by the i-IGT and i-IFG groups (Table 2). After a median of 2.5 years (interquartile range 2.3, 2.8), the pooled HR for diabetes incidence in i-IFG was 0.97 (95% CI 0.66, 1.44; I2 = 0), i-IGT 0.65 (0.44, 0.96; I2 = 0), and IFG+IGT 0.51 (0.38, 0.68; I2 = 0) (Pinteraction = 0.01) (Fig. 1 and Supplementary Fig. 2). The main results were not materially altered in sensitivity analyses (Supplementary Tables 4 and 5). The risk of bias was low in all four studies (Supplementary Fig. 3), and the certainty of the evidence was moderate (Supplementary Table 6). There are minor discrepancies in effect estimates between the original articles (12,14–16) and the current study, the reasons for which are explained in Supplementary Table 7.
Table 2.
Pooled incidence rate of diabetes across studies by prediabetes phenotype
| Prediabetes phenotype | Study arm | No. of participants | No. of events | IR (95% CI) per 1,000 person-years |
|---|---|---|---|---|
| i-IFG | Total | 1,240 | 161 | 54.77 (20.34, 89.20) |
| Control arm | 634 | 83 | 55.47 (16.40, 94.53) | |
| Intervention arm | 606 | 78 | 53.13 (21.98, 84.28) | |
| i-IGT | Total | 796 | 105 | 65.21 (19.96, 110.45) |
| Control arm | 405 | 58 | 72.16 (14.71, 129.60) | |
| Intervention arm | 391 | 47 | 49.17 (20.28, 78.05) | |
| IFG+IGT | Total | 758 | 250 | 147.01 (93.07, 200.95) |
| Control arm | 373 | 149 | 180.46 (113.84, 247.09) | |
| Intervention arm | 385 | 101 | 107.02 (63.22, 150.82) |
IR, incidence rate; CI, confidence interval; i-IFG, isolated impaired fasting glucose; i-IGT, isolated impaired glucose tolerance; IFG+IGT, impaired fasting glucose plus impaired glucose tolerance.
Figure 1.
Forest plot for the effect of conventional lifestyle interventions on type 2 diabetes incidence by prediabetes phenotype. Con, control; Int, intervention; i-IFG, isolated impaired fasting glucose; i-IGT, isolated impaired glucose tolerance; IFG+IGT, impaired fasting glucose plus impaired glucose tolerance. n refers to the number of events, and N refers to the sample size.
Conclusions
The findings of this systematic review and meta-analysis show that the effect of conventional lifestyle interventions on type 2 diabetes incidence varies among prediabetes phenotypes, with a significant risk reduction in people with i-IGT and with IFG+IGT but not in those with i-IFG.
These differences in risk reduction could be attributed mainly to the variations in the pathophysiological abnormalities between prediabetes phenotypes (17). People with i-IFG have decreased early-phase insulin secretion and increased hepatic insulin resistance, whereas i-IGT is characterized by reduced early- and late-phase insulin secretion and elevated skeletal muscle insulin resistance and IFG+IGT includes a combination of defects seen in i-IFG and i-IGT (17). These pathophysiological abnormalities that differentiate individuals with IGT and i-IFG might mean that different therapeutic interventions are likely required to prevent progression to diabetes (2,17).
People with i-IFG constitute a substantial proportion of the global prediabetes population. A recent meta-analysis of 14 studies with 27,112 individuals with prediabetes found that the proportional prevalence of i-IFG (ADA criteria) was 58% in Caucasians and 48% in Asians (18). The proportional prevalence of i-IFG among adults in India was much higher (84% with ADA criteria), as reported in a nationwide study (19). In addition to its high prevalence, i-IFG increases the risk of developing diabetes four- to sixfold in comparison with normoglycemia (20) and is a high-risk state for cardiovascular disease and all-cause mortality (2). Thus, more research is required to identify effective interventions for this large group at high risk. Some promising strategies include a low-calorie diet (∼1,200 kcal/day) or high-intensity interval training, as they have been shown to normalize fasting plasma glucose and reverse the pathophysiology in people with type 2 diabetes (2).
