Abstract
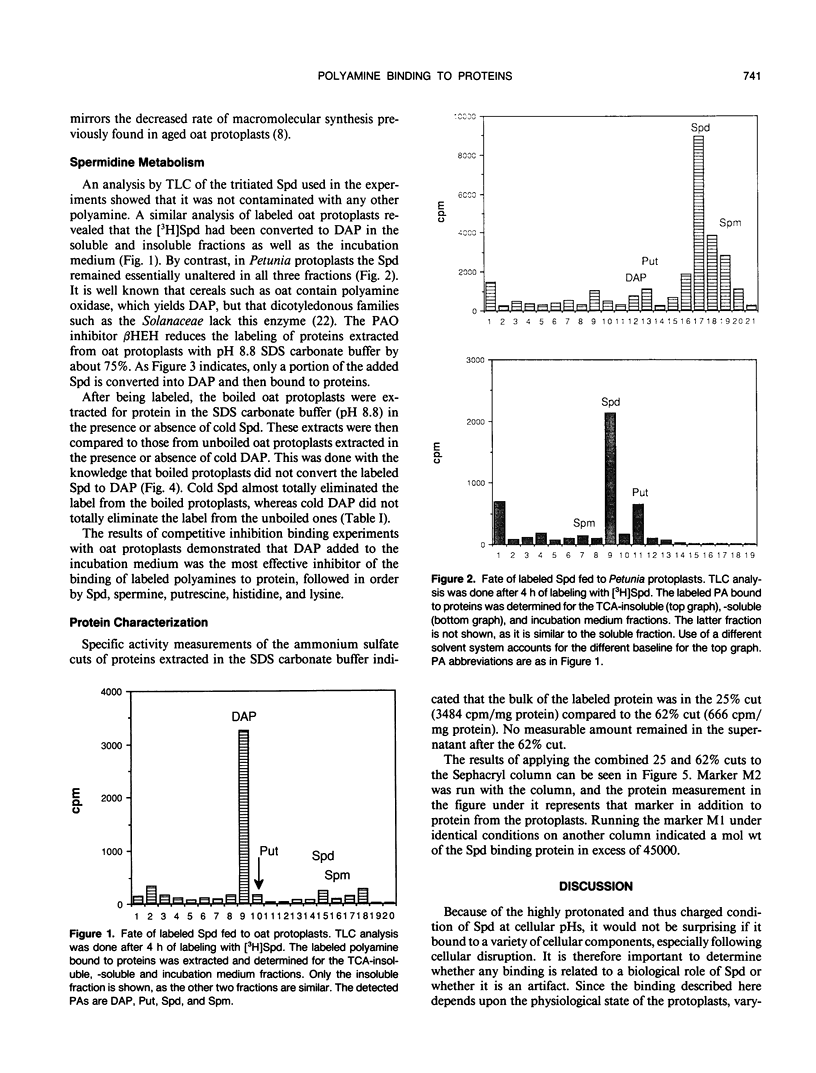
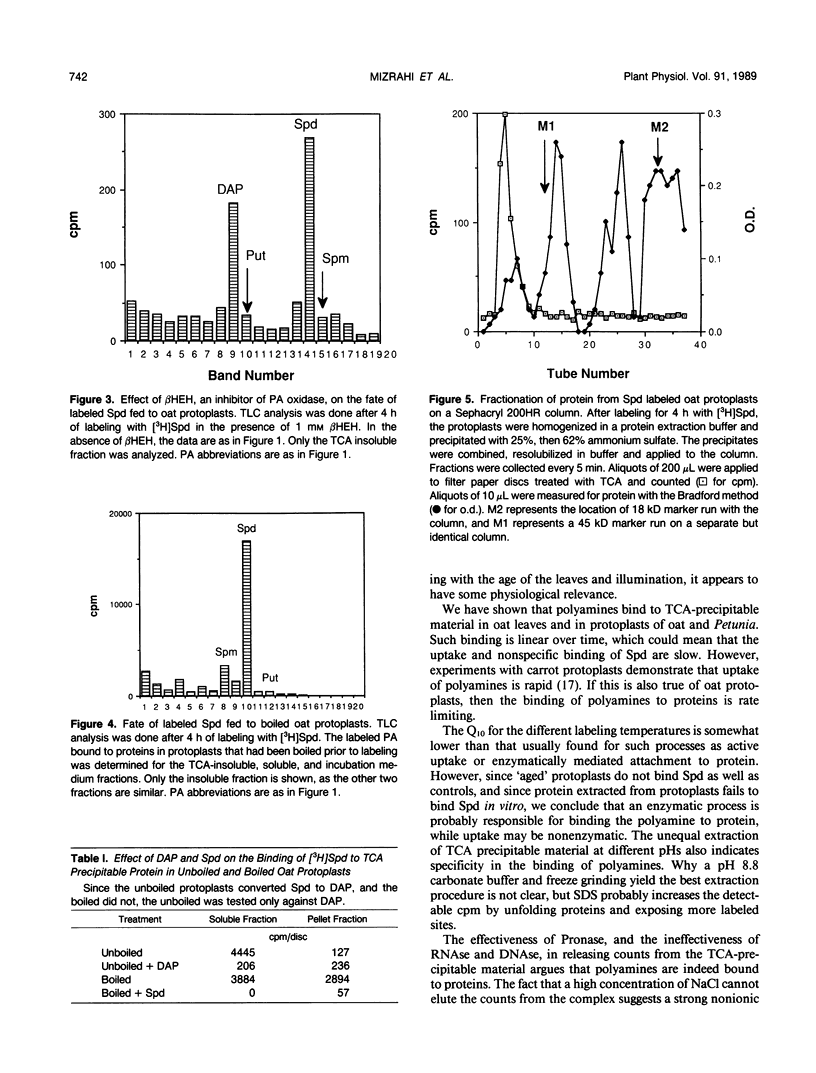
Previous work (A Apelbaum et al. [1988] Plant Physiol 88: 996-998) has demonstrated binding of labeled spermidine (Spd) to a developmentally regulated 18 kilodalton protein in tobacco tissue cultures derived from thin surface layer explants. To assess the general importance of such Spd-protein complexes, we attempted bulk isolation from protoplasts of Petunia and oat (Avena sativa). In Petunia, as in tobacco, fed radioactive Spd is bound to protein, but in oat, Spd is first converted to 1,3,-diaminopropane (DAP), probably by polyamine oxidase action. In oat, binding of DAP to protein depends on age of donor leaf and conditions of illumination and temperature, and the extraction of the DAP-protein complex depends upon buffer and pH. The yield of the DAP-protein complex was maximized by extraction of frozenthawed protoplasts with a pH 8.8 carbonate buffer containing SDS. Its molecular size, based on Sephacryl column fractionation of ammonium sulfate precipitated material, exceeded 45 kilodaltons. Bound Spd or DAP can be released from their complexes by the action of Pronase, but not DNAse, RNAse, or strong salt solutions, indicating covalent attachment to protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman A., Kaur-Sawhney R., Galston A. W. Stabilization of Oat Leaf Protoplasts through Polyamine-mediated Inhibition of Senescence. Plant Physiol. 1977 Oct;60(4):570–574. doi: 10.1104/pp.60.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apelbaum A., Canellakis Z. N., Applewhite P. B., Kaur-Sawhney R., Galston A. W. Binding of spermidine to a unique protein in thin-layer tobacco tissue culture. Plant Physiol. 1988;88:996–998. doi: 10.1104/pp.88.4.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagni N., Adamo P., Serafini-Fracassini D. RNA, proteins and polyamines during tube growth in germinating apple pollen. Plant Physiol. 1981 Sep;68(3):727–730. doi: 10.1104/pp.68.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beninati S., Piacentini M., Cocuzzi E. T., Autuori F., Folk J. E. Covalent incorporation of polyamines as gamma-glutamyl derivatives into CHO cell protein. Biochim Biophys Acta. 1988 Feb 10;952(3):325–333. doi: 10.1016/0167-4838(88)90134-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Canellakis Z. N., Bondy P. K., Infante A. A. Spermidine is bound to a unique protein in early sea urchin embryos. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7613–7615. doi: 10.1073/pnas.82.22.7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores H. E., Galston A. W. Analysis of polyamines in higher plants by high performance liquid chromatography. Plant Physiol. 1982 Mar;69(3):701–706. doi: 10.1104/pp.69.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrer J., Kaur-Sawhney R., Shih L. M., Galston A. W. Effects of Exogenous 1,3-Diaminopropane and Spermidine on Senescence of Oat Leaves : II. Inhibition of Ethylene Biosynthesis and Possible Mode of Action. Plant Physiol. 1982 Dec;70(6):1597–1600. doi: 10.1104/pp.70.6.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icekson I., Apelbaum A. Evidence for transglutaminase activity in plant tissue. Plant Physiol. 1987 Aug;84(4):972–974. doi: 10.1104/pp.84.4.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur-Sawhney R., Flores H. E., Galston A. W. Polyamine oxidase in oat leaves: a cell wall-localized enzyme. Plant Physiol. 1981 Aug;68(2):494–498. doi: 10.1104/pp.68.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur-Sawhney R., Flores H. E., Galston A. W. Polyamine-induced DNA Synthesis and Mitosis in Oat Leaf Protoplasts. Plant Physiol. 1980 Feb;65(2):368–371. doi: 10.1104/pp.65.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn G. D., Affolter H. U., Atmar V. J., Seebeck T., Gubler U., Braun R. Polyamine-mediated phosphorylation of a nucleolar protein from Physarum polycephalum that stimulates rRNA synthesis. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2541–2545. doi: 10.1073/pnas.76.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M. H., Cooper H. L., Folk J. E. Identification of hypusine, an unusual amino acid, in a protein from human lymphocytes and of spermidine as its biosynthetic precursor. Proc Natl Acad Sci U S A. 1981 May;78(5):2869–2873. doi: 10.1073/pnas.78.5.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistocchi R., Keller F., Bagni N., Matile P. Transport and subcellular localization of polyamines in carrot protoplasts and vacuoles. Plant Physiol. 1988 Jun;87(2):514–518. doi: 10.1104/pp.87.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slocum R. D., Kaur-Sawhney R., Galston A. W. The physiology and biochemistry of polyamines in plants. Arch Biochem Biophys. 1984 Dec;235(2):283–303. doi: 10.1016/0003-9861(84)90201-7. [DOI] [PubMed] [Google Scholar]
- Swift T. A., Dias J. A. Effects of the polyamine spermine on binding of follicle-stimulating hormone to membrane-bound immature bovine testis receptors. Biochim Biophys Acta. 1986 Feb 21;885(2):221–230. doi: 10.1016/0167-4889(86)90092-3. [DOI] [PubMed] [Google Scholar]