The strengths of this analysis included the ability to obtain IPD, permitting standardization of the effect measure and outcome definition across studies, and imputation of missing outcome data. We used the “within-trial interactions” approach to assess the differences in the intervention effect between prediabetes phenotypes, thereby eliminating aggregation bias (3). However, the analyses are post hoc and observational, so the results should be considered hypothesis generating. We combined studies with i-IFG defined based on the ADA (three studies) or WHO (one study) criteria for the meta-analysis. However, this did not affect our results, as the pooled HRs for i-IFG defined only according to ADA criteria and WHO criteria were similar (1.01 vs. 0.96, respectively), and they were also similar to the pooled HR in Fig. 1 (0.97). Further, the meta-analysis is constrained by a small number of studies, the majority of which were conducted among Asian Indians or Japanese, and so the effect of lifestyle interventions in i-IFG may be different for other ethnicities. Finally, there is a possibility of confirmation bias based on findings from the individual studies included in the systematic review. However, the meta-analysis mitigated any substantial risk from this bias.
In conclusion, conventional lifestyle interventions significantly reduced type 2 diabetes incidence in people with IGT (with or without IFG) but not in those with i-IFG. Further confirmation and efforts to lower diabetes incidence in people with i-IFG are needed.
This article contains supplementary material online at https://doi.org/10.2337/figshare.23675406.
Article Information
Funding and Duality of Interest. T.S. is supported by the Woodruff Health Sciences Center Synergy Awards and the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under award number UL1TR002378. T.S. and M.K.A. are partially supported by grant #75D30120P0742 from the Centers for Disease Control and Prevention (CDC) Atlanta. K.K., M.J.D., and T.Y. are supported by the National Institute for Health and Care Research (NIHR) Applied Research Collaboration East Midlands (ARC EM) and the NIHR Leicester Biomedical Research Centre. J.E.S. is supported by a National Health and Medical Research Council (NHMRC) Investigator Grant. R.B. is affiliated with the NIHR ARC EM. M.K.A. and K.M.V.N. are partially supported by the Georgia Center for Diabetes Translation Research, which is funded by the NIH (P30DK111024). K.K. was Chair and M.J.D. and T.Y. were members of the National Institute for Health and Care Excellence (NICE) Public Health Guidance (PH38): Type 2 Diabetes: Prevention in people at high risk. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. T.S., K.K., K.M.V.N., V.M., M.J.D., T.Y., B.O., K.R.T., R.J.T., R.M.A., M.B.W., M.K.A., and J.E.S. were involved in the conception of the study. T.S. wrote the initial draft of the manuscript and conducted the statistical analyses with guidance from the statistician (R.B.). T.S. and R.B. developed the search strategies and performed the literature search, study selection, data extraction, and risk-of-bias and certainty-of-the-evidence assessment with disagreements resolved by R.J.T. All authors provided intellectual input for the manuscript, contributed to revising the manuscript, and approved the final content. T.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding Statement
T.S. is supported by the Woodruff Health Sciences Center Synergy Awards and the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under award number UL1TR002378. T.S. and M.K.A. are partially supported by grant #75D30120P0742 from the Centers for Disease Control and Prevention (CDC) Atlanta. K.K., M.J.D., and T.Y. are supported by the National Institute for Health and Care Research (NIHR) Applied Research Collaboration East Midlands (ARC EM) and the NIHR Leicester Biomedical Research Centre. J.E.S. is supported by a National Health and Medical Research Council (NHMRC) Investigator Grant. R.B. is affiliated with the NIHR ARC EM. M.K.A. and K.M.V.N. are partially supported by the Georgia Center for Diabetes Translation Research, which is funded by the NIH (P30DK111024). K.K. was Chair and M.J.D. and T.Y. were members of the National Institute for Health and Care Excellence (NICE) Public Health Guidance (PH38): Type 2 Diabetes: Prevention in people at high risk. No other potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying article, p. 1894.
M.K.A. and J.E.S. are joint senior authors.
References
- 1. Haw JS, Galaviz KI, Straus AN, et al. Long-term sustainability of diabetes prevention approaches: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med 2017;177:1808–1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Campbell MD, Sathish T, Zimmet PZ, et al. Benefit of lifestyle-based T2DM prevention is influenced by prediabetes phenotype. Nat Rev Endocrinol 2020;16:395–400 [DOI] [PubMed] [Google Scholar]
- 3. Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, U.K., John Wiley & Sons, 2019 [Google Scholar]
- 4. Stewart LA, Clarke M, Rovers M, et al.; PRISMA-IPD Development Group . Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: the PRISMA-IPD Statement. JAMA 2015;313:1657–1665 [DOI] [PubMed] [Google Scholar]
- 5. ElSayed NA, Aleppo G, Aroda VR, et al.; American Diabetes Association . 2. Classification and diagnosis of diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2023;46(Suppl. 1):S19–S40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization, International Diabetes Federation . Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of a WHO/IDF Consultation. Geneva, World Health Org., 2006 [Google Scholar]
- 7. Aziz Z, Mathews E, Absetz P, et al. A group-based lifestyle intervention for diabetes prevention in low- and middle-income country: implementation evaluation of the Kerala Diabetes Prevention Program. Implement Sci 2018;13:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 9. Guyatt GH, Oxman AD, Vist GE, et al.; GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gorfine M, Zucker DM. Shared frailty methods for complex survival data: a review of recent advances. Annu Rev Stat Appl 2023;10:51–73 [Google Scholar]
- 11. Riley RD, Debray TPA, Fisher DJ, et al.; Individual participant data meta-analysis to examine interactions between treatment effect and participant-level covariates: statistical recommendations for conduct and planning. Stat Med. 2020;39:2115–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weber MB, Ranjani H, Staimez LR, et al. The stepwise approach to diabetes prevention: results from the D-CLIP randomized controlled trial. Diabetes Care 2016;39:1760–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res 2011;20:40–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thankappan KR, Sathish T, Tapp RJ, et al. A peer-support lifestyle intervention for preventing type 2 diabetes in India: a cluster-randomized controlled trial of the Kerala Diabetes Prevention Program. PLoS Med 2018;15:e1002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davies MJ, Gray LJ, Troughton J, et al.; Let’s Prevent Diabetes Team . A community based primary prevention programme for type 2 diabetes integrating identification and lifestyle intervention for prevention: the Let’s Prevent Diabetes cluster randomised controlled trial. Prev Med 2016;84:48–56 [DOI] [PubMed] [Google Scholar]
- 16. Saito T, Watanabe M, Nishida J, et al.; Zensharen Study for Prevention of Lifestyle Diseases Group . Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose levels: a randomized controlled trial. Arch Intern Med 2011;171:1352–1360 [DOI] [PubMed] [Google Scholar]
- 17. Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of β-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 2006;29:1130–1139 [DOI] [PubMed] [Google Scholar]
- 18. Yip WCY, Sequeira IR, Plank LD, Poppitt SD. Prevalence of pre-diabetes across ethnicities: a review of impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) for classification of dysglycaemia. Nutrients 2017;9:1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Anjana RM, Deepa M, Pradeepa R, et al.; ICMR–INDIAB Collaborative Study Group . Prevalence of diabetes and prediabetes in 15 states of India: results from the ICMR-INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol 2017;5:585–596 [DOI] [PubMed] [Google Scholar]
- 20. Richter B, Hemmingsen B, Metzendorf MI, Takwoingi Y. Development of type 2 diabetes mellitus in people with intermediate hyperglycaemia. Cochrane Database Syst Rev 2018;10:CD012661. [DOI] [PMC free article] [PubMed] [Google Scholar